Published online Jun 7, 2015. doi: 10.3748/wjg.v21.i21.6728
Peer-review started: October 18, 2014
First decision: November 14, 2014
Revised: November 20, 2014
Accepted: January 30, 2015
Article in press: January 30, 2015
Published online: June 7, 2015
Processing time: 238 Days and 16.1 Hours
AIM: To analyze the incidence and possible risk factors in hospitalized patients treated with Clostridium difficile infection (CDI).
METHODS: A total of 11751 patients were admitted to our clinic between 1 January 2010 and 1 May 2013. Two hundred and forty-seven inpatients were prospectively diagnosed with CDI. For the risk analysis a 1:3 matching was used. Data of 732 patients matched for age, sex, and inpatient care period and unit were compared to those of the CDI population. Inpatient records were collected from an electronic hospital database and comprehensively reviewed.
RESULTS: Incidence of CDI was 21.0/1000 admissions (2.1% of all-cause hospitalizations and 4.45% of total inpatient days). The incidence of severe CDI was 12.6% (2.63/1000 of all-cause hospitalizations). Distribution of CDI cases was different according to the unit type, with highest incidence rates in hematology, gastroenterology and nephrology units (32.9, 25 and 24.6/1000 admissions, respectively) and lowest rates in 1.4% (33/2312) in endocrinology and general internal medicine (14.2 and 16.9/1000 admissions) units. Recurrence of CDI was 11.3% within 12 wk after discharge. Duration of hospital stay was longer in patients with CDI compared to controls (17.6 ± 10.8 d vs 12.4 ± 7.71 d). CDI accounted for 6.3% of all-inpatient deaths, and 30-d mortality rate was 21.9% (54/247 cases). Risk factors for CDI were antibiotic therapy [including third-generation cephalosporins or fluoroquinolones, odds ratio (OR) = 4.559; P < 0.001], use of proton pump inhibitors (OR = 2.082, P < 0.001), previous hospitalization within 12 mo (OR = 3.167, P < 0.001), previous CDI (OR = 15.32; P < 0.001), while presence of diabetes mellitus was associated with a decreased risk for CDI (OR = 0.484; P < 0.001). Treatment of recurrent cases was significantly different from primary infections with more frequent use of vancomycin alone or in combination (P < 0.001), and antibiotic therapy duration was longer (P < 0.02). Severity, mortality and outcome of primary infections and relapsing cases did not significantly differ.
CONCLUSION: CDI was accounted for significant burden with longer hospitalization and adverse outcomes. Antibiotic, PPI therapy and previous hospitalization or CDI were risk factors for CDI.
Core tip:Clostridium difficile infection (CDI) is one of the most common healthcare-associated infections. It has a high economic burden and its incidence is rapidly increasing in long-term care facilities and acute care hospitals. In the present study, we reported an epidemic of CDI with one of the highest incidences to date. Previous antibiotic treatment, proton pump inhibitor use, previous hospitalization, higher Charlson Comorbidity Index, and previous CDI were identified as predictive factors. CDI was associated with a high healthcare burden, long hospital stay and high mortality.
-
Citation: Kurti Z, Lovasz BD, Mandel MD, Csima Z, Golovics PA, Csako BD, Mohas A, Gönczi L, Gecse KB, Kiss LS, Szathmari M, Lakatos PL. Burden of
Clostridium difficile infection between 2010 and 2013: Trends and outcomes from an academic center in Eastern Europe. World J Gastroenterol 2015; 21(21): 6728-6735 - URL: https://www.wjgnet.com/1007-9327/full/v21/i21/6728.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i21.6728
Clostridium difficile infection (CDI) is one of the most common antibiotic-associated complications nowadays and a leading cause of healthcare associated infections[1]. The incidence of CDI is dramatically increasing since 2000[2] and its rising severity is well represented by more frequent transfer to the intensive care unit, colectomy and infection associated mortality[3]. It results in remarkable healthcare system costs and eventually leads to an important healthcare burden[4-6].
Previously increasing incidence was only reported in long-term care facilities. In contrast, recent studies report both community onset CDI[7] and acute hospital care onset[8]. Annual incidence of Clostridium difficile associated diarrhea and colitis (CDAD and CDAC, respectively) sharply increased from 35 to 156/100000 in past twenty years in Quebec[9]. The increase was more significant in elderly patients, 65 years and above, (number of CDI reports furthermore elevated via mandatory surveillance healthcare systems)[10].
Not only did the incidence, but also the number of complicated cases and mortality rates increased[11]. Of note, asymptomatic carriers and colonization of colon microbial flora is observed in about 3% of the population, although in a much higher proportion of patients after long hospital stays and surgery[12]. The spectrum of clinical manifestations associated to Clostridium difficile can diverge from asymptomatic carriers to life-threatening infection. CDI symptoms can vary between diarrhea and colitis or enteritisto even life-threatening complicated forms, pseudomembranosus, fulminant colitis or toxic megacolon. Some studies reported decreasing incidence of severe CDI. Feuerstadt et al[12] reported improved prognosis and decreased mortality (30-d mortality decreased significantly in both the overall (17.1% vs 13.1%, P < 0.01) and in the severe CDI (31.3% vs 23.3%, P < 0.05) cohorts between CDI 2006-2008 and 2009-2011.
Recently reported epidemic and wide-spreading of Clostiridum difficile infections are associated with health care associated factors and resistant strains (e.g., NAP1/B027)[13]. Suggested risk factors for developing CDI include prior antibiotic use, acid suppressive agents[14,15], previous CDI[16], comorbidities, malignancies, gastrointestinal disorders[17] and inflammatory bowel diseases[18].
Since there are only limited retrospective data are available from Eastern Europe, our aim was to analyze prospectively the incidence, possible risk factors, treatment strategy and outcome of CDI infections in hospitalized patients, treated at the 1st Department of Medicine, Semmelweis University, Budapest, Hungary.
All patients admitted to the 1st Department of Medicine, Semmelweis University, Budapest, Hungary between 1 January 2010 and 1 May 2013 prospectively evaluated. Our institution is an academic center with a secondary referral center including all specialities of internal medicine and oncology and provides primary internal medical care for a region of about 225000 inhabitants with a secondary referral area of 750000 to 1500000 inhabitants for specialized care. A total of 11751 inpatients were admitted in our clinic during the follow-up period, including all cause of hospital admission. Total 601 stool sample tested for CDI in Microbiology Department of Semmelweis University, microbiological serology test, including 168 positive and 433 negative result and including recidive cases. Testing density was 5.11/10000 patient-days.
A total of 247 inpatients had a confirmed diagnosis of CDI based on the clinical symptoms, laboratory results and cytotoxin stool testing and/or stool culture. Patient data were collected from the hospital electronic database.
CDI was defined as an acute diarrheal disease (more than three liquid stools per day) with a positive cytotoxin stool assay or a positive cytotoxin stool assay associated with the diagnosis of pseudomembranous colitis by imaging or endoscopic methods, surgery, or autopsy[19]. Repeated exotoxin positivity in 3 mo were defined as recurrence. In our department we apply standardized medical protocols and surveillance guidelines for healthcare associated infections (HAI) including CDI, and thus evaluation of symptomatic patients and treatment strategy is harmonized.
For defining the possible risk factors a 1:3 matching was used. Data of 732 inpatients matched for age, gender, inpatient care period and unit were compared to the CDI population. Inpatient records were collected and comprehensively reviewed, including inpatient ward, co-morbidities (according to Charlson Comorbitiy Index and age-adjusted Charlson Comorbidity Index[20]), medication use (including previous or current antibiotic treatment, proton pump inhibitors and any medication for the treatment of co-morbidities and the current CDI episode), laboratory parameters [white blood cell count (WBC), creatinine, C-reactive protein (CRP), serum albumin level].
Severe CDI was defined as WBC 15 G/L or above and serum albumin level 30 g/L or below based on previous guidelines[21].
Community acquired CDI defined as symptoms developed before hospital admission or less than 48 h after[22].
Three different outcomes were uses, such as recovered, recurrence after healing (within 12 wk), and death. Recurrence was defined as a clinical relapse including symptoms and positive stool test within 12 wk from the discharge.
The study protocol was approved by the Semmelweis University Regional and Institutional Committee of Science and Research Ethics (TUKEB 56/2013).
SPSS version 20 (SPSS Inc., Chicago, IL, United States) was used for statistical analysis. D-test, ANOVA-Scheffe test were used to compare continuous variables, Khi2, Fischer-exact tests were used to compare categorical variables. Categorical variables if appropriate were further tested in a multivariate analysis by using logistic regression analysis. Variables with a P vaule < 0.1 were included in the multivariate testing. Kaplan-Meier curve was plotted to analyse mortality outcomes with LogRank test. A P value of < 0.05 was considered significant.
The crude incidence of CDI infection was 21.0 per 1000 all-cause hospital admissions (2.1% of all-cause hospitalizations), 4.45% of total inpatient days were related to CDI (4326/96284 d, equaling 25.6 cases per 10000 patient-days) during the observed period. The majority of the patients were 60 years or older (< 40 years old: 4.7%, 40-60 years old: 11.9%, > 60 years old: 83.4%). Community acquired infection rate was 45.3%. Symptoms were detected at hospitalization in 82 patients (33.2%) and within 3 d from admission in further 30 patients (12.1%). Mean time to presence of CDI symptoms was 2.75 ± 5.3 d from hospital admission.
Total 601 stool sample tested for Clostridium difficile infection in Microbiology Department of Semmelweis University, microbiological serology test, including 168 positive and 433 negative result and including relapses. Testing density was 5.11/10000 patient-days.
The incidence of CDI was different according to the unit type, with highest incidence rates in hematology, gastroenterology and nephrology units (32.9, 25 and 24.6/1000 admissions) and lowest rates in 1.4% (33/2312) in endocrinology and general internal medicine (14.2 and 16.9/1000 admissions) units. Incidence did not differ between genders.
The incidence of severe CDI was 12.6% (2.63/1000 of all cause hospitalizations). In severe CDI patients were older (severe: 84.2% vs all: 69.6% of patients were > 65 years, P < 0.001) and duration of hospitalization was longer (18.4 ± 11.7 inpatient days vs 17.3 ± 10.3 inpatient days, P < 0.001).
Serum creatinine level, WBC and CRP were higher while serum albumin level was lower in patients with CDI compared to controls. Charlson Comorbity Index and age-adjusted Charlson Comorbidity Index were also significantly higher in CDI cases compared to controls (5.6 ± 3.1 and 6.8 ± 2.7 vs 4.8 ± 3.0 and 5.9 ± 2.7, P < 0.001). More detailed description of clinical and laboratory parameters are shown in Table 1.
| CDI cases(n = 247) | Controls(n = 732) | P value | |
| Age (yr) | 72.4 (14.2) | 70.6 (13.8) | NS |
| Male/female | 90/157 | 276/455 | NS |
| Charlson Index | 5.6 (3.1) | 4.8 (3.0) | < 0.001 |
| Age-adjusted Charlson Comorbidity Index | 6.8 (2.7) | 5.9 (2.7) | < 0.001 |
| CRP (mg/L) | 108.3 (101.3) | 49.8 (74.0) | < 0.001 |
| Procalcitonin (mg/L) | 1.8 (8.1) | 1.2 (12.4) | NS |
| WBC count (G/L) | 14.3 (20.9) | 9.9 (8.4) | < 0.001 |
| Albumin (g/L) | 29.5 (9.9) | 36.2 (11.2) | < 0.001 |
| Creatinine (μmol/l) | 158.3 (158.2) | 124.1 (117.7) | < 0.001 |
| Na (mmol/L) | 137.7 (14.7) | 136.1 (25.1) | NS |
| K (mmol/L) | 4.1 (4.4) | 4.4 (4.8) | NS |
Risk factors for CDI included previous “risk” antibiotic therapy (clindamycin, penicillins, third generation cephalosporins and fluoroquinolones, P < 0.001), use of proton pump inhibitors (OR = 2.08, P < 0.001), previous hospitalization within 12 mo (OR = 3.16, P < 0.001), previous CDI (OR = 15.3, P < 0.001). The presence of diabetes mellitus was associated with a decreased risk for CDI (OR = 0.48, P < 0.001) (Table 2).
| Univariate analysis | Multivariate analysis | |
| Gender | P = 0.77 | P = 0.47 |
| Previous Clostridium difficile infection | P < 0.001, | P = 0.08 |
| OR = 15.3 | ||
| 95%CI: 2.03-48.7 | ||
| Previous hospitalization1 | P < 0.001, | P < 0.001, |
| OR = 3.17 | OR = 2.39, | |
| 95%CI: 2.19-4.57 | 95%CI: 1.61-3.51 | |
| Healthcare facility or nursery home | P = 0.06 | P = 0.81 |
| Treatment with "risk" antibiotics | P < 0.001 , | P < 0.001 |
| OR = 4.56 | OR = 4.09, | |
| 95%CI: 3.36-6.19 | 95%CI: 2.98-5.61 | |
| Proton pump inhibitor therapy | P < 0.001, | P = 0.006 |
| OR = 2.08 | OR = 1.62, | |
| 95%CI: 1.52-2.85 | 95%CI: 1.15-2.29 | |
| Charlson Comorbidity Index | P = 0.001 | P = 0.004 |
| OR = 1.08, | ||
| 95%CI: 1.03-1.14 |
Treatment of primary infection was started with metronidazole in 70.8% of the patients (28.4% iv and 42.4% oral), vancomycin alone in 7.7% or combination therapy in 21.5%. Change in the antibiotic treatment was required in 11.9%. The mean length of antibiotic treatment was 12.1 ± 6.9 d. The initial treatment of severe CDI included combination therapy in 31.6%, metronidazole in 60.5% or vancomycin alone in 7.9% of the cases. Change in the antibiotic therapy was required in 15.8% of the patients. The length of the treatment was 13.6 ± 5.9 d, and 12.6 ± 7.1 d in severe cases.
Treatment strategy was different in community vs hospital-acquired cases with a tendency towards higher metronidazole (P = 0.07) and lower vancomycin (P = 0.004) and/or combination therapy (P = 0.04) rates in the community acquired cases. A similar proportion of the patients required a change of the first therapy.
The treatment strategy was not significantly different according to the unit type, age or gender (data not shown).
Treatment of recurrent cases was significantly different from primary infections (86.7% vancomycin based including 53.3% combination vancomycin-metronidazole vs 29.2% vancomycin-based therapy in primary CDI, P < 0.001). Length of treatment recurrent infections was 16.6 days, longer compared to the primary cases (P = 0.03 vs primary CDI).
Duration of hospital stay was longer (17.6 ± 10.8 d vs 12.4 ± 7.7 d, P < 0.01) in patients with CDI infection compared to the controls. Length of hospitalization was not different between age-groups (data not shown); 8.1% of the patients required ICU therapy during the CDI infection.
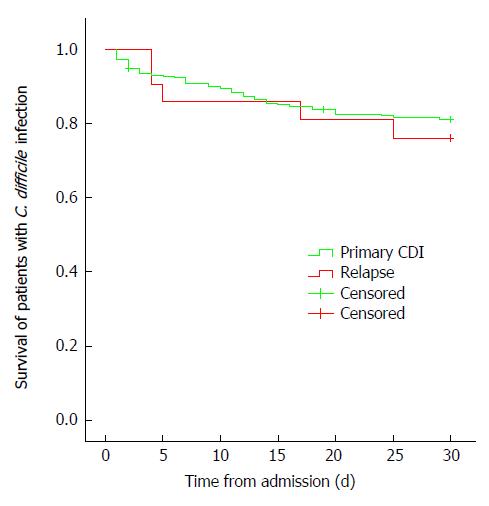
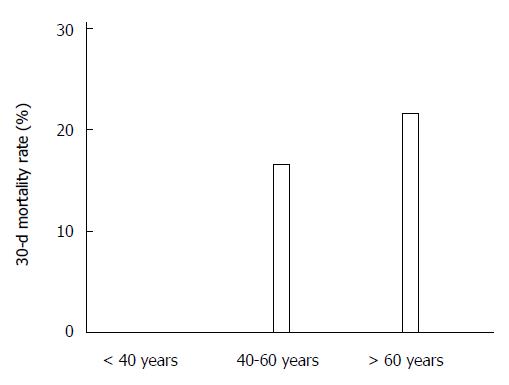
The 30-d mortality rate was 21.9% in CDI patients (54/247 cases), equaling 6.3% of all-inpatient deaths (37/555) (Figure 1). In addition, mortality rates were different according to age with highest mortality in the eldest patients (21.7% in > 60 years old, 16.7% in 40-60 years old and 0% in younger patients, P = 0.053, Figure 2).
Recurrence of CDI infection was 11.3% (n = 26) 12 wk after discharge. The outcome of recurrent cases was not significantly different from that of the primary infection. The rate of severe CDI was 5.9% and 30-d mortality was 23.8% (Figure 1).
This is the first prospective systematic evaluation of the incidence, risk factors, treatment and outcomes of CDI in a tertiary academic center in Eastern Europe in inpatients. CDI was associated with a high health care burden and it was responsible for 4.5% of inpatients stays, leading to long hospital stay and high mortality (21.9%). The incidence of severe CDI was 12.6% while recurrence of CDI infection was 11.3% within 12 wk after discharge. Primary CDI infection was initially treated by metronidazole-based regimen, while severe or recurrent cases were significantly more often treated initially with vancomycin, alone or in combination.
Previous studies reported increasing incidence rates from Western Europe and North America. The mean prevalence of CDI increased from 261 to 546 discharged cases per 100000 patients in a nationwide study from the United States between 1993 and 2003[23] (Estimations were based on the discharge data from the Nationwide Inpatient Sample. Similar results were published from another multicenter study between 2000 and 2006. The incidence of hospital-onset CDI increased from 7 to 8.5 cases/10000 patient-days, similarly the community-onset CDI increased from 1.1 to 1.3 cases/10000 patient-days[24]. A striking rise in incidence was reported from the Quebec region: 35.6 per 100000 population in 1991 to 156.3 per 100000 in 2003, with some local geographic variation. Of note, the highest increase was observed among patients aged 65 years or above (from 102.0 to 866.5 per 100000)[9].
In concordance, the incidence rate of CDI was 21/1000 all cause hospital admission and the majority (83.4%) of patients were elderly (older than 60 years). Importantly, the diagnosis of CDI in the present study was based on clinical symptoms and positive cytotoxin assay. A systematic stool evaluation by patients with non-suggestive clinical symptoms was not performed. Therefore, a proportion of mild cases may have been unnoticed.
In a multinational European study, the mean incidence of CDI was 4.1 per 10000 patient-days, with 63% of the patients aged 65 years or more as extrapolated from the results obtained in November 2008, from single hospitals[25]. Of note, a significant geographic variation was reported with the highest rate in Finland (19.1 per 10000 patient-days) and the lowest in Turkey and Bulgaria (0 and 0.6 per 10000 patient-days). Interestingly, very low incidences were reported from Eastern European countries including Bulgaria, Croatia, Czech Republic, Romania, Slovakia and Hungary (2 per 10000 patient-days). In contrast, Poland reported one of the highest incidences (12.5 per 10000 patient-days). The incidence in the present study equals 25.6 cases per 10000 patient-days, which is one of the highest reported in Europe, 5 to 10-fold higher compared to the Eastern European data in the multicenter study from 2008.
Few other data are available from Eastern Europe. Surprisingly low, 0.6 per 10000 patient-days incidence rate was reported from a university center from Croatia by Novak et al[26] in 2010. Similarly low incidence was reported in a Czech tertiary referral center by Balihar et al[27] in 2014, with an incidence of 0.6 per 10000 and 15.8% severe cases, in a retrospective observational study[27]. Finally, the incidence rate in the present study was almost 5-times higher than in the recently published data from Austria (5.23 per 10000 patient-days)[28]. The rate of severe CDI was similar in the present study (12.6%) to that reported from Austria (16.5%). Patients with severe CDI were older and CDI was associated with longer hospital stay. Interestingly, even higher severe CDI rates were reported from the US (20.1%) between 2006 and 2011[12] and from Canada (rising from 7.1% in 1991-1993 to 18.2% in 2003)[9].
Previous antibiotic treatment (clindamycin, amocillin/clavulanic acid, cephalosporins, ciprofloxacin and fluoroquinolones), acid suppressive agents, previous hospitalizations, long-term care home residence and comorbidities were previously reported as risk factors for CDI[14-16,29-31]. In concordance, in the present study previous antibiotic treatment with the above compounds, proton pump inhibitor use, previous hospitalization, higher Charlson Comorbidity Index, and previous CDI were identified as predictive factors for CDI. In addition, higher creatinine, lower serum albumin level and higher white blood cell count was associated with CDI and in previous studies with adverse outcomes[9]. Male gender, elevated CRP and fluoroquinolone exposure was associated with severe CDI and more frequent transition to intensive care unit[32]. The protective association found for diabetes mellitus might represent a selection bias, since the chance for being hospitalized with any gastrointestinal symptoms, especially diarrhea (as well as the CDI incidence) was lower in the endocrinology unit compared to the gastroenterology, nephrology or hematology units.
Previous studies suggested a benefit from vancomycin-based treatment strategy, especially in patients with severe CDI preventing adverse outcomes and the development of complicated CDI[9]. In a recent paper from the US, authors reported a shift in the treatment patterns, with shorter duration of oral metronidazole (P < 0.001), longer duration of intravenous metronidazole (P = 0.04), more frequent use of vancomycin (P < 0.001) and more frequent switching from metronidazole to vancomycin (P < 0.001) between 2006 and 2011[12]. In the present study, patients received a tailored therapy with increased and earlier use of vancomycin in severe and recurrent cases. Largely similarly treatment data were presented from the Czech Republic[27]. Interestingly, in the present study, treatment strategy was different in community vs hospital-acquired cases, with higher metronidazole and lower vancomycin/combination rates in community acquired cases.
Readmission rate (11.3%) in the present study was lower compared to that reported from North America (16%-18%)[12,33] and the Czech Republic (16.4%). However, even lower readmission rates were reported in a multicenter study from Canada (7%)[34]. The average total length of hospital stay in the present study was in the range of previous findings with a mean incremental length of stay of 5.0-13.6 and 2.7-21.3 d for CDI requiring admission and hospital acquired CDI episodes[35]. Of note, much longer mean hospital stay was reported recently from the Czech Republic (median 35 d)[27].
Despite the relatively aggressive treatment strategy, the 30-d mortality rate in the present study was as high as 21.9%. The higher Charlson Comorbidity Index and overall high proportion of elderly patients may at least partly explain this finding. Similar mortality rates were reported recently from the Czech Republic (overall: 19.7%, hospital-acquired: 22.4% and in severe-CDI: 62%) in a cohort with similar age distribution and comorbidity pattern. A mortality rate of 15.2% was reported in a multicenter study from Canada[35]. In another Canadian study death rate in complicated and non-complicated CDI was between 19%-25.5% and 4.2%-11.3% without a clear time trend between 1991 and 2003[9]. In addition, increasing adjusted mortality rates were reported in the US in the Nationwide Inpatient Sample between 1993 and 2003. Mortality rates increased from 20.3 deaths per 100000 discharges in 1993 to 50.2 deaths per 100000 discharges in 2003[23]. In contrast, lower and improving mortality rates were reported more recently from the US (overall: 17.1% vs 13.1%, P < 0.01 and severe CDI: 31.3% vs 23.3%, P < 0.05) between 2006 and 2011, despite a higher Charlson Comorbidity Scores and older population in the more recent cohort[12]. Finally, mortality rate (11.0%) was lower in a case-control study from Japan, while risk factors for CDI were not different from Europe and North America[36]. Of note, in the latter study only the use of vancomycin but not metronidazole was associated with a decreased risk for mortality.
Authors are aware of potential limitations of this study including the possible underestimation of the incidence due to the strict inclusion criteria. Cases were identified by suggestive symptoms and cytotoxin test positivity, therefore milder cases might have remained unidentified. Demographic data was only partly registered, e.g., nursery home care was not always documented. Conventional treatment methods were used in our university hospital for CDI, including vancomycin and/or metronidazole therapy and patient isolation. The use of new antibiotics, e.g., fidaxomicin, tigecyclin or fecal microbiota transplantation was exceptional with only one patient evaluated for fecal microbiota transplantation. In the present study, definition of severe CDI was based on Society for Healthcare Epidemiology of America (SHEA) guidelines, but this severity based evaluation was not validated previously. In contrast, the strengths of the present study include the prospective, complete capture. Cases were identified through the full electronic online in- and outpatient medical records, which is linked to the microbiology and laboratory data, making the search, data capture and analysis extremely reliable. The system contains all out- and inpatient records related to the patient including laboratory data, imaging, hospitalization and/or surgery related hospitalization records from all departments of the Semmelweis University since 2005. In addition, we apply standardized medical protocols and surveillance guidelines in our Department for HAI including CDI, and thus evaluation of symptomatic patients and treatment strategy is harmonized in the different units of the department.
In conclusion, the incidence of CDI was high in this prospective study, and was associated with longer hospital stay and adverse outcomes. Early readmission rates were comparable to findings of previous studies. A relatively high proportion of patients received aggressive antibiotic therapy and this was tailored to the severity of the cases. Antibiotic therapy, proton pump inhibitor treatment, previous hospitalization and CDI were identified as risk factors for CDI.
Clostridium difficile infections (CDI) are one of the most important healthcare associated infections. Its incidence is increasing and CDI is associated with an important healthcare burden.
There are only limited retrospective data available from Eastern Europe on incidence rates, risk factors, treatment strategy and outcome of CDI.
In the present prospective study, authors report an epidemic of CDI infection with exceedingly high incidence rates in a tertiary academic center in Eastern Europe. In our department we apply standardized medical protocols and surveillance guidelines for HAI including CDI, and thus evaluation of symptomatic patients and treatment strategy is harmonized. CDI infection was associated with adverse outcomes, longer hospital stay and high mortality rate. Antibiotic therapy, proton pump inhibitor treatment, previous hospitalization and CDI were risk factors for CDI.
Understanding the possible risk factors, disease course and outcomes of CDI and treatment strategy in these patient cohort may lead to better optimized treatment strategy and reduced healthcare associated complications.
Diagnosis of CDI based on clinical symptoms of diarrhea with positive cytotoxin stool assay or with diagnosis of pseudomembranosus colitis. Comorbidities were categorized according to Charlson Comorbidity and age adjusted Comorbidity Index. Severe infections were defined according to current infection specialists’ guidelines (severe leukocytosis and hypoalbuminaemia). Recurrence was defined as relapse of symptoms and positive stool test within 12 wk from discharge.
This is an epidemiological study regarding Clostridium difficile infection in Eastern Europe where its incidence is unclear. The authors present prospective data regarding incidence, risk factors, treatment and outcomes of Clostridium difficile infection. The paper covers an interesting topic and includes a considerable number of patients. They found that antibiotics and proton pump inhibitors were associated with CDI, which confirms the results of previous studies. The epidemiology of CDI is important because CDI remains a major nosocomial infection in the Western world and the epidemiology of CDI appears to be shifting more from healthcare- to community-acquired disease.
P- Reviewer: Freedberg DE, Germer CT, Honda H S- Editor: Yu J L- Editor: A E- Editor: Liu XM
| 1. | Miller BA, Chen LF, Sexton DJ, Anderson DJ. Comparison of the burdens of hospital-onset, healthcare facility-associated Clostridium difficile Infection and of healthcare-associated infection due to methicillin-resistant Staphylococcus aureus in community hospitals. Infect Control Hosp Epidemiol. 2011;32:387-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 284] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 2. | Honda H, Dubberke ER. The changing epidemiology of Clostridium difficile infection. Curr Opin Gastroenterol. 2014;30:54-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Abou Chakra CN, Pepin J, Sirard S, Valiquette L. Risk factors for recurrence, complications and mortality in Clostridium difficile infection: a systematic review. PLoS One. 2014;9:e98400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 258] [Cited by in RCA: 246] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 4. | Hall AJ, Curns AT, McDonald LC, Parashar UD, Lopman BA. The roles of Clostridium difficile and norovirus among gastroenteritis-associated deaths in the United States, 1999-2007. Clin Infect Dis. 2012;55:216-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 222] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 5. | Dubberke ER, Butler AM, Reske KA, Agniel D, Olsen MA, D’Angelo G, McDonald LC, Fraser VJ. Attributable outcomes of endemic Clostridium difficile-associated disease in nonsurgical patients. Emerg Infect Dis. 2008;14:1031-1038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 133] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 6. | Dubberke ER, Olsen MA. Burden of Clostridium difficile on the healthcare system. Clin Infect Dis. 2012;55 Suppl 2:S88-S92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 409] [Cited by in RCA: 459] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 7. | Centers for Disease Control and Prevention (CDC). Vital signs: preventing Clostridium difficile infections. MMWR Morb Mortal Wkly Rep. 2012;61:157-162. [PubMed] |
| 8. | Campbell RJ, Giljahn L, Machesky K, Cibulskas-White K, Lane LM, Porter K, Paulson JO, Smith FW, McDonald LC. Clostridium difficile infection in Ohio hospitals and nursing homes during 2006. Infect Control Hosp Epidemiol. 2009;30:526-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 9. | Pépin J, Valiquette L, Alary ME, Villemure P, Pelletier A, Forget K, Pépin K, Chouinard D. Clostridium difficile-associated diarrhea in a region of Quebec from 1991 to 2003: a changing pattern of disease severity. CMAJ. 2004;171:466-472. [PubMed] |
| 10. | Dudeck MA, Weiner LM, Malpiedi PJ, Edwards JR, Peterson KD. Sievert DM Risk Adjustment for Healthcare Facility-Onset C. difficile and MRSA Bacteremia Laboratory-identified Event Reporting in NHSN. Available from: http://www.cdc.gov/nhsn/pdfs/mrsa-cdi/RiskAdjustment-MRSA-CDI.pdf. |
| 11. | Kazanowski M, Smolarek S, Kinnarney F, Grzebieniak Z. Clostridium difficile: epidemiology, diagnostic and therapeutic possibilities-a systematic review. Tech Coloproctol. 2014;18:223-232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Feuerstadt P, Das R, Brandt LJ. The evolution of urban C. difficile infection (CDI): CDI in 2009-2011 is less severe and has better outcomes than CDI in 2006-2008. Am J Gastroenterol. 2014;109:1265-1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Cloud J, Kelly CP. Update on Clostridium difficile associated disease. Curr Opin Gastroenterol. 2007;23:4-9. [PubMed] |
| 14. | Dubberke ER, Reske KA, Yan Y, Olsen MA, McDonald LC, Fraser VJ. Clostridium difficile--associated disease in a setting of endemicity: identification of novel risk factors. Clin Infect Dis. 2007;45:1543-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 205] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 15. | Dial S, Delaney JA, Barkun AN, Suissa S. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA. 2005;294:2989-2995. [PubMed] |
| 16. | Huang SS, Datta R, Platt R. Risk of acquiring antibiotic-resistant bacteria from prior room occupants. Arch Intern Med. 2006;166:1945-1951. [PubMed] |
| 17. | Ben-Horin S, Margalit M, Bossuyt P, Maul J, Shapira Y, Bojic D, Chermesh I, Al-Rifai A, Schoepfer A, Bosani M. Combination immunomodulator and antibiotic treatment in patients with inflammatory bowel disease and clostridium difficile infection. Clin Gastroenterol Hepatol. 2009;7:981-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 18. | Lo Vecchio A, Zacur GM. Clostridium difficile infection: an update on epidemiology, risk factors, and therapeutic options. Curr Opin Gastroenterol. 2012;28:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 139] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 19. | Debast SB, Bauer MP, Kuijper EJ. European Society of Clinical Microbiology and Infectious Diseases: update of the treatment guidance document for Clostridium difficile infection. Clin Microbiol Infect. 2014;20 Suppl 2:1-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 766] [Cited by in RCA: 793] [Article Influence: 72.1] [Reference Citation Analysis (0)] |
| 20. | Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373-383. [PubMed] |
| 21. | Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, Pepin J, Wilcox MH. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31:431-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2285] [Cited by in RCA: 2200] [Article Influence: 220.0] [Reference Citation Analysis (0)] |
| 22. | Lee L, Cohen SH. Community-Acquired Clostridium difficile Infection: An Emerging Problem. Current Emergency and Hospital Medicine Reports. 2013;149-153. |
| 23. | Ricciardi R, Rothenberger DA, Madoff RD, Baxter NN. Increasing prevalence and severity of Clostridium difficile colitis in hospitalized patients in the United States. Arch Surg. 2007;142:624-631; discussion 631. [PubMed] |
| 24. | Dubberke ER, Butler AM, Yokoe DS, Mayer J, Hota B, Mangino JE, Khan YM, Popovich KJ, Fraser VJ. Multicenter study of Clostridium difficile infection rates from 2000 to 2006. Infect Control Hosp Epidemiol. 2010;31:1030-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 25. | Bauer MP, Notermans DW, van Benthem BH, Brazier JS, Wilcox MH, Rupnik M, Monnet DL, van Dissel JT, Kuijper EJ. Clostridium difficile infection in Europe: a hospital-based survey. Lancet. 2011;377:63-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 803] [Cited by in RCA: 831] [Article Influence: 59.4] [Reference Citation Analysis (0)] |
| 26. | Novak A, Spigaglia P, Barbanti F, Goic-Barisic I, Tonkic M. First clinical and microbiological characterization of Clostridium difficile infection in a Croatian University Hospital. Anaerobe. 2014;30:18-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Balihar K, Kozak F, Kozeluhova J, Hejda V, Fremundova L, Krcma M, Geigerova L, Bergerova T, Matejovic M. Clostridium difficile infection in hospitalized patients at a Czech tertiary center: analysis of epidemiology, clinical features, and risk factors of fulminant course. Eur J Gastroenterol Hepatol. 2014;26:880-887. [PubMed] |
| 28. | Starzengruber P, Segagni Lusignani L, Wrba T, Mitteregger D, Indra A, Graninger W, Presterl E, Diab-Elschahawi M. Severe Clostridium difficile infection: incidence and risk factors at a tertiary care university hospital in Vienna, Austria. Wien Klin Wochenschr. 2014;126:427-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Marwick CA, Yu N, Lockhart MC, McGuigan CC, Wiuff C, Davey PG, Donnan PT. Community-associated Clostridium difficile infection among older people in Tayside, Scotland, is associated with antibiotic exposure and care home residence: cohort study with nested case-control. J Antimicrob Chemother. 2013;68:2927-2933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 30. | Depestel DD, Aronoff DM. Epidemiology of Clostridium difficile infection. J Pharm Pract. 2013;26:464-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 31. | Friedman HS, Navaratnam P, Reardon G, High KP, Strauss ME. A retrospective analysis of clinical characteristics, hospitalization, and functional outcomes in residents with and without Clostridium difficile infection in US long-term care facilities. Curr Med Res Opin. 2014;30:1121-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Khanafer N, Touré A, Chambrier C, Cour M, Reverdy ME, Argaud L, Vanhems P. Predictors of Clostridium difficile infection severity in patients hospitalised in medical intensive care. World J Gastroenterol. 2013;19:8034-8041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 33. | Aitken SL, Joseph TB, Shah DN, Lasco TM, Palmer HR, DuPont HL, Xie Y, Garey KW. Healthcare resource utilization for recurrent Clostridium difficile infection in a large university hospital in Houston, Texas. PLoS One. 2014;9:e102848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | Miller MA, Hyland M, Ofner-Agostini M, Gourdeau M, Ishak M. Morbidity, mortality, and healthcare burden of nosocomial Clostridium difficile-associated diarrhea in Canadian hospitals. Infect Control Hosp Epidemiol. 2002;23:137-140. [PubMed] |
| 35. | Gabriel L, Beriot-Mathiot A. Hospitalization stay and costs attributable to Clostridium difficile infection: a critical review. J Hosp Infect. 2014;88:12-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 36. | Takahashi M, Mori N, Bito S. Multi-institution case-control and cohort study of risk factors for the development and mortality of Clostridium difficile infections in Japan. BMJ Open. 2014;4:e005665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |