Published online Jun 7, 2015. doi: 10.3748/wjg.v21.i21.6764
Peer-review started: November 24, 2014
First decision: December 26, 2014
Revised: January 14, 2015
Accepted: February 5, 2015
Article in press: February 5, 2015
Published online: June 7, 2015
Processing time: 199 Days and 7.7 Hours
Gastric cancer (GC) is the most prevalent malignancy in the world, especially in China. GC has been postulated to spread via several different routes, including through hematogenous channels, lymphatic vessels, the seeding of peritoneal surfaces, direct extension through the gastric wall, and retrograde extension through the vas deferens or lymphatics. Testicular metastasis is rare. We show here a 53-year-old patient with GC who underwent a radical total gastrectomy approximately 22 mo ago after he presented with a sensation of heaviness and swelling of the right hemiscrotum. The diagnosis of metastatic adenocarcinoma was made after a right-side orchiectomy. We report the first case of testicular metastasis from gastric adenocarcinoma in mainland China and summarize the clinicopathologic features of the disease based on previously published papers.
Core tip: Gastric cancer has been postulated to spread via several different routes, including through hematogenous channels, lymphatic vessels, the seeding of peritoneal surfaces, direct extension through the gastric wall, and retrograde extension through the vas deferens or lymphatics. This paper presents the clinicopathologic features of a case of testicular metastasis from gastric carcinoma in a 53-year-old man. This particular pattern of spread in gastric carcinoma is rare. We report the first such case in mainland China and summarize the clinicopathologic features of the disease based on the current literature .
- Citation: Li B, Cai H, Kang ZC, Wu H, Hou JG, Ma LY. Testicular metastasis from gastric carcinoma: A case report. World J Gastroenterol 2015; 21(21): 6764-6768
- URL: https://www.wjgnet.com/1007-9327/full/v21/i21/6764.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i21.6764
The incidence of secondary neoplasia of testicular cancers ranges from 0.02% to 2.5%. Other than leukemia and lymphoma, the most common origins of secondary testicular tumors are the prostate (35%), the lung (18%), the skin (melanoma, 11%) and the kidney (9%)[1]. The testis is a rare site of metastasis from gastric carcinoma. A testicular secondary may present as a mass that resembles a primary testicular tumor and is usually diagnosed by a pathological examination. We report a male patient who presented with a testicular lump that was determined to be a metastasis from a primary gastric tumor.
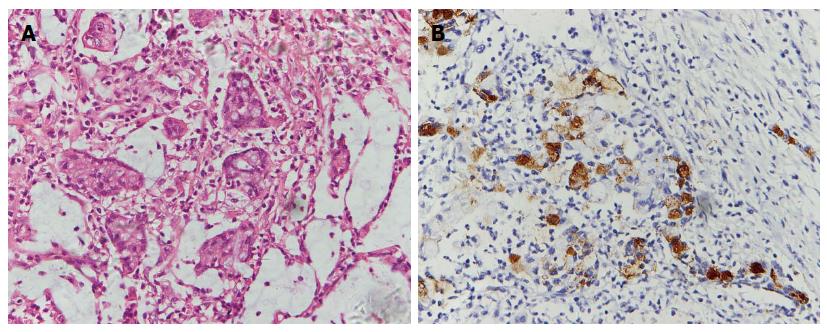
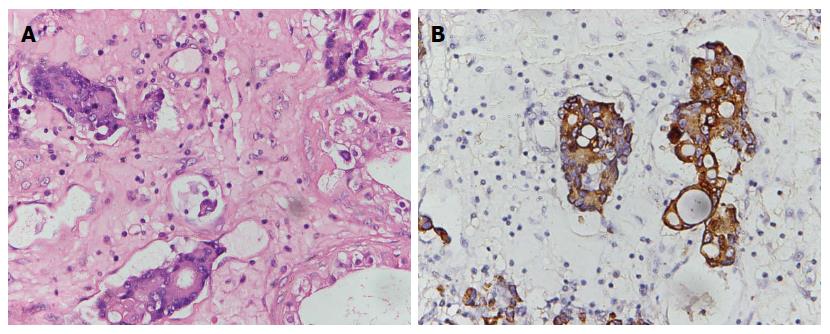
A 53-year-old man, who was diagnosed with gastric adenocarcinoma and who underwent a radical total gastrectomy, presented with a sensation of heaviness and swelling of the right hemiscrotum for approximately 6 mo. A physical examination revealed a moderate hydrocele on the right side, and the testis was impalpable. Ultrasonography indicated a 5-cm mass in the right testis, with numerous fronds surrounded by the hydrocele, while a computerized tomography (CT) scan revealed a small volume of ascites which indicated peritoneal metastasis, a hydrocele on the right side, and no para-aortic lymphadenopathy. These appearances were consistent with a testicular tumor. However, the level of β-HCG and AFP in serum were within normal limits. The patient had undergone an examination for epigastralgia 22 mo earlier. Gastroscopy and biopsy had revealed a poorly differentiated gastric adenocarcinoma in the body of stomach, and then a radical total gastrectomy plus esophagojejunal Roux-en-Y anastomosis was performed. A pathological examination revealed that the lesser and greater curvature of the stomach was 11 cm and 21 cm in length, respectively. The ulcerative lesion measuring 2.5 cm × 2 cm × 1 cm and was located 3 cm away from the proximal resection margin and 5 cm away from the distal resection margin. A histological examination of the postsurgical specimen confirmed that the full-thickness of gastric wall had been infiltrated by poorly differentiated adenocarcinoma cells; lymph nodes metastasis (22/36) was also shown. Hyperchromatic and pleomorphic neoplastic cells of the signet ring cell type were aggregated in clusters that floated in the mucinous lakes among the tumor tissue (Figure 1A). The tumor cells were immunoreactive for Muc-5 (partly+) (Figure 1B) and Ki-67 (30%+). No cancerous cells were found within the greater omentum, in the proximal resection margin or in the distal resection margin according to the histopathology examination. The patient received 6 cycles of chemotherapy after the operation, and the regimen was FOLFOX4 (oxaliplatin 85 mg/m2 per day and calcium folinate 200 mg/m2 per day were administered intravenously by a drip on the first day, fluorouracil 400 mg/m2 per day was injected intravenously, and fluorouracil 600 mg/m2 per day was injected intravenously via a pump for 22 h over the first 2 d. This regimen was performed once every 2 wk and twice as 1 cycle.) His chemotherapy was completed on 29th May 2013, and the PET-CT scan showed no recurrence of the tumor after 1 mo. He was then followed-up by regular check-ups, and no abnormalities were detected until six months ago. On 18th September 2014, the patient underwent an orchiectomy of the right testis. Macroscopically, the right testis measured 5 cm × 3 cm × 2 cm and its incisal surface contained a gray solid tumor. The right epididymis measured 4 cm × 1.5 cm × 1 cm and its incisal surface showed an isabeling solid tumor. The spermatic cord was 6 cm in length. Microscopy revealed extensive infiltrates of a poorly differentiated adenocarcinoma that involved the testicular interstitium (Figure 2A), tunica albuginea, epididymis, and spermatic cord. Hyperchromatic and pleomorphic neoplastic tumor cells were observed especially in the testicular interstitium. Some signet ring cells were clustered and had invaded the cancerous tissue; focal mucinous lakes were also observed. Those tumor cells were immunoreactive for CAM5.2 (scattered+) (Figure 2B), CK8/18 (scattered+), Muc-1 (manipulus+), Ki-67 (focal 40%+), but were nonreactive for Vimentin (VI), Oct 3/4, placental alkaline phosphatase (PLAP), inhibin hormone (INH), C-Kit and AFP. These features suggested a metastatic mucinous adenocarcinoma that was consistent with an origin in the patient’s gastric carcinoma. The patient received 2 cycles of the FOLFOX4 chemotherapy after the orchiectomy of the right testis. The disease was stable until 31st December 2014.
The incidence on biopsy of secondary neoplasms that are testicular in nature is 0.02% to 2.5%. Other than leukemia and lymphoma, the most common origins of secondary testicular tumors are the prostate (35%), the lung (18%), the skin (melanoma, 11%) and the kidney (9%)[1]. Gastric cancer (GC) with testicular metastasis is not frequently described, and only approximately 14 cases worldwide have been reported. GC has been postulated to spread by several routes, including through hematogenous channels, lymphatic vessels, the seeding of peritoneal surfaces, direct extension through the gastric wall, and retrograde extension through the vas deferens or lymphatics. The possible dissemination pathways of gastric tumor metastasis to the testicles are hematogenous channels, retrograde through the lymph vessels and along the spermatic cord and the seeding of the peritoneal surfaces. Then, the tumor cells may migrate through the abdominal ring and over an open Haller’s habenula to reach the testis, which is a similar pattern of migration as the Krukenberg tumor in women[2]. Ford et al[3] reported that the left testis and spermatic cord were involved by the gastric carcinoma and this was accompanied by a hard, nontender Blumer’s shelf. However, the incidence of secondary metastasis to the testis is very low compared with metastasis to the ovary, which can be explained by its distinct anatomy. The testes are suspended in the scrotum by scrotal tissue and the spermatic cords, and are invaginated by the serosal tunica vaginalis, which becomes sequestered from the processus vaginalis and the peritoneal cavity just before birth[4]. The right testis descends to the scrotum later compared with the left testis in children, and the right processus vaginalis may be more prone to insufficiency. This may lead to an insufficient obliteration of the peritoneal gap in the abdominal inguinal ring and the generation of a temporary patent processus vaginalis testis, whereas in adulthood, the regressive obliteration of these structures allows for tumor spread through a patent processus vaginalis. Inga-Marie Schaefer reported an elderly male patient who underwent an orchidopexy for left-sided cryptorchidism before the tumor metastasized to the testis, and only the non-cryptorchid right testis was affected, possibly because previous operative obliteration of the left inguinal canal prevented metastatic spread to that side[5]. The age of the patients at the onset of symptoms ranged from 23 to 68 years. The average age of onset of testicular metastasis of GC was 55.9 ± 13.7 years, whereas most patients with primary testicular tumors are rarely over 40 years of age. Complaints of unilateral testicular swelling with or without pain are frequent: 4 of 8 cases occurred on the right side[1,2,5,6] and 4 of 9 cases occurred on the left side[3,4,7,8]. Most of the cases occurred 2 mo to 9 years after the diagnosis of the gastric carcinoma, and some of the patients were misdiagnosed with primary testicular tumors before the histopathology examination[1,5] because of the similar clinical manifestations, such as swelling and pain of the scrotum and sudden acute pain of the testis[4]. Hydrocele is also a typical sign of metastatic tumors of the testis[3,4,5,7,8]. A case report showed that the yellowish fluid that was collected by aspiration from the hydrocele contained metastatic adenocarcinoma cells, which were consistent with those of a gastric primary tumor[4]. The negative effects of the hydrocele may include its role as an obstacle to lymphatic return by the metastatic cancer cells and the insufficiency of the processus vaginalis, which may lead to contact with the ascites[5]. The tumor markers in the serum that are related to the gastric carcinoma such as CA19-9[6,7] and CEA[2] may be elevated, which may indicate a recurrence of the gastric tumor. The levels of AFP and β-HCG in the serum, which are typically elevated in primary tumors of the testis, were within normal limits. Ota et al[7] reported an atypical patient with an elevated level of β-HCG in the serum (9.48 ng/mL) and in the hydrocele (83.2 ng/mL), which likely occurred because of the expression of β-HCG in the gastric cancerous tissue, as demonstrated by an immunohistochemical analysis. The positive expression rate of β-HCG in gastric cancer tissue is approximately 10.5%-23.5%[7], and some types of gastric carcinoma also express AFP. Therefore, it may be prudent to identify this marker in primary testicular tumors. Most of the patients underwent an orchiectomy. Due to the strong capsule around the testes termed the “tunica albuginea”, metastasis to the testes rarely occurs, but when it does, the tumor cells are prone to infiltrate the tunica albuginea, the epididymis, and the spermatic cord[6]; only 4 reported cases thus far have involved the testicular interstitium[1,2,5,8], and 1 case has involved the rete testis[5]. A histologic examination of postsurgical samples confirmed poorly differentiated adenocarcinomas (including the presence of signet ring cells and mucinous cells), which were similar to the primary gastric tumor; however, one case showed a degree of differentiation in the metastatic tumor cells that was lower than that of the primary tumor[3]. The tumor cells were immunoreactive for CEA and pan-keratin, but were nonreactive for AFP, β-HCG, CD117 and PLAP, which is a characteristic of a primary testicular tumor[1], except for those markers that are also expressed in primary gastric carcinoma[7]. The immunohistochemical markers of cancerous tissue are the most significant rationale for diagnosis. The prognosis of patients with testicular metastasis from gastric carcinoma is poor because of the advanced stage of the disease, and the overall survival is only 2-51 mo.
In conclusion, we present the clinicopathologic features of a testicular metastasis from gastric carcinoma in a 53-year-old man. This particular pattern of spread in gastric carcinoma is rare, and the diagnosis is usually based on pathological examination.
A 53-year-old man who was diagnosed with gastric adenocarcinoma and who underwent a radical total gastrectomy, presented with a sensation of heaviness and swelling of the right hemiscrotum.
A physical examination revealed a moderately-sized hydrocele on the right side, and the testis was impalpable.
The differential diagnosis of this disease is usually primary testicular tumor, which is based on the histopathology examination.
The levels of β-HCG and AFP in the serum were within normal limits.
Ultrasonography indicated a 5-cm mass in the right testis, with numerous fronds surrounded by the hydrocele. A computerized tomography scan revealed a small volume of ascites, a hydrocele on the right side, and no paraaortic lymphadenopathy.
Microscopy revealed extensive infiltrates of a poorly differentiated adenocarcinoma that involved the testicular interstitium, tunica albuginea, epididymis, and spermatic cord. In addition, the tumor cells were immunoreactive for CAM5.2 (scattered+) and CK8/18 (scattered+).
The patient underwent an orchiectomy of the right testis and was subsequently treated with FOLFOX 4 chemotherapy.
Gastric cancer with testicular metastasis is rarely reported, and the clinical and pathological characteristics of the disease remain unclear.
Orchiectomy is a surgical method of castration in which one or both testicles are removed. There are three main types of orchiectomy: simple, subcapsular, and inguinal.
Testicular tumors in patients with a history of gastric carcinoma should be diagnosed based on the histopathologic examination.
The authors describe a case of a 53-year-old patient with gastric cancer with testicular metastasis. They have also summarized the clinicopathologic features of the disease based on previously published papers.
P- Reviewer: Herszenyi L S- Editor: Qi Y L- Editor: O’Neill M E- Editor: Wang CH
| 1. | Yang KC, Chao Y, Luo JC, Kuo JY, Lee RC, Li AF, Li CP. The unusual presentation of gastric adenocarcinoma as a testicular mass: a favorable response to docetaxel and Cisplatin plus oral tegafur/uracil and leucovorin. J Chin Med Assoc. 2010;73:88-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Alois M, Valentina P, Andreas G. The “Krukrnberg” tumor in male. Arch Esp Urol. 2005;58:971-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (1)] |
| 3. | Ford ML, Tandan B. Metastatic tumor of the spermatic cord and testis from carcinoma of the stomach: a case report. South Med J. 1969;62:352-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 4. | Wai HP, Yau TK, Sze WM, Yeung MW, Hioe F, Lee AW. Metastatic tumour of the tunica vaginalis testis from carcinoma of the stomach. Int J Clin Pract. 2000;54:685-686. [PubMed] |
| 5. | Schaefer IM, Sauer U, Liwocha M, Schorn H, Loertzer H, Füzesi L. Occult gastric signet ring cell carcinoma presenting as spermatic cord and testicular metastases: “Krukenberg tumor” in a male patient. Pathol Res Pract. 2010;206:519-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Civelek B, Aksoy S, Kös T, Seker MM, Arik Z, Sendur MA, Yaman S, Cihan S, Ozdemir NY, Uncu D. Isolated Testicular Metastasis of Gastric Cancer. J Gastrointest Cancer. 2011;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Ota T, Shinohara M, Tanaka M, Date Y, Itakura H, Munakata A, Kinoshita K, Hishima T, Koike M, Kitamura M. Spermatic cord metastases from gastric cancer with elevation of serum hCG-beta: a case report. Jpn J Clin Oncol. 2000;30:239-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |