Published online Jun 7, 2015. doi: 10.3748/wjg.v21.i21.6736
Peer-review started: November 8, 2014
First decision: November 26, 2014
Revised: January 9, 2015
Accepted: February 13, 2015
Article in press: February 13, 2015
Published online: June 7, 2015
Processing time: 217 Days and 3 Hours
AIM: To investigate the utility of 1H magnetic resonance spectroscopy (1H MRS) as a noninvasive test for steatosis in patients infected with hepatitis C virus.
METHODS: Ninety patients with chronic hepatitis C and pathology data underwent 3.0T 1H MRS, and the results of MRS and pathological analysis were compared.
RESULTS: This group of patients included 26 people with mild fatty liver (28.89%), 16 people with moderate fatty liver (17.78%), 18 people with severe fatty liver (20.0%), and 30 people without fatty liver (33.33%). The water peak was near 4.7 parts per million (ppm), and the lipid peak was near 1.3 ppm. Analysis of variance revealed that differences in the lipid peak, the area under the lipid peak, ratio of the lipid peak to the water peak, and ratio of the area under the lipid peak to the area under the water peak were statistically significant among the groups. Specifically, as the severity of fatty liver increased, the value of each index increased correspondingly. In the pairwise comparisons, the mean lipid peak, area under the lipid peak, ratio of the lipid peak to the water peak, and ratio of the area under the lipid peak to the area under the water peak were significantly different between the no fatty liver and moderate fatty liver groups, whereas no differences were noted between the severe fatty liver group and the mild or moderate fatty liver group. Area under the ROC curve (AUC) of area ratio in lipid and water and ratio in lipid and water in the no fatty liver group to mild fatty liver group, mild fatty liver group to moderate fatty liver group, and moderate fatty liver disease group to severe fatty liver group, were 0.705, 0.900, and 0.975, respectively.
CONCLUSION: 1H MRS is a noninvasive technique that can be used to provide information on the effect of liver steatosis on hepatic metabolic processes. This study indicates that the 1H MRS can be used as an indicator of steatosis in patients with chronic hepatitis C.
Core tip: The aim of this study was to investigate the utility of 1H magnetic resonance imaging spectroscopy (1H MRS) as a noninvasive test of steatosis in patients infected with hepatitis C virus. Ninety chronic hepatitis C patients with pathology data underwent 3.0T 1H MRS. 1H MRS is a noninvasive technique that can be used to provide liver steatosis information on hepatic metabolic processes. This study indicates that the 1H MRS can be used as an indicator of steatosis in chronic hepatitis C patients.
- Citation: Zhang Q, Zhang HM, Qi WQ, Zhang YG, Zhao P, Jiao J, Wang JB, Zhang CY. 3.0T 1H magnetic resonance spectroscopy for assessment of steatosis in patients with chronic hepatitis C. World J Gastroenterol 2015; 21(21): 6736-6744
- URL: https://www.wjgnet.com/1007-9327/full/v21/i21/6736.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i21.6736
As a result of obesity and insulin resistance in patients with nonalcoholic fatty liver disease (NAFLD), the prevalence of hepatic steatosis is increasing rapidly throughout the world[1,2]. Simple nonalcoholic steatosis can progress to more serious liver disease [nonalcoholic steatohepatitis (NASH) and cirrhosis], representing a threat to public health.
Hepatitis C virus (HCV) is one of the leading causes of liver disease worldwide. It is estimated that approximately 3% of the global population is infected with HCV, many of whom develop chronic liver disease, cirrhosis, or even hepatitis carcinoma[3-5]. The prognosis of hepatitis and the efficacy of antiviral therapy vary among individuals, and recently, the presence of fatty liver was also found to affect these variables. The incidence of HCV infection with overlapping steatosis ranges internationally between 22% and 76%. Fatty liver and viral hepatitis can exist simultaneously and promote liver fibrosis, which is an important risk factor for cirrhosis and hepatocellular carcinoma[6,7]. In addition, in recent years, studies illustrated that hepatic steatosis also affects antiviral efficacy, and American Association for the Study of Liver Diseases (AASLD) HCV treatment guidelines suggested that fatty liver is one of the factors that affect the likelihood of a virologic response following HCV treatment.
Liver biopsy remains the gold standard for evaluating hepatic steatosis, despite well-established drawbacks regarding its invasiveness and sampling errors due to small sample sizes and inter-observer variability[8,9]. However, this invasive procedure is not without risk. The procedure is associated with a low mortality rate and high error rate, predominantly owing to undersampling, whereby less than 1/50000th of the liver volume is typically obtained for histologic evaluation. Histological assessment of a needle biopsy specimen is potentially inaccurate because the heterogenic manifestation of hepatic steatosis can lead to underscoring of the severity of steatosis or result in false-positive results[10]. These factors highlight the need for a noninvasive test to characterize diffuse liver disease. For ethical reasons and because most patients are unwilling to undergo repeated procedures, treatment algorithms rarely allow serial liver biopsy.
Noninvasive modalities such as ultrasound, computed tomography, and magnetic resonance imaging (MRI) have been employed for the assessment of hepatic steatosis[11-13]. However, these modalities do not specifically measure hepatic fat content, they are semiquantitative, and they lack a high sensitivity and specificity[12]. Many studies have focused on the role of imaging techniques as noninvasive alternatives to liver biopsy for detecting and quantifying hepatic steatosis[14]. The reported sensitivities and specificities of different imaging techniques and different studies investigating the same technique vary substantially.
1H magnetic resonance spectroscopy (1H MRS) is a safe and noninvasive alternative for quantifying hepatic fat content. The modality offers good reproducibility and a detailed investigation of different liver lobes, and it has been evaluated in various clinical studies. 1H MRS is widely used to measure intramyocellular and intrahepatocellular lipid content in vivo[15,16]. 1H MRS measures the resonance signals derived from protons in triglycerides (TGs), which can be quantified and used as a noninvasive marker of the severity of steatosis. The lipids observed in 1H MRS arise mainly from TGs in lipid droplets, as these are nuclear magnetic resonance-visible, whereas lipids bound to membranes and proteins are too rigid to generate a 1H MRS-observable signal. This property of 1H MRS to detect mobile lipids in lipid droplets has made it the standard method for quantifying liver fat content[17,18]. The purpose of this study was to assess the value of 1H MRS in diagnosing hepatic steatosis in patients with NAFLD.
From January 2010 to June 2010, 90 patients with chronic hepatitis C were enrolled. The diagnosis of hepatitis C was based on the AASLD Clinical Guideline for Hepatitis C (2004). This study was conducted in accordance with the Declaration of Helsinki. This study was conducted with approval from the Ethics Committee of Jilin University. Written informed consent was obtained from all participants.
All enrolled patients were also naïve to any anti-viral treatment. The other inclusion criteria were as follows: (1) HCV RNA > 500 copies/mL; (2) absence of complications such as gastrointestinal bleeding, hepatic encephalopathy, and primary liver cancer; and (3) liver function defined as Child-Pugh grade A or B based on serum bilirubin and albumin levels, the presence of ascites and hepatic encephalopathy, and the prothrombin time. Patients with hypersplenism were also enrolled. The exclusion criteria were as follows: (1) infection with hepatitis A, B, D, or F virus, Epstein-Barr virus, cytomegalovirus, or human immunodeficiency virus; and (2) the presence of alcoholic or drug-induced liver diseases or severe heart, brain, or kidney disease.
According to the 2003 branch of the Chinese Medical Association to develop liver fatty liver disease classification criteria for grading liver fat content[19], 30%-50% hepatic steatosis was classified as mild fatty liver; 50%-75% steatosis as moderate fatty liver, and greater than 75% as severe fatty liver. Meanwhile, fatty degeneration of the field of vision affecting less than 30% of liver cells was classified as the absence of fatty liver. The severity of disease was scored according to the Ishak system. The classification of patients with mild and moderate diseases was based on the Ishak fibrosis (F) and necroinflammatory (NI) scoring system as follows: mild hepatitis (F ≤ 2 and NI ≤ 3), moderate hepatitis (3 ≤ F < 6 and NI > 4), and cirrhosis (F = 6). Liver disease was evaluated using 3.0T MRI 1H MRS. According to each area under the peak, we can calculate the percentage area and compare the values with those obtained via pathologic analysis to determine whether the aforementioned parameters differ among the groups.
MRI measurements were performed using a clinical Philips Achieva 3.0 T TX scanner (Philips Healthcare, Best, The Netherlands). The Sense Torso coil was positioned on the abdomen, and scout images were acquired to localize the liver and surrounding structures. T1- and T2-weighted images were acquired for all patients and controls. The images were acquired using the following parameters: TR/TE, 2000/40 ms; field of view = 35 mm × 35 mm × 35 mm, 96 averages, 3.4 mm, PA w/s exc angle 250.
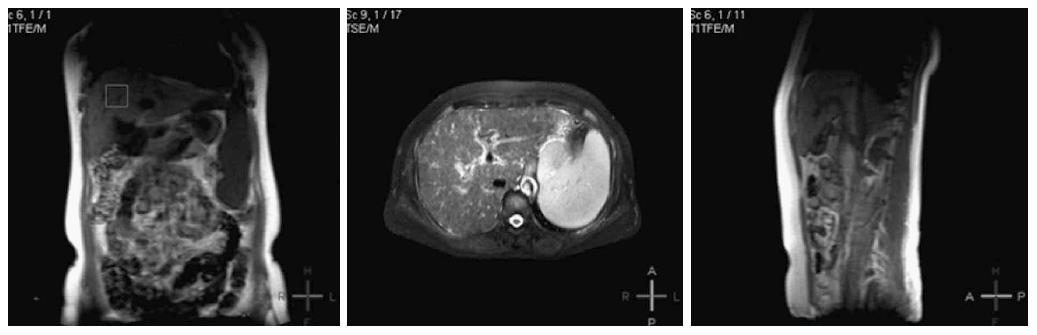
1H MRS was performed with and without water suppression. Localized breath-hold single voxel point resolved spectroscopy (PRESS) with TR/TE = 3000 ms/35 ms and number of averages = 64 were taken. A voxel of 2 cm × 2 cm × 2 cm was located mainly in the right parietal region of the liver in all subjects. Data acquisition was performed with breath holding to ensure that the scanning area of interest was constant and to reduce the impact of cardiac pulsatility. Liver tissue contains more water and fat, and the strongest 1H MRS signals detected are water and fat.
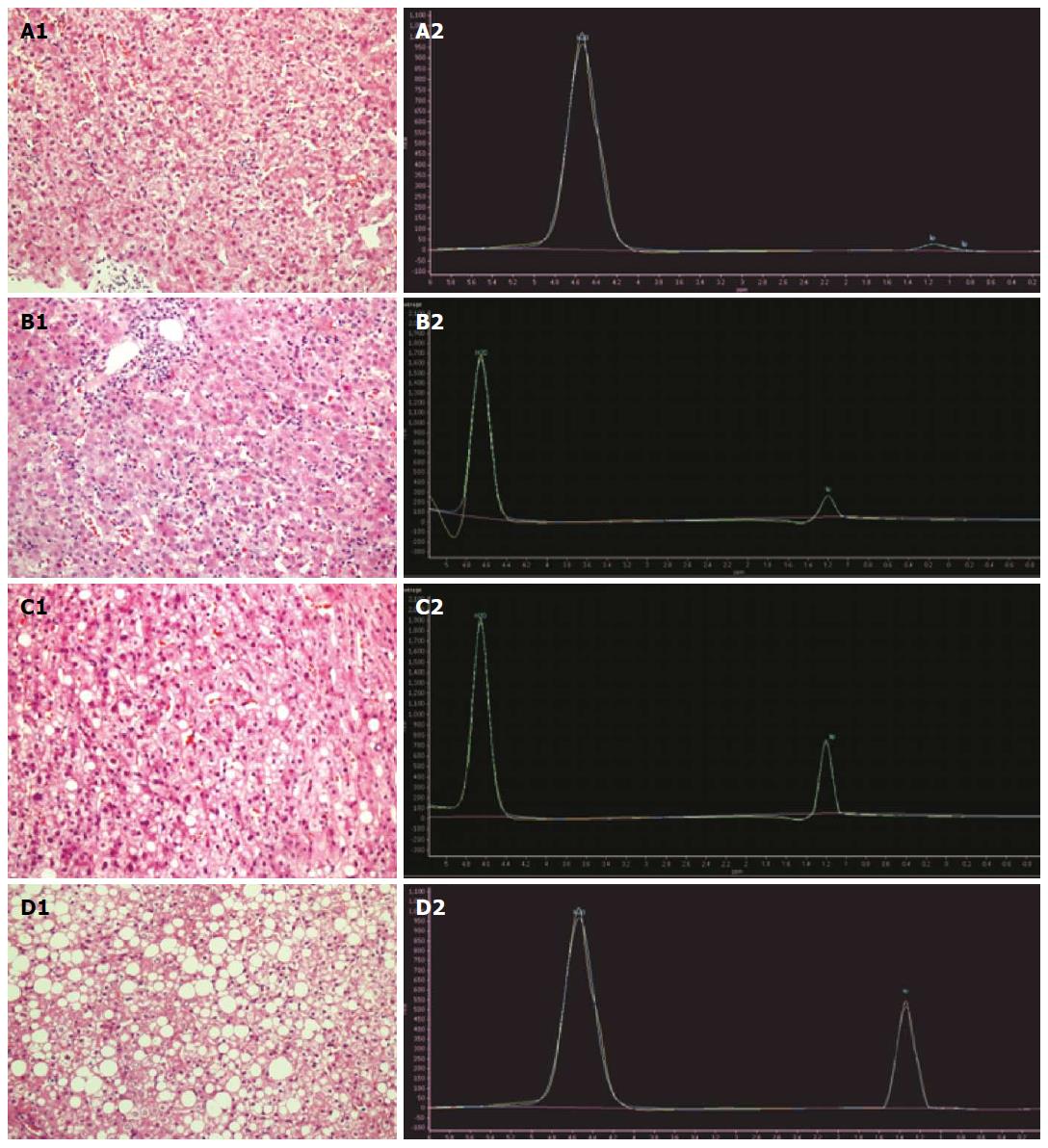
All data were calibrated and calculated using the spectroview of extended MR workspace 2.6.3.2. The peak lipid value, peak water value, area under the lipid peak, and lipid/water ratios of all patients were analyzed and compared among the different groups. In Philips workstation, the collection of the original data was proceeded with Spectroview software. After a baseline correction and frequency correction, with water peak as a reference, 1H MRS water peak is about near 4.7 parts per million (ppm), and fat peak is about 1.3 ppm. Then, the lipid peak/water peak ratio, the area under the lipid peak, and the ratio of the area under the lipid peak to the area under the water peak were calculated (Figure 1).
All statistical analyses were performed using SPSS 19.0 software (SPSS Inc., Chicago, IL). Mean data were analyzed using the t-test. All statistical tests were two-sided, and P < 0.05 was considered statistically significant. The statistical methods of this study were reviewed by Yong-Gui Zhang from 3rd Hospital of Jilin University.
This group of patients included 26 (28.89%), 16 (17.78%), and 18 patients (20.0%) with mild, moderate, and severe fatty liver, respectively, and 30 patients without fatty liver (33.33%). In terms of gender differences, the proportion of males was higher than that of females in the moderate and severe fatty liver groups (P < 0.05), whereas no gender differences were observed in the no fatty liver and mild fatty liver groups. In addition, total bilirubin (TBIL), alanine aminotransferase (ALT), aspartate transaminase (AST), triglyceride (TG), cholesterol, and fasting blood glucose levels were higher in the moderate and severe fatty liver groups than in the no fatty liver and mild fatty liver groups (P < 0.05; Table 1).
| No fatty liver (n = 30) | Mild fatty liver (n = 26) | Moderate fatty liver (n = 16) | Severe fatty liver (n = 9) | |
| Age (yr) | 49.8 ± 10.6 | 53.4 ± 9.8 | 52.7 ± 10.4 | 55.6 ± 11.2 |
| Gender | Male 8 (53.33%) | Male 6 (46.15%) | Male 5 (62.5%)a | Male 7 (77.78%)a |
| HCV-RNA(copies/mL) | 5.18 ± 1.20 | 5.43 ± 1.14 | 5.23 ± 1.65 | 5.43 ± 1.47 |
| Child-Plug | 3 ± 1 | 4 ± 1 | 6 ± 1 | 7 ± 1 |
| TBIL | 12.7 ± 6.7 | 13.9 ± 6.4 | 26.6 ± 6.9a | 38.7 ± 11.6a |
| ALT | 12.9 ± 10.6 | 18.8 ± 12.3 | 62.4 ± 13.2a | 103.8 ± 20.3a |
| AST | 10.7 ± 9.9 | 16.5 ± 10.1 | 52.5 ± 12.3a | 87.7 ± 16.2a |
| TG | 1.2 ± 0.3 | 1.8 ± 0.4 | 3.2 ± 1.1a | 5.4 ± 2.1a |
| Chol | 2.7 ± 1.2 | 3.3 ± 1.5 | 5.4 ± 1.8a | 6.7 ± 2.2a |
| Blood glucose | 4.1 ± 0.2 | 4.3 ± 0.3 | 5.8 ± 2.0a | 7.4 ± 3.3a |
In the lipid and water peak curve, the water peak was near 4.7 ppm, and the lipid peak was near 1.3 ppm. Fat peak increased in patients with fatty liver, and the peak obviously increased with the severity of fatty liver (Figure 2).
1H MRS parameter analysis revealed that the lipid peak, area under the lipid peak, lipid peak/water peak ratio, and the ratio of the area under the lipid peak to the area under the water peak were significantly different among the groups, as each index increased with increasing severity of liver disease (P < 0.05). Pairwise comparisons revealed significant differences in the lipid peak, area under the lipid peak, lipid peak/water peak ratio, and the ratio of the area under the lipid peak to the area under the water peak between the no fatty liver and moderate fatty liver groups, the no fatty liver and severe fatty liver groups, the mild and severe fatty liver groups, and the moderate and severe fatty liver groups (P < 0.05). Meanwhile, no significant differences were noted between the no fatty liver and mild fatty liver groups (P > 0.05; Table 2).
| Group | Peak of fat | Fat area under the peak | Peak of water | Water area under the peak | Fat/water peak ratio | Fat/water under the peak area ratio |
| No fatty liver | 81.4 ± 46.1 | 32.56 ± 18.44 | 1450 ± 540 | 575.9 ± 216.4 | 0.0789 ± 0.0612 | 0.0846 ± 0.0531 |
| Mild fatty liver | 181.5 ± 87.7 | 71.87 ± 35.14 | 1340 ± 590 | 528.4 ± 223.8 | 0.2038 ± 0.1552 | 0.2124 ± 0.1588 |
| Moderate fatty liver | 596.4 ± 293.8a | 238.6 ± 117.5a | 1460 ± 670 | 582.6 ± 247.9 | 0.6344 ± 0.4924a | 0.5968 ± 0.4326a |
| Severe fatty liver | 1155.6 ± 250.2a | 462.2 ± 120.16a | 1420 ± 480 | 568.7 ± 197.2 | 0.8856 ± 0.4795a | 0.8742 ± 0.4528a |
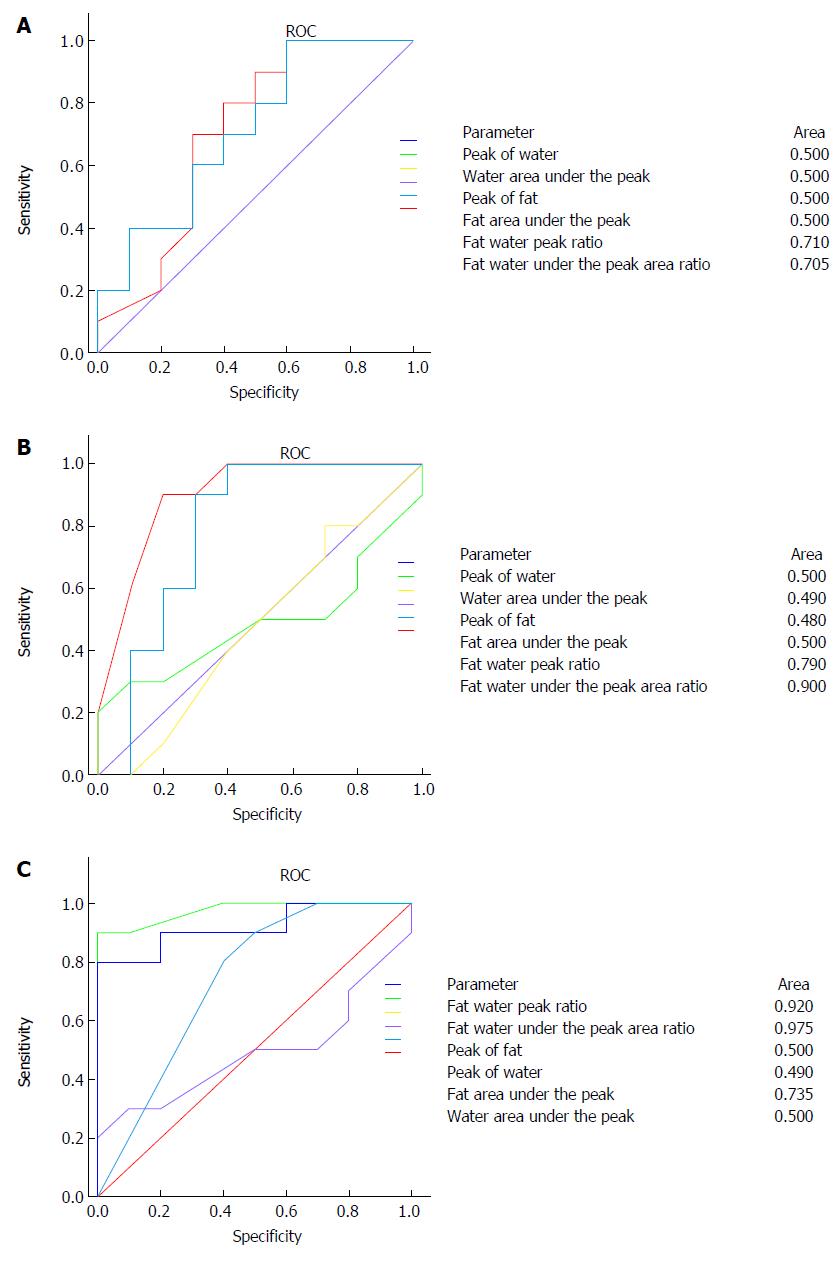
ROC curve of 1H MRS parameter was used to different the degree of fatty liver. When comparing the no fatty liver group and mild fatty liver groups, area under the ROC curve (AUC) of area ratio in lipid and water and ratio in lipid and water were 0.705 and 0.71, which have certain reference significance, and the other parameters are not sensitive (Figure 3A). When comparing the mild fatty liver group and moderate fatty liver group, area under the ROC curve (AUC) of area ratio in lipid and water and ratio in lipid and water were 0.900 and 0.780, respectively, which showed a good sensitivity and specificity (Figure 3B). To compare the moderate fatty liver disease and severe fatty liver groups, area under the ROC curve (AUC) of area ratio in lipid and water and ratio in lipid and water were 0.975 and 0.920, respectively, which showed a good sensitivity and specificity, and lipid peak area under the ROC curve AUC was 0.735 (Figure 3C).
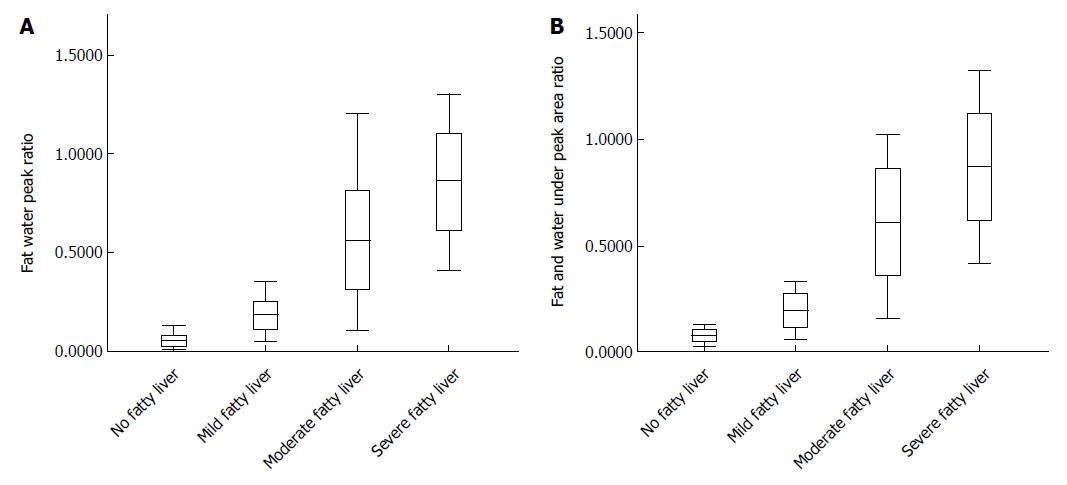
By analysis of peak ratio of fat and water, and area ratio of fat and water in patients with different degrees of fatty liver, it was shown that peak ratio of fat and water and the area ratio of fat and water between groups were statistically significant (P < 0.05; Figure 4).
It is estimated that approximately 3% of the global population is chronically infected with HCV and that approximately 4 million persons are newly infected each year. In 55%-85% of patients, HCV infection progresses to chronic liver disease, with many patients remaining asymptomatic. In approximately 20% of cases, fibrosis develops into cirrhosis, which leads to hepatocellular cancer in 5% of patients each year[20,21]. Liver biopsy is the reference standard for staging and grading chronic liver disease, but this invasive procedure is not without risk. There is a low mortality rate and high error rate associated with this procedure, predominantly owing to undersampling, as typically less than 1/50000th of the liver volume is obtained for histologic evaluation[22]. As a result of the problems associated with biopsy, a steady drive to identify an effective noninvasive method of evaluating liver damage has led to developments both in testing with serologic biomarkers of disease and in imaging. For ethical reasons and because most patients are unwilling to undergo repeated procedures, treatment algorithms in the United Kingdom rarely allow serial liver biopsy. The impetus to find a reliable and repeatable biomarker of disease activity and response to treatment thus has a renewed focus[23].
MRS is a valuable tool for the noninvasive assessment of metabolic processes in vivo. Because of the presence of certain compounds in the organization of nuclear protons, these compounds or metabolites produce certain chemical shifts in specific chemical environments. Small changes in the magnetic resonance peak caused by these changes could be collected by a magnetic resonance scanner and converted to numerical spectra. Neuronal markers, membrane constituents, osmolytes, and the energy status can be measured for the diagnosis of various diseases and therapeutic monitoring in humans[24]. 1H MRS generates a spectrum of the various resonances of protons that are embedded in different chemical bonds. Because the protons are surrounded by various nuclei and electrons with their own magnetic properties, small magnetic field perturbations occur in a systematic manner, leading to slight differences in the received frequencies of protons in different chemical bonds. Thus, the chemical shifts occur essentially as a consequence of the variable electronegativity of adjacent chemical moieties in the molecule. The chemical shift scale describes the position of resonances in the spectrum in ppm, irrespective of the field strength, relative to a reference set at 0 ppm. The underlying frequency shift, however, measured in Hertz (Hz), is directly proportional to the strength of the magnetic field, e.g., 1 ppm of the proton spectrum at 1.5T refers to 64 Hz and to 128 Hz at 3.0T. Therefore, with higher magnetic fields, the resonances are better separated. The frequency separation of the resonances or peaks describes the resolution of the spectrum[19,25].
The clinical use of localized 1H MRS in vivo, first in the brain and then in the prostate, has been well established and refined over the last two decades[26,27]. Single volume spectroscopy with a stimulated echo acquisition mode or the PRESS technique is recommended because of longer acquisition times and reduced SNR for multivoxel liver MRS with chemical shift imaging[28,29].
The ratio of the fat peak (1.3 ppm) to the water peak (4.7 ppm) is a common definition of the hepatic fat percentage as determined by 1H MRS[30]. Using this definition, Thomas et al[30] reported the relationship between body adiposity and steatosis in 11 patients with NASH and identified hepatic fat percentages of up to 75%. In a clinical study by Longo et al, an equation (AUCtotal fat peaks/AUCtotal peaks) for calculating hepatic fat content from 1H MR spectra was advocated. The same method was applied in a large study by Szczepaniak et al, who evaluated the prevalence of hepatic steatosis in over 2300 participants of the Dallas Heart Study population[31].
In this study, a Philips Achieva 3.0T TX scanner and 1H torso toil were used to obtain the signal. Localized breath-hold single-voxel PRESS was used. In this study, data were analyzed using the Philips Achieva 3.0T spectroview of extended MR workspace 2.6.3.2, quantitative spectral analysis of chemical shifts, calculation of the product of the metabolite peak and the area under the peak, and other variables. The peak lipid value, area under the lipid peak, peak lipid/peak water ratio, and ratio of the area under the lipid peak to the area under the water peak were statistically different between the control group and antiviral group at baseline and between baseline and 6 mo after the start of therapy in the antiviral therapy group. 1H MRS parameter analysis revealed that that the lipid peak, area under the lipid peak, fat peak/water peak ratio, and ratio of the area under the lipid peak to the area under the water peak were statistically significant among the groups, as each index increased with increasing severity of fatty liver disease. Pairwise comparisons revealed significant differences in the lipid peak, area under the lipid peak, lipid peak/water peak ratio, and the ratio of the area under the lipid peak to the area under the water peak between the no fatty liver and moderate fatty liver groups, the no fatty liver and severe fatty liver groups, the mild and severe fatty liver groups, and the moderate and severe fatty liver groups. Meanwhile, no significant differences were noted between the no fatty liver and mild fatty liver groups. The findings suggested that liver steatosis was modified significantly by antiviral therapy in patients with chronic HCV-linked steatosis, which is the same as the result reported by van Werven[32].
In short, 3.0T 1H MRS may be an effective technology for assessing lipid metabolism in patients with chronic HCV. However, the study samples are relatively small, necessitating further in-depth exploration.
Hepatitis C virus (HCV) is one of the leading causes of liver disease worldwide. Liver biopsy remains the gold standard for providing the stage (extent of fibrosis) and grade (degree of NI activity) of HCV-related liver disease, but this invasive procedure is not without risk. The impetus to find a reliable and repeatable biomarker of disease activity and response to treatment thus has a renewed focus
Clinical (in vivo) 1H magnetic resonance spectroscopy (1H MRS) is a noninvasive technique that can be used to assess the degree of liver steatosis.
This study was the first attempt to use 1H MRS to assess the steatosis of the liver in hepatitis C during the antiviral therapy. 1H MRS is a noninvasive technique that can be used to assess the degree of liver steatosis.
1H MRS is a noninvasive technique that can be used to assess the degree of liver steatosis.
1H MRS is a safe and noninvasive alternative for quantifying hepatic fat content. The modality offers good reproducibility and a detailed investigation of different liver lobes, and it has been evaluated in various clinical studies. 1H MRS is widely used to measure intramyocellular and intrahepatocellular lipid content in vivo. 1H MRS measures the resonance signals derived from protons in triglycerides (TGs), which can be quantified and used as a noninvasive marker of the severity of steatosis. The lipids observed in 1H MRS arise mainly from TGs in lipid droplets, as these are nuclear magnetic resonance-visible, whereas lipids bound to membranes and proteins are too rigid to generate a 1H MRS-observable signal. This property of 1H MRS to detect mobile lipids in lipid droplets has made it the standard method for quantifying liver fat content
This is a good descriptive study in which authors attempt to use 3.0T 1H MR spectroscopy for assessment of steatosis during antiviral therapy for chronic hepatitis C. It provided a new noninvasive technique for assessing the steatosis of the liver and response to antiviral therapy for chronic hepatitis C.
P- Reviewer: Chuang WL, Lau WY, Skrypnyk IN, Sugimura H S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Liu XM
| 1. | Angulo P. Current best treatment for non-alcoholic fatty liver disease. Expert Opin Pharmacother. 2003;4:611-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Williams R. Global challenges in liver disease. Hepatology. 2006;44:521-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 546] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 3. | Rector RS, Thyfault JP, Wei Y, Ibdah JA. Non-alcoholic fatty liver disease and the metabolic syndrome: an update. World J Gastroenterol. 2008;14:185-192. [PubMed] |
| 4. | Bashir MR, Merkle EM, Smith AD, Boll DT. Hepatic MR imaging for in vivo differentiation of steatosis, iron deposition and combined storage disorder: single-ratio in/opposed phase analysis vs. dual-ratio Dixon discrimination. Eur J Radiol. 2012;81:e101-e109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Irimia E, Mogoantă L, Predescu IO, Efrem IC, Stănescu C, Streba LA, Georgescu AM. Liver steatosis associated with chronic hepatitis C. Rom J Morphol Embryol. 2014;55:351-356. [PubMed] |
| 6. | Rubbia-Brandt L, Fabris P, Paganin S, Leandro G, Male PJ, Giostra E, Carlotto A, Bozzola L, Smedile A, Negro F. Steatosis affects chronic hepatitis C progression in a genotype specific way. Gut. 2004;53:406-412. [PubMed] |
| 7. | Cheng FK, Torres DM, Harrison SA. Hepatitis C and lipid metabolism, hepatic steatosis, and NAFLD: still important in the era of direct acting antiviral therapy? J Viral Hepat. 2014;21:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Moritou Y, Ikeda F, Iwasaki Y, Baba N, Takaguchi K, Senoh T, Nagano T, Takeuchi Y, Yasunaka T, Ohnishi H. Impact of comorbid hepatic steatosis on treatment of chronic hepatitis C in Japanese patients and the relationship with genetic polymorphism of IL28B, PNPLA3 and LDL receptor. Acta Med Okayama. 2014;68:17-22. [PubMed] |
| 9. | Macaluso FS, Maida M, Cammà C, Cabibbo G, Cabibi D, Alduino R, Di Marco V, Craxì A, Petta S. Steatosis affects the performance of liver stiffness measurement for fibrosis assessment in patients with genotype 1 chronic hepatitis C. J Hepatol. 2014;61:523-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 10. | Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, Grimaldi A, Capron F, Poynard T. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898-1906. [PubMed] |
| 11. | Mehta SR, Thomas EL, Bell JD, Johnston DG, Taylor-Robinson SD. Non-invasive means of measuring hepatic fat content. World J Gastroenterol. 2008;14:3476-3483. [PubMed] |
| 12. | Cho CS, Curran S, Schwartz LH, Kooby DA, Klimstra DS, Shia J, Munoz A, Fong Y, Jarnagin WR, DeMatteo RP. Preoperative radiographic assessment of hepatic steatosis with histologic correlation. J Am Coll Surg. 2008;206:480-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Bohte AE, van Werven JR, Bipat S, Stoker J. The diagnostic accuracy of US, CT, MRI and 1H-MRS for the evaluation of hepatic steatosis compared with liver biopsy: a meta-analysis. Eur Radiol. 2011;21:87-97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 326] [Cited by in RCA: 395] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 14. | Charatcharoenwitthaya P, Lindor KD. Role of radiologic modalities in the management of non-alcoholic steatohepatitis. Clin Liver Dis. 2007;11:37-54, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 147] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 15. | Bastati N, Feier D, Wibmer A, Traussnigg S, Balassy C, Tamandl D, Einspieler H, Wrba F, Trauner M, Herold C. Noninvasive differentiation of simple steatosis and steatohepatitis by using gadoxetic acid-enhanced MR imaging in patients with nonalcoholic fatty liver disease: a proof-of-concept study. Radiology. 2014;271:739-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 16. | Chabanova E, Bille DS, Thisted E, Holm JC, Thomsen HS. (1)H MRS assessment of hepatic steatosis in overweight children and adolescents: comparison between 3T and open 1T MR-systems. Abdom Imaging. 2013;38:315-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Opstad KS, Bell BA, Griffiths JR, Howe FA. An investigation of human brain tumour lipids by high-resolution magic angle spinning 1H MRS and histological analysis. NMR Biomed. 2008;21:677-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Hwang I, Lee JM, Lee KB, Yoon JH, Kiefer B, Han JK, Choi BI. Hepatic steatosis in living liver donor candidates: preoperative assessment by using breath-hold triple-echo MR imaging and 1H MR spectroscopy. Radiology. 2014;271:730-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Brook G, Soriano V, Bergin C. European guideline for the management of hepatitis B and C virus infections, 2010. Int J STD AIDS. 2010;21:669-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Gambarota G, Tanner M, van der Graaf M, Mulkern RV, Newbould RD. 1H-MRS of hepatic fat using short TR at 3T: SNR optimization and fast T2 relaxometry. MAGMA. 2011;24:339-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696-699. [PubMed] |
| 23. | Cobbold J, Lim A, Wylezinska M, Cunningham C, Crossey M, Thomas H, Patel N, Cox J, Taylor-Robinson S. Magnetic resonance and ultrasound techniques for the evaluation of hepatic fibrosis. Hepatology. 2006;43:1401-1402; author reply 1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Godfrey EM, Patterson AJ, Priest AN, Davies SE, Joubert I, Krishnan AS, Griffin N, Shaw AS, Alexander GJ, Allison ME. A comparison of MR elastography and 31P MR spectroscopy with histological staging of liver fibrosis. Eur Radiol. 2012;22:2790-2797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Karlas T, Petroff D, Garnov N, Böhm S, Tenckhoff H, Wittekind C, Wiese M, Schiefke I, Linder N, Schaudinn A. Non-invasive assessment of hepatic steatosis in patients with NAFLD using controlled attenuation parameter and 1H-MR spectroscopy. PLoS One. 2014;9:e91987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 116] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 26. | Cunningham CH, Vigneron DB, Marjanska M, Chen AP, Xu D, Hurd RE, Kurhanewicz J, Garwood M, Pauly JM. Sequence design for magnetic resonance spectroscopic imaging of prostate cancer at 3 T. Magn Reson Med. 2005;53:1033-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Hom JJ, Coakley FV, Simko JP, Qayyum A, Lu Y, Schmitt L, Carroll PR, Kurhanewicz J. Prostate cancer: endorectal MR imaging and MR spectroscopic imaging--distinction of true-positive results from chance-detected lesions. Radiology. 2006;238:192-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Fischbach F, Bruhn H. Assessment of in vivo 1H magnetic resonance spectroscopy in the liver: a review. Liver Int. 2008;28:297-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 29. | Lee Y, Kim H. Assessment of diffusion tensor MR imaging (DTI) in liver fibrosis with minimal confounding effect of hepatic steatosis. Magn Reson Med. 2015;73:1602-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Thomas EL, Hamilton G, Patel N, O’Dwyer R, Doré CJ, Goldin RD, Bell JD, Taylor-Robinson SD. Hepatic triglyceride content and its relation to body adiposity: a magnetic resonance imaging and proton magnetic resonance spectroscopy study. Gut. 2005;54:122-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 240] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 31. | Tang A, Tan J, Sun M, Hamilton G, Bydder M, Wolfson T, Gamst AC, Middleton M, Brunt EM, Loomba R. Nonalcoholic fatty liver disease: MR imaging of liver proton density fat fraction to assess hepatic steatosis. Radiology. 2013;267:422-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 420] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 32. | van Werven JR, Nederveen AJ, Stoker J. [No-invasive determination of liver fat with 1H-MR spectroscopy]. Ned Tijdschr Geneeskd. 2011;155:A2756. [PubMed] |