Published online Jun 7, 2015. doi: 10.3748/wjg.v21.i21.6660
Peer-review started: May 26, 2014
First decision: June 27, 2014
Revised: July 17, 2014
Accepted: September 5, 2014
Article in press: September 5, 2014
Published online: June 7, 2015
Processing time: 380 Days and 17.3 Hours
AIM: To establish the safety and feasibility of laparoscopic splenectomy (LS) for littoral cell angioma (LCA).
METHODS: From September 2003 to November 2013, 27 patients were diagnosed with LCA in our institution. These patients were divided into two groups based on operative procedure: LS (13 cases, Group 1) and open splenectomy (14 cases, Group 2). Data were collected retrospectively by chart review. Comparisons were performed between the two groups in terms of demographic characteristics (sex and age); operative outcomes (operative time, estimated blood loss, transfusion, and conversion); postoperative details (length of postoperative stay and complications); and follow-up outcome.
RESULTS: LS was successfully carried out in all patients except one in Group 1, who required conversion to hand-assisted LS because of perisplenic adhesions. The average operative time for patients in Group 1 was significantly shorter than that in Group 2 (127 ± 34 min vs 177 ± 25 min, P = 0.001). The average estimated blood loss in Group 1 was significantly lower than in Group 2 (62 ± 48 mL vs 138 ± 64 mL, P < 0.01). No patient in Group 1 required a blood transfusion, whereas one in Group 2 required a transfusion. Two patients in Group 1 and four in Group 2 suffered from postoperative complications. All the complications were cured by conservative therapy. There were no deaths in our series. All patients were followed up and no recurrence or abdominal metastasis were found.
CONCLUSION: LS for patients with LCA is safe and feasible, with preferable operative outcomes and long-term tumor-free survival.
Core tip: Littoral cell angioma (LCA) is a rare splenic tumor. Consequently, there is a paucity of data in the literature on laparoscopic splenectomy (LS) for LCA. We successfully performed LS in 13 patients with LCA. Compared with patients who underwent open splenectomy, patients who underwent LS required shorter operative time and suffered lower blood loss. No patient had tumor recurrence. LS is safe and feasible in patients with LCA.
- Citation: Cai YQ, Wang X, Ran X, Liu XB, Peng B. Laparoscopic splenectomy for splenic littoral cell angioma. World J Gastroenterol 2015; 21(21): 6660-6664
- URL: https://www.wjgnet.com/1007-9327/full/v21/i21/6660.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i21.6660
Littoral cell angioma (LCA) is a rare splenic tumor that was first reported by Falk et al[1] in 1991. It arises from cells in the red-pulp sinuses and usually presents with anemia, pyrexia or thrombocytopenia with splenomegaly[2]. Clinically, most of the LCAs described in the literature have been benign; however, several reports have described malignant LCA[3]. Two kinds of morphological presentations of LCA are reported, including the more commonly encountered diffuse multiple nodular form and the rare solitary form[4].
Generally, splenic tumors are rare, and it is difficult to establish a definite preoperative diagnosis of whether they are malignant or benign. Splenectomy is generally indicated for patients with splenic tumors because of the possibility that the lesion is malignant[5]. Since laparoscopic splenectomy (LS) was introduced in 1991[6], it has shown advantages over open splenectomy in terms of lower blood loss, shorter postoperative stay and fewer surgery-related complications[7]. However, it is still controversial to perform total LS for a splenic tumor[5]. There is a paucity of data in the literature on LS for LCA because of its rarity.
In this study, we reported the largest series of LS for LCA, aiming to acquire a better understanding of LCA and to establish the safety and feasibility of LS in this setting.
From September 2003 to May 2013, 27 patients underwent splenectomy and were diagnosed with LCA by postoperative pathological and immunohistological examinations. The data were collected retrospectively by chart review in terms of demographic characteristics, operative details, postoperative details and follow-up outcomes. This study was approved by the Ethics Committee of Sichuan University, Chengdu, China.
Patients received general anesthesia and were placed in the right semi-decubitus position, with the left side elevated by approximately 60° and the operating table slightly tilted to the reverse Trendelenburg position. The surgical procedure for LS has been described previously[8]. Four ports were used for all patients. A 10-mm trocar was placed at the upper umbilicus for a 10-mm, 30° camera. A 5-mm trocar was placed at the subxiphoid position, and another 5-mm trocar was placed in the left axillary line below the lower pole of the spleen. A 12-mm trocar was placed at the left mid-clavicular line, below the margin of the spleen, for the use of the ultrasonic dissector and linear laparoscopic vascular stapler. We dissected the perisplenic ligaments in the order of splenogastric ligament (including the short gastric arteries), splenophrenic ligament, splenic flexure attachment and splenorenal ligament. After all the attachments and ligaments were entirely dissected, the splenic hilum was transected with a linear laparoscopic vascular stapler (Echelon 60 ENDOPATH Stapler; Ethicon Endo-Surgery, Cincinnati, OH, United States). The spleen was placed in a retrieval bag, morcellated with forceps and retracted via a 12-mm incision. A closed suction drain was routinely placed in the splenic fossa. The amylase level of the drainage fluid was measured.
Operative time was defined as the time from the first incision to skin closure. The splenic size was defined as the longitudinal diameter. Splenomegaly was defined as longitudinal diameter > 12 cm[9]. Morbidity was defined as any complication associated with the operation within 30 d of surgery. Pancreatic fistula (graded A-C) was defined by the International Study Group on Pancreatic Fistula (ISGPF)[10].
Numerical data are expressed as mean ± standard deviation. Statistical analyses were performed using SPSS for Windows version 16.0. Differences between variables were compared using the nonparametric Mann-Whitney U test, Student’s t test, χ2 test, and Fisher’s exact test. P < 0.05 was considered statistically significant.
The demographic and clinical characteristics of the patients are shown in Table 1. There were 15 female and 12 male patients, with a female-to-male ratio of 1.25 to 1. The median age was 45 years (range: 7-65 years). There was no significant difference between the two groups in terms of demographic characteristics.
| Variables | Group 1 | Group 2 | P value |
| Cases | 13 | 14 | - |
| Age (yr) | 47.2 ± 11.0 | 43.2 ± 19.2 | NS |
| Sex (M/F) | 4/9 | 8/6 | NS |
| Platelet count (× 109/L) | 93.3 ± 46.1 | 103.7 ± 45.9 | NS |
| Clinical symptoms | NS | ||
| No symptoms | 6 (46.2) | 6 (42.8) | |
| Abdominal pain | 5 (38.5) | 4 (28.6) | |
| Hypersplenism | 8 (61.5) | 6 (42.9) | |
| Radiographical features | NS | ||
| Isolated mass | 1 (7.7) | 2 (15.4) | |
| Multiple masses | 12 (92.3) | 13 (84.6) | |
| Splenomegaly | NS | ||
| Yes | 9 (69.2) | 9 (64.3) | |
| No | 4 (30.8) | 5 (35.7) | |
| Thrombocytopenia | NS | ||
| Yes | 8 (61.5) | 6 (42.9) | |
| No | 5 (38.5) | 8 (57.1) |
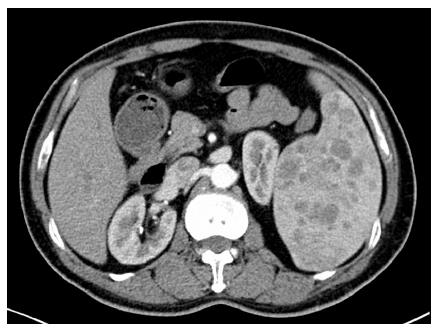
Overall, 12 patients (44.4%) were asymptomatic, with splenic lesions detected by routine physical examination. The most frequent symptom was abdominal pain (33.3%). In terms of radiographical character, only three patients (11.1%) presented with a solitary lesion. A typical computed tomography image is shown in Figure 1. Splenomegaly (18 cases, 66.7%) was a common presentation of LCA, and thrombocytopenia (14 cases, 51.9%) was also frequent. No significant difference was found between the two groups in terms of clinical and radiographical characteristics.
The operative and postoperative details are shown in Table 2. We successfully performed LS for 12 of 13 patients with LCA. One patient required conversion to hand-assisted LS (HALS) because of extensive perisplenic adhesion. The spleen size was comparable between the two groups (17.2 ± 4.8 cm vs 16.1 ± 3.9 cm, P = 0.524). The mean operative time for patients in Group 1 was 128 ± 37 min (range: 80-230 min). The patients in Group 2 required a significantly longer operative time (177 ± 25 min, range: 150-230 min, P < 0.001). The average estimated blood loss for patients in Group 1 was also significantly lower (62 ± 48 mL vs 138 ± 64 mL, P = 0.002). No patient in Group 1 required blood transfusion, whereas one patient (7.1%) in Group 2 required blood transfusion. There were no deaths in either group.
| Variables | Group 1 | Group 2 | P value |
| Operating time (min) | 128 ± 37 | 177 ± 25 | < 0.001 |
| Estimated blood loss (mL) | 62 ± 48 | 138 ± 64 | 0.002 |
| Transfusion | 0 (0) | 1 (7.1) | NS |
| Conversion | 1 (7.7) | - | - |
| Spleen size (cm) | 17.2 ± 4.8 | 16.1 ± 3.9 | NS |
| Length of stay (d) | 4.9 ± 1.1 | 7.1 ± 2.4 | 0.005 |
| Time to oral intake (d) | |||
| Complications | 2 (15.4) | 4 (28.5) | NS |
| Incision infection | 0 (0) | 1 (7.1) | |
| Pancreatic fistula | 1 (7.7) | 0 (0) | |
| Portal vein thrombosis | 1 (7.7) | 0 (0) | |
| Pulmonary infection | 0 (0) | 1 (7.1) | |
| Abdominal fluid collection | 0 (0) | 2 (14.3) |
Six patients in our series suffered from complications, including two (15.4%) in Group 1 and four (28.5%) in Group 2. One patient in Group 1 suffered from Grade A pancreatic fistula, which was diagnosed by routine examination of amylase levels in the drainage fluid. One patient suffered from portal vein thrombosis, which was diagnosed by ultrasonographic examination. Both patients were cured by conservative therapy. Four patients in Group 2 suffered from incision infection, pulmonary infection and abdominal fluid collection. All patients were cured by conservative therapy. There were no deaths in either group.
All patients were followed up via outpatient visits and/or telephone interview. The mean follow-up period was 42 mo (range: 9-125 mo). No patient suffered from tumor recurrence or abdominal dissemination. The platelet count returned to normal in 13 of 14 patients.
LCA is a rare primary splenic vascular tumor that originates from the littoral cells lining the splenic red pulp sinuses. Although the majority of LCA tumors are benign, there are two subtypes of malignant LCA reported in the literature, including littoral cell angiosarcoma and littoral cell hemangioendothelioma[11]. There are few clinical data regarding laparoscopic management of LCA. To date, our study included the largest series of LS for LCA, which enabled us to establish more definite conclusions regarding the safety and feasibility of LS in this setting.
The clinical presentations of LCA ranges from completely asymptomatic to symptoms such as abdominal pain, splenomegaly and thrombocytopenia[12,13]. Although there is no age predilection, LCA usually occurs in adults and appears to be rare in children. Only two patients (7.4%) who suffered from LCA were children in our series. The majority of LCAs were multiple, although solitary lesions were also reported. It is difficult to establish an accurate diagnosis of LCA preoperatively. At present, the final diagnosis is only possible via histopathological examination[14,15]. Fine-needle aspiration is performed for cytological diagnosis of splenic tumors[16]. However, this procedure is not routinely recommended for a mass in the spleen because of poor specificity, the risk of bleeding and tumor cell dissemination if the tumor is malignant[5].
Splenectomy and long-term follow-up are indicated for LCA because of its malignant potential[17]. Given the lower blood loss, shorter postoperative stay and fewer surgery-related complications, LS has become the gold standard for many hematological disorders, such as immune thrombocytopenic purpura and hemolytic anemia. However, there are just a few case reports regarding LS for LCA[5,18,19]. Rosen et al[19]. reported the first case of LS for LCA in 2002. Blansfield et al[18] reported the second case of LS for LCA in 2005. Both of those patients underwent successful LS, with favorable operative outcomes and no morbidity. Yano et al[5] reported one case of hand-assisted splenectomy for LCA in 2003. That patient was also discharged uneventfully.
However, there are still many concerns about LS in the setting of splenic tumors. LCA may be associated with splenomegaly, even a massive splenomegaly. It is a technical challenge to perform LS for patients with a massive splenomegaly. Furthermore, some surgeons have stated that an intact specimen is necessary for histological examination, and others argue that tumors treated laparoscopically may deteriorate the oncological outcome[20].
In our study, there were 13 patients in the LS group, including nine with splenomegaly. All patients, including those with splenomegaly, underwent successful LS. The mean operating time was 128 min and the mean blood loss was 62 mL, which were comparable with the data in the literature. No patient required conversion to open surgery or blood transfusion. Only one patient converted to HALS because of perisplenic adhesion. The introduction of HALS has enabled surgeons to insert their hands into the abdomen while maintaining the pneumoperitoneum. This technique allows surgeons to recover tactile sensation, enables them to obtain quick access to hemorrhages, and facilitates the retrieval of the spleen from the abdomen. HALS enables surgeons to carry out LS successfully without conversion to open surgery[21]. Overall, it is technically feasible to perform LS for patients with LCA.
Fais et al[22] stated that laparoscopic procedures for tumors are not suitable because of high local recurrence after long-term follow-up. They concluded that tumor recurrence might be caused by a pneumoperitoneum. Fortunately, the LCA was located in the splenic parenchyma. The splenic capsule can prevent the dissemination of tumor cells. Subsequently, to prevent tumor cell dissemination, additional attention should be paid to prevent splenic capsule rupture during dissection. We also highlighted the importance of sufficient elevation of the upper pole of the spleen, which is crucial so that the first stapler can cross the splenic hilum. Kawanaka et al[21] reported seven cases of uncontrollable bleeding during transection of the splenic hilar pedicles with an endoscopic linear vascular stapler because the first stapler failed to cross the entire splenic hilar pedicles. In our practice, we experienced a patient with hypersplenism caused by liver cirrhosis, which required conversion to open surgery, because of the stapler crossing the splenic parenchyma, caused by insufficient elevation of the upper pole of the spleen. This could be a major problem if we were dealing with splenic tumors, especially malignant tumors.
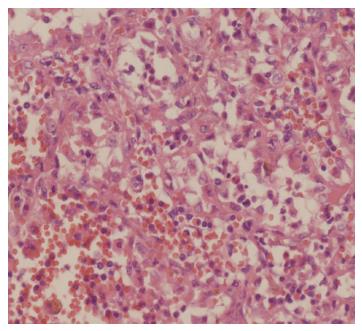
All our specimens were put into a retrieval bag, morcellated and retrieved via the 12-mm incision. During this procedure, additional attention should be paid to keep the retrieval bag intact, which is crucial to prevent tumor cell dissemination. The morcellated specimens did not interfere with the final histological diagnosis of LCA (Figure 2). However, if an intact specimen is required, an additional incision is required for retrieval. All patients were followed up and no patient suffered from tumor cell implantation in the trocar incisions or abdominal dissemination.
There were several limitations associated with our study. The study was retrospective. The sample size was relatively small, which precluded us from establishing a definite conclusion regarding the safety and feasibility of LS in the setting of LCA. Thus, multicenter prospective studies are required to achieve a better understanding of LCA and identify the safety and feasibility of LS in this setting.
In conclusion, LCA is a rare splenic neoplasm. Splenectomy is an effective therapeutic strategy, with long-term tumor-free survival. It is safe and feasible to perform LS in patients with LCA. However, LS for LCA should be performed by experienced laparoscopic surgeons and additional attention should be paid to prevent tumor cell dissemination.
Littoral cell angioma (LCA) is a rare splenic neoplasm. Consequently, there is a paucity of data in the literature on laparoscopic splenectomy (LS) for LCA.
The authors included the largest number of LCAs reported in the literature and presented the demographic characteristics, clinical presentation, radiological characteristics and long-term outcomes of splenectomy for LCA.
This is the first study concerning the safety and feasibility of LS in patients with LCA. Compared with open splenectomy, patients who underwent LS required shorter operative time and had lower blood loss. Patients who underwent LS suffered from fewer complications and required a shorter postoperative stay. The patients in the two groups had comparable long-term tumor-free survival rates.
LS is safe and feasible for patients with LCA. However, LS for LCA should be performed by experienced laparoscopic surgeons and additional attention should be paid to prevent tumor cell dissemination.
LCA is a rare splenic tumor that arises from cells in the red-pulp sinuses of the spleen.
Although a retrospective small case series, it was a well-conducted study concerning a rare splenic tumor, approached laparoscopically. The authors describe the feasibility and usefulness of LS for splenic LCA.
P- Reviewer: Agresta F, Fabozzi M, Liu QD, Yokoyama N S- Editor: Ma YJ L- Editor: Stewart G E- Editor: Wang CH
| 1. | Falk S, Stutte HJ, Frizzera G. Littoral cell angioma. A novel splenic vascular lesion demonstrating histiocytic differentiation. Am J Surg Pathol. 1991;15:1023-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 160] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 2. | Mac New HG, Fowler CL. Partial splenectomy for littoral cell angioma. J Pediatr Surg. 2008;43:2288-2290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Abbott RM, Levy AD, Aguilera NS, Gorospe L, Thompson WM. From the archives of the AFIP: primary vascular neoplasms of the spleen: radiologic-pathologic correlation. Radiographics. 2004;24:1137-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 238] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 4. | Emir S, Sozen S, Yazar MF, Altınsoy HB, Arslan Solmaz O, Ozkan Z. Littoral-cell angioma of the spleen. Arch Iran Med. 2013;16:189-191. [PubMed] |
| 5. | Yano H, Imasato M, Monden T, Okamoto S. Hand-assisted laparoscopic splenectomy for splenic vascular tumors: report of two cases. Surg Laparosc Endosc Percutan Tech. 2003;13:286-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Delaitre B, Maignien B. [Splenectomy by the laparoscopic approach. Report of a case]. Presse Med. 1991;20:2263. [PubMed] |
| 7. | Silecchia G, Boru CE, Fantini A, Raparelli L, Greco F, Rizzello M, Pecchia A, Fabiano P, Basso N. Laparoscopic splenectomy in the management of benign and malignant hematologic diseases. JSLS. 2006;10:199-205. [PubMed] |
| 8. | Cai YQ, Zhou J, Chen XD, Wang YC, Wu Z, Peng B. Laparoscopic splenectomy is an effective and safe intervention for hypersplenism secondary to liver cirrhosis. Surg Endosc. 2011;25:3791-3797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | O’Reilly RA. Splenomegaly in 2,505 patients at a large university medical center from 1913 to 1995. 1963 to 1995: 449 patients. West J Med. 1998;169:88-97. [PubMed] |
| 10. | Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W, Buchler M; International Study Group on Pancreatic Fistula D. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3282] [Cited by in RCA: 3512] [Article Influence: 175.6] [Reference Citation Analysis (34)] |
| 11. | Heese J, Bocklage T. Specimen fine-needle aspiration cytology of littoral cell angioma with histologic and immunohistochemical confirmation. Diagn Cytopathol. 2000;22:39-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Suto H, Imai H, Sato E, Ando J, Nobukawa B, Sugimoto K. Severe thrombocytopenia caused by littoral cell angioma. Int J Hematol. 2008;88:253-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Ziske C, Meybehm M, Sauerbruch T, Schmidt-Wolf IG. Littoral cell angioma as a rare cause of splenomegaly. Ann Hematol. 2001;80:45-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Musgrave NJ, Williamson RM, O’Rourke NA, Searle JW. Test and teach. Incidentally discovered splenic vascular lesion. Littoral cell angioma of the spleen. Pathology. 2002;34:579-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Veillon DM, Williams RB, Sardenga LJ, Harrison GK, Cotelingam JD. ‘Little’ littoral cell angioma of the spleen. Am J Surg Pathol. 2000;24:306-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Barbazza R, De Martini A, Mognol M, Banzi A, D’Agata G. Fine needle aspiration biopsy of a splenic hemangioma. A case report with review of the literature. Haematologica. 1990;75:278-281. [PubMed] |
| 17. | Matuszczak E, Reszec J, Dębek W, Hermanowicz A, Chyczewski L. Is littoral cell angioma of the spleen as rare as previously believed in the pediatric population? Folia Histochem Cytobiol. 2012;50:480-485. [PubMed] |
| 18. | Blansfield JA, Goldhahn RT, Josloff RK. Littoral cell angioma of the spleen treated by laparoscopic splenectomy. JSLS. 2005;9:222-224. [PubMed] |
| 19. | Rosen M, Brody F, Walsh RM, Tarnoff M, Malm J, Ponsky J. Outcome of laparoscopic splenectomy based on hematologic indication. Surg Endosc. 2002;16:272-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 75] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Yano H, Nakano Y, Tono T, Ohnishi T, Iwazawa T, Kimura Y, Kanoh T, Monden T. Hand-assisted laparoscopic splenectomy for splenic tumors. Dig Surg. 2004;21:215-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Kawanaka H, Akahoshi T, Kinjo N, Konishi K, Yoshida D, Anegawa G, Yamaguchi S, Uehara H, Hashimoto N, Tsutsumi N. Technical standardization of laparoscopic splenectomy harmonized with hand-assisted laparoscopic surgery for patients with liver cirrhosis and hypersplenism. J Hepatobiliary Pancreat Surg. 2009;16:749-757. [PubMed] |
| 22. | Fais PO, Carricaburu E, Sarnacki S, Berrebi D, Orbach D, Baudoin V, de Lagausie P. Is laparoscopic management suitable for solid pseudo-papillary tumors of the pancreas? Pediatr Surg Int. 2009;25:617-621. [PubMed] |