Published online Jun 7, 2015. doi: 10.3748/wjg.v21.i21.6649
Peer-review started: September 5, 2014
First decision: October 14, 2014
Revised: December 30, 2014
Accepted: January 16, 2015
Article in press: January 16, 2015
Published online: June 7, 2015
Processing time: 282 Days and 20.3 Hours
AIM: To investigate whether neuron-glial antigen 2 (NG2) could be an effective prognostic marker in hepatocellular carcinoma (HCC).
METHODS: NG2 expression was semi-quantitatively scored from the immunohistochemistry (IHC) data based on the number of positive cells and the staining intensity. A total of 132 HCC specimens and 96 adjacent noncancerous tissue samples were analyzed by IHC for NG2 protein expression. To confirm the NG2 expression levels observed by IHC, we measured NG2 expression in 30 randomly selected tumor and adjacent noncancerous tissue samples by quantitative real-time polymerase chain reaction and Western blot. The correlations between NG2 protein expression and the clinicopathological features of HCC patients were analyzed using the χ2 test. To assess the prognostic value of NG2 for HCC, the association between NG2 expression and survival was analyzed using the Kaplan-Meier method with the log-rank test. To further evaluate the prognostic value of NG2 expression, a Cox multivariate proportional hazards regression analysis was performed with all the variables to derive risk estimates related to disease-free and overall survival and to control for confounders.
RESULTS: High NG2 expression was observed in significantly more primary tumor samples (63.6%; 84/132) compared with the adjacent noncancerous tissue samples (28.1%; 27/96) (P < 0.0001). Moreover, high NG2 protein expression was closely associated with tumor differentiation (χ2 = 9.436, P = 0.0089), recurrence (χ2 = 5.769, P = 0.0163), tumor-node-metastasis (TNM) stage (χ2 = 8.976, P = 0.0027), and invasion (χ2 = 5.476, P = 0.0193). However, no significant relationship was observed between NG2 protein expression in HCC and other parameters, such as age, sex, tumor size, serum alpha fetoprotein (AFP), tumor number, or tumor capsule. The log-rank test indicated a significant difference in the overall survival of HCC patients with high NG2 expression compared with those with low NG2 expression (29.2% vs 9.5%, P < 0.001). Moreover, NG2 expression in HCC tissue significantly correlated with disease-free survival (15.2% vs 6.7%, P < 0.001). Multivariate analysis showed that NG2 expression (HR = 2.035, P = 0.002), serum AFP (HR = 1.903, P = 0.003), TNM stage (HR = 2.039, P = 0.001), and portal vein invasion (HR = 1.938, P = 0.002) were independent prognostic indicators for OS in HCC patients. Furthermore, NG2 expression (HR = 1.974, P = 0.003), serum AFP (HR = 1.767, P = 0.008), TNM stage (HR = 2.078, P = 0.001), tumor capsule (HR = 0.652, P = 0.045), and portal vein invasion (HR = 1.941, P = 0.002) were independent prognostic indicators for DFS in HCC patients.
CONCLUSION: The up-regulation of NG2 is associated with poor prognosis in HCC. Therefore, NG2 could be useful as an additional prognostic marker to increase the resolution of traditional approaches.
Core tip: To help predict which patients have an unfavorable prognosis, it is critical to identify a dependable prognostic biomarker that correlates with hepatocellular carcinoma (HCC) progression, invasion, metastasis and recurrence. Despite recent evidence showing that neuron-glial antigen 2 (NG2) is expressed on the surface of differentiated malignant cells, progenitor cells, and cancer stem cells in various tumors and promotes the growth and metastasis of melanoma cells, whether it is associated with HCC progression remains elusive. The results of the present study showed that NG2 is useful as an additional prognostic marker to increase the resolution of current approaches.
- Citation: Lu LL, Sun J, Lai JJ, Jiang Y, Bai LH, Zhang LD. Neuron-glial antigen 2 overexpression in hepatocellular carcinoma predicts poor prognosis. World J Gastroenterol 2015; 21(21): 6649-6659
- URL: https://www.wjgnet.com/1007-9327/full/v21/i21/6649.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i21.6649
Hepatocellular carcinoma (HCC) is a common type of cancer, and in China, its associated morbidity and mortality rates have become among the highest in the world, accounting annually for 55% of new cases and deaths worldwide[1]. HCC treatment is extremely difficult because patients are asymptomatic until the cancer rapidly progresses into its terminal phase, creating a challenging and ineffective treatment. In most cases, relatively favorable curative effects are achieved by surgical liver resection, and even after liver resection, the 5-year survival rate is less than 35% because of recurrence and metastasis[2,3]. To help predict which patients have an unfavorable prognosis, it is critical to identify a dependable prognostic biomarker that correlates with HCC progression, invasion, metastasis and recurrence.
Neuron-glial antigen 2 (NG2)/chondroitin sulfate proteoglycan (CSPG-4) is an integral membrane proteoglycan located on the surface of many types of progenitor cells[4]. NG2 is a phylogenetically conserved protein[5] composed of a 280-kDa N-linked glycoprotein and a 450-kDa proteoglycan[6] that plays numerous mediatory roles in the proliferation, migration, and metastasis of melanoma cells[7]. The human homologue of NG2 is expressed in most human melanomas and promotes the growth and metastasis of melanoma cells[7]. NG2 expression is associated with metastasis in soft-tissue sarcoma patients[8] and with the progression of glioma[9], astrocytoma[10], and myeloid leukemia[11]. NG2 also regulates cell adhesion to collagen and other extracellular matrix (ECM) components[12-14]. NG2 is expressed on the surface of differentiated malignant cells, progenitor cells, and cancer stem cells in various tumors[15]. Furthermore, NG2 plays a critical role in the growth, migration, and metastasis of tumor cells[8]. In the normal adult brain, NG2 is expressed by 5% of all neural cells, has a wide anatomical distribution, and is expressed in more than 70% of cycling progenitors. NG2+ progenitors have the ability to proliferate, self-renew, and produce different types of neural cells under normal and pathological conditions. Collectively, these characteristics have led to a reevaluation of the lineage potential and function of the previously considered unipotent oligodendrocyte precursors. It remains unknown whether HCC migration and metastasis can be attributed to NG2 expression and function. Thus, this study investigated whether NG2 is overexpressed in HCC, whether it is associated with HCC progression, invasion, metastasis, and recurrence, and whether it could be an effective prognostic marker.
All patients provided written informed consent to use tumor tissue for clinical research. Tissue samples were collected from 132 HCC patients who underwent a hepatectomy at the Department of Hepatobiliary Surgery between January and December 2007, according to protocols approved by the Medical Ethics Committee of the Southwest Hospital and the First Affiliated Hospital of the Third Military Medical University. No patients received radiotherapy or chemotherapy before hepatectomy. The survivors were followed for 6 years. The tumors were pathologically graded according to the World Health Organization classification system, and the pathological staging after hepatectomy was performed according to the 2002 tumor-node-metastasis (TNM) classification of malignant tumors from the International Union Against Cancer. The clinicopathological characteristics of the patients are listed in Table 1. Immediately after resection, 30 fresh tumor tissue specimens were snap-frozen in liquid nitrogen and stored at -80 °C. Overall survival (OS) was defined as the period between surgery and death or the last contact. Survivor data at the last follow-up were abridged. Relapse-free survival (RFS) was defined as the period between surgery and any form of recurrence[16].
| Characteristic | n | NG2 | χ2 | P value | |
| High | Low | ||||
| Hepatocellular carcinoma | 132 | 84 | 48 | < 0.0001 | |
| Non-tumor liver | 96 | 27 | 69 | ||
| Total cases | 132 | 84 | 48 | ||
| Age (yr) | |||||
| ≥ 50 | 62 | 40 | 22 | 0.039 | 0.843 |
| < 50 | 70 | 44 | 26 | ||
| Sex | |||||
| Male | 106 | 69 | 37 | 0.494 | 0.482 |
| Female | 26 | 15 | 11 | ||
| Tumor size | |||||
| ≥ 5 cm | 90 | 56 | 34 | 0.244 | 0.621 |
| < 5 cm | 42 | 28 | 14 | ||
| TNM stage | |||||
| I, II | 52 | 25 | 27 | 8.976 | 0.002 |
| III, IV | 80 | 59 | 21 | ||
| Tumor differentiation | |||||
| Well | 33 | 15 | 18 | 9.436 | 0.008 |
| Moderate | 52 | 32 | 20 | ||
| Poor | 47 | 37 | 10 | ||
| Serum AFP | |||||
| ≥ 200 ng/dL | 42 | 30 | 12 | 1.616 | 0.203 |
| < 200 ng/dL | 90 | 54 | 36 | ||
| Recurrence | |||||
| Yes | 65 | 48 | 17 | 5.769 | 0.016 |
| No | 67 | 36 | 31 | ||
| Invasion | |||||
| Yes | 70 | 51 | 19 | 5.476 | 0.019 |
| No | 62 | 33 | 29 | ||
| Tumor number | |||||
| Multiple | 31 | 19 | 12 | 0.096 | 0.756 |
| Single | 101 | 65 | 36 | ||
| Tumor capsule | |||||
| Yes | 58 | 33 | 25 | 2.031 | 0.154 |
| No | 74 | 51 | 23 | ||
Tissue sections were deparaffinized in xylene and rehydrated in ethanol. In vivo peroxidase activity was blocked with 3% H2O2 for 10 min. The sections were subjected to antigen retrieval in a microwave oven in 10 mmol/L citrate buffer (pH 6.0). The sections were incubated in 10% goat serum albumin in phosphate-buffered saline (PBS) for 30 min, then in an anti-human NG2 polyclonal antibody (1:100 dilution; Abcam, Burlingame, United States) at 4 °C overnight, and finally with appropriate secondary antibodies for 60 min and washed with PBS. Negative controls were incubated with PBS instead of the primary antibody under identical conditions.
Total RNA was extracted with the TRIzol reagent (TaKaRa, Dalian, China). qRT-PCR was performed using the SYBR Green PCR Master Mix (TaKaRa, Dalian, China) on a Rotor-Gene 6000 RT-PCR System (Applied Biosystems) with the following cycling conditions: 95 °C for 30 s and 40 cycles of 95 °C for 5 s and 60 °C for 60 s. The primer pair sequences are provided below: NG2 forward 5’-CCTGTCCTCACCAATGTCCTCCT-3’, NG2 reverse 5’-GCTGGCACTGTTGAGACTCTTGAC-3’, β-actin forward 5’-ATAGCACAGCCTGGATAGCAACGTAC-3’ and β-actin reverse 5’-CACCTTCTACAATGAGCTGCGTGTG-3’. The β-actin gene was used as the reference control. The relative mRNA expression levels were calculated based on the Ct values using the 2-ΔΔCt method. Each experiment was repeated in triplicate.
Western blot was performed as previously described[17]. After electrophoresis, the proteins were transferred to a polyvinylidene fluoride membrane. The membrane was blocked with 5% nonfat milk in Tris-buffered saline with Tween (TBST) and incubated with an anti-human NG2 polyclonal antibody (1:1000 dilution; Abcam, Burlingame, United States) in TBST containing 5% bovine serum albumin at 4 °C overnight. After washing three times with TBST, the membrane was incubated with a horseradish peroxidase-conjugated secondary antibody diluted in TBST at room temperature for 2 h. The protein signals were visualized using an electrochemiluminescence system. The gray values were calculated using Image J2x. The NG2 protein levels were normalized to β-actin protein levels.
Significant relationships between NG2 expression and the clinicopathological parameters were evaluated using the χ2 test or Fisher’s exact test. Survival rates were calculated using the Kaplan-Meier method and compared using the log-rank test. The independent prognostic factors for survival were identified by a multivariate analysis using the Cox proportional hazard regression model. All the analyses were performed using SPSS 17.0 for Windows.
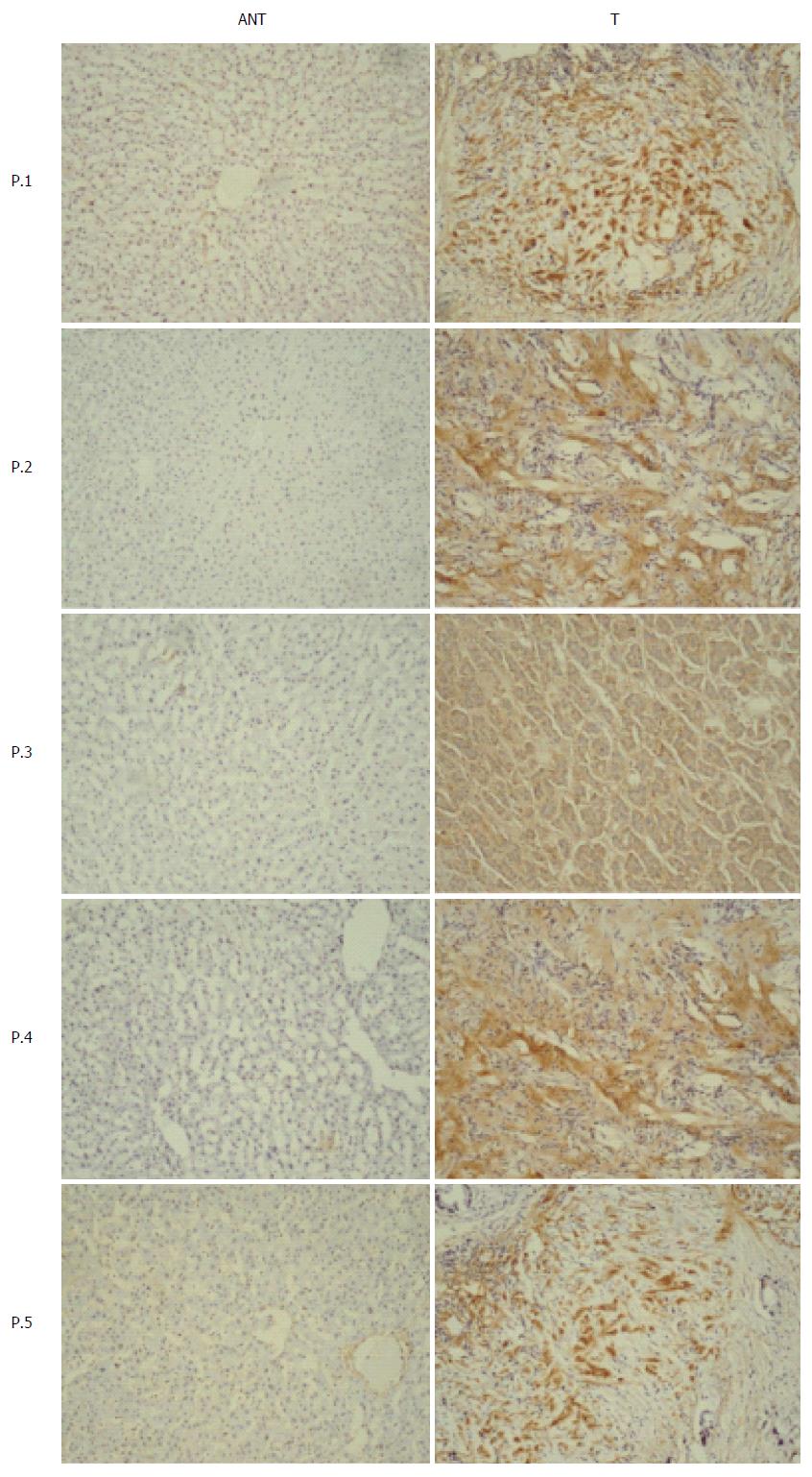
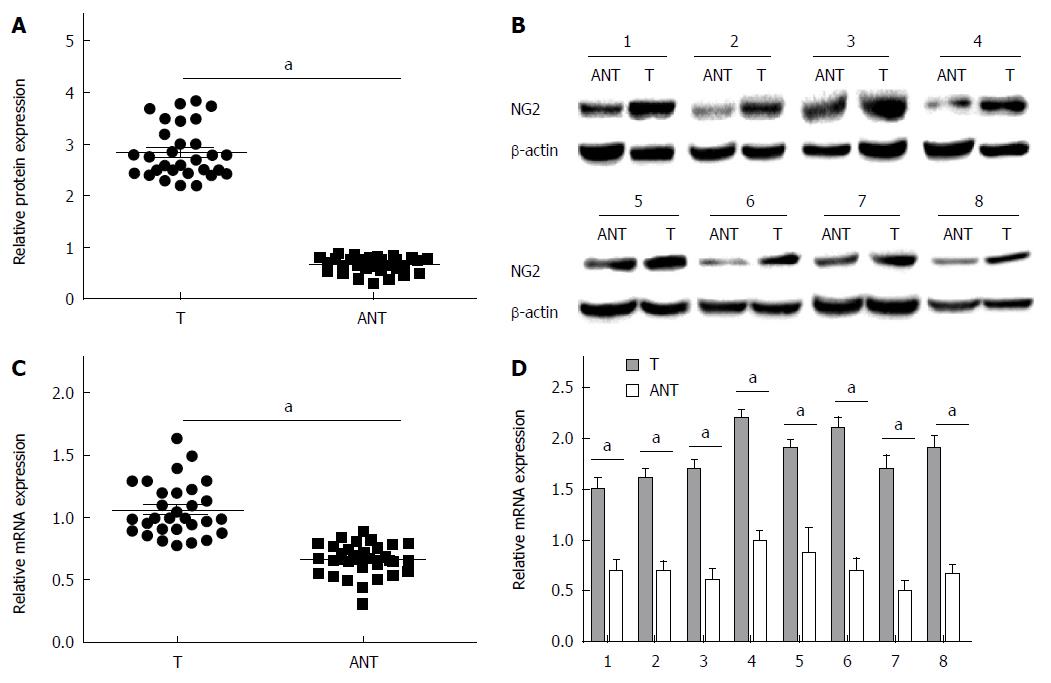
NG2 expression was semi-quantitatively scored from the immunohistochemistry (IHC) data based on the number of positive cells and the staining intensity (Table 2). Samples were considered NG2-positive if either the cell nucleus or cytoplasm stained positive. As shown in Table 1, high NG2 expression was observed in significantly more primary tumor samples (63.6%; 84/132) compared with the adjacent noncancerous tissue samples (28.1%; 27/96) (P < 0.0001). NG2 expression was determined by IHC in 5 representative pairs of hepatic carcinoma tissue and adjacent non-tumorous tissue (Figure 1). To confirm the NG2 expression levels observed by IHC, we measured NG2 expression in 30 randomly selected tumor samples by qRT-PCR and Western blot. The results show that NG2 protein and mRNA expression markedly increased in primary tumor samples compared with the adjacent noncancerous tissue samples (Figure 2).
| Score | |
| Percent positive | |
| 0% | 0 |
| 1%-10% | 1 |
| 11%-50% | 2 |
| 51%-80% | 3 |
| > 80% | 4 |
| Staining intensity | |
| No staining | 0 |
| Weakly stained | 1 |
| Moderately stained | 2 |
| Strongly stained | 3 |
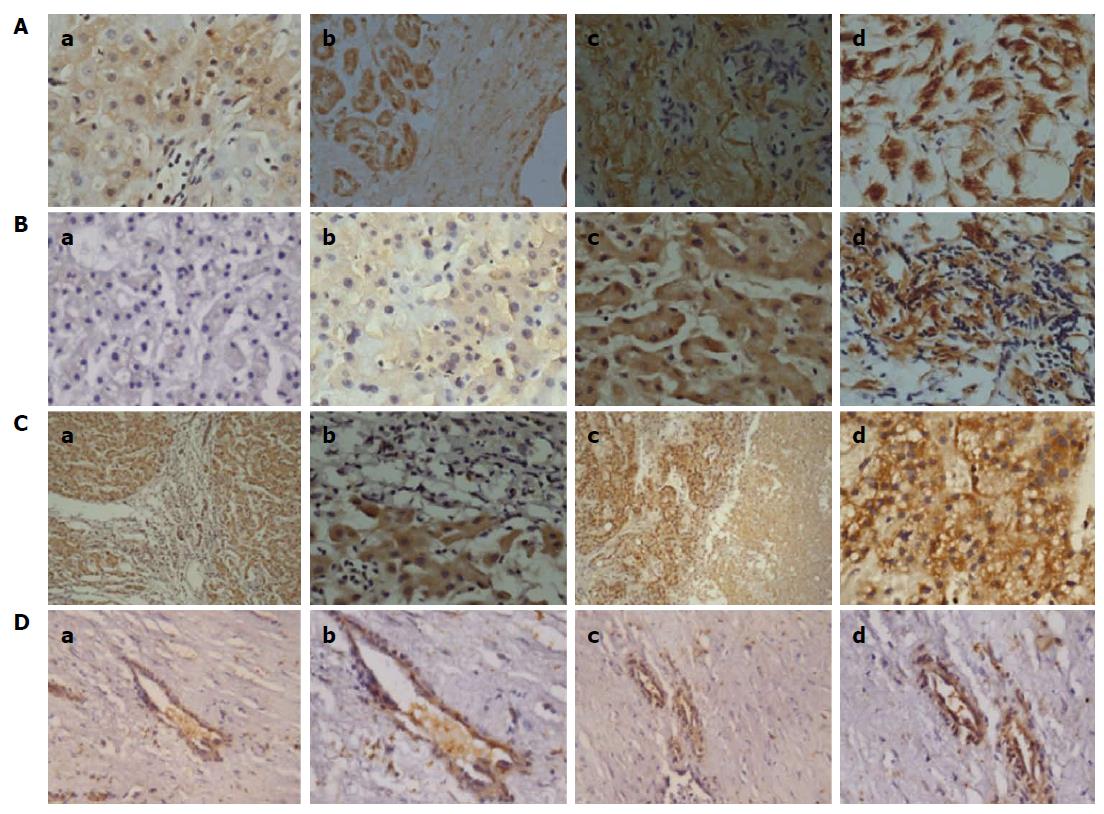
As presented in Table 1, the correlations between NG2 protein expression and the clinicopathological features of HCC patients were analyzed using the χ2 test. High NG2 protein expression was closely associated with tumor differentiation (P = 0.0089), recurrence (P = 0.0163), TNM stage (P = 0.0027), and invasion (P = 0.0193). However, no significant relationship was observed between NG2 protein expression in HCC and other parameters, such as age, sex, tumor size, serum AFP, tumor number, or tumor capsule. NG2 expression in human hepatic carcinoma tissues at TNM III/IV stages was significantly increased compared with TNM I/II stage tissues (Figure 3A). Additionally, NG2 expression in human hepatic carcinoma tissues with a poor degree of differentiation was significantly increased compared with moderate and high degrees of differentiation (Figure 3B). Moreover, NG2 expression increased in human hepatic carcinoma tissues with portal vein invasion (Figure 3C), and interestingly, NG2 was highly expressed in vessels within human hepatic carcinoma tissue (Figure 3D).
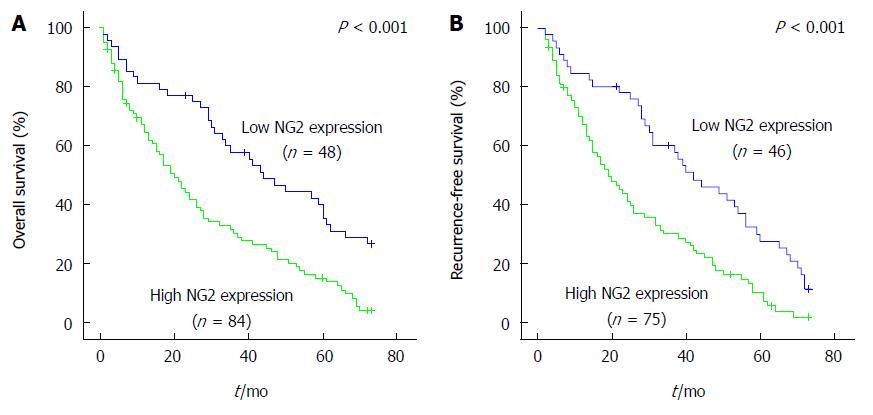
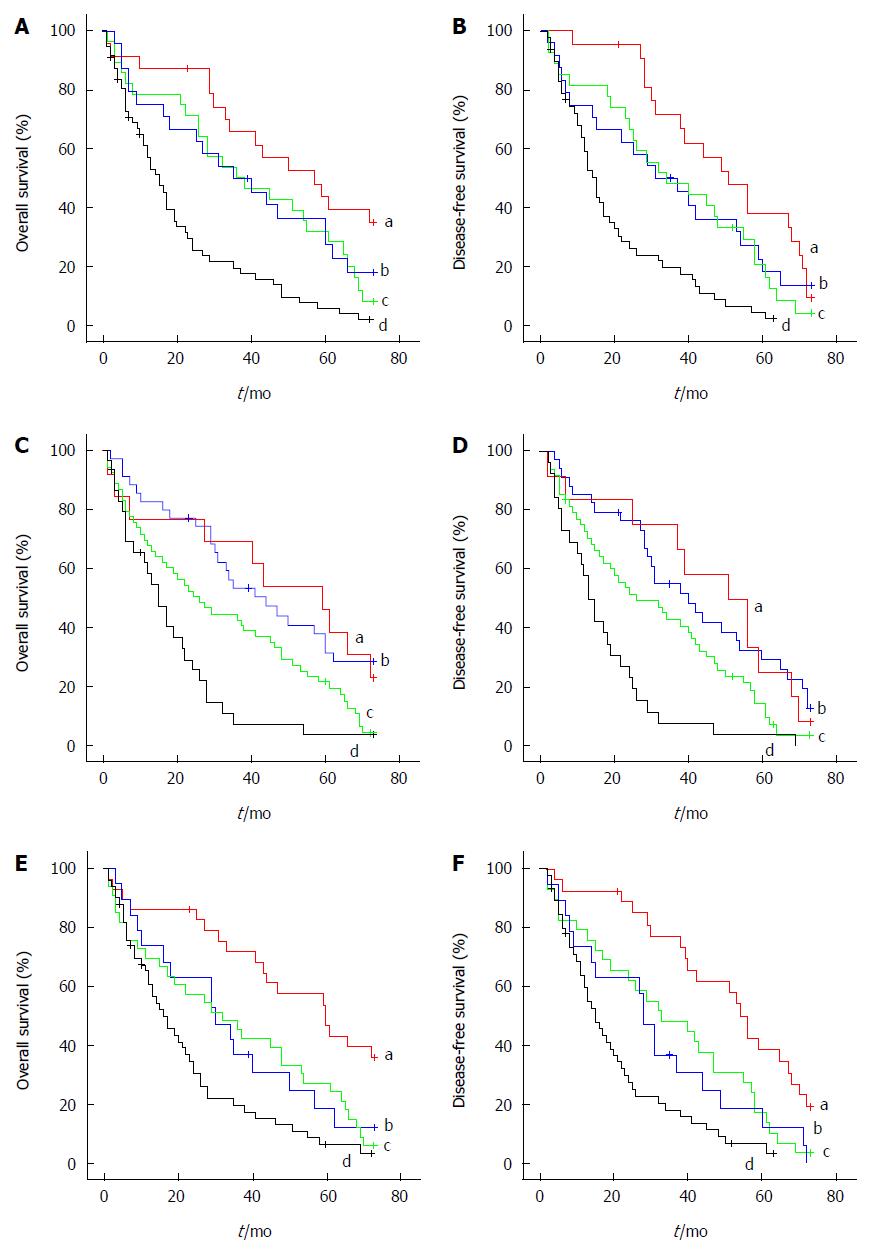
To assess the prognostic value of NG2 for HCC, the association between NG2 expression and survival was analyzed using the Kaplan-Meier method with the log-rank test. Patients were separated into two groups, an NG2-high group (n = 84) and an NG2-low group (n = 48). The log-rank test indicated a significant difference in the overall survival of HCC patients with high NG2 expression compared with those with low NG2 expression (P < 0.001; Figure 4A). Moreover, NG2 expression in HCC tissue significantly correlated with disease-free survival (P < 0.001; Figure 4B). Other prognostic factors were investigated, and a univariate analysis revealed that invasion, serum AFP and TNM stage adversely affected the disease-free and overall survival (Table 3). Moreover, serum AFP and tumor number adversely affected progression-free but not overall survival (Table 3). Combination analysis of the survival data revealed that 25 (48.1%) of the 52 HCC patients with TNM stage I/II disease were NG2-positive and had an unfavorable outcome (Figure 5A and B). In contrast, 59 (73.7%) of the 80 HCC patients with TNM stage III/IV disease were NG2-positive but had an unfavorable outcome (Figure 5A and B). Similarly, of the 90 patients with low serum AFP, 54 (60%) had NG2-positive tumors and an unfavorable outcome (Figure 5C and D). Interestingly, 51 (72.8%) of the 70 HCC patients with venous invasion were NG2-positive and had a poor outcome (Figure 5E and F). Conversely, of the 62 patients without venous invasion, 33 (53.2%) with NG2-positive tumors did not have a favorable outcome (Figure 5E and F). These results indicate that high NG2 expression correlated with shorter survival time.
| Factor | n | 6-yr disease-free survival | P value | 6-yr overall survival | P value |
| NG2 | 0.000 | 0.000 | |||
| Negative | 84 | 15.2% | 29.2% | ||
| Positive | 48 | 6.7% | 9.5% | ||
| Age (yr) | 0.946 | 0.356 | |||
| ≥ 50 | 62 | 10.2% | 21.0% | ||
| < 50 | 70 | 9.7% | 31.4% | ||
| Sex | 0.281 | 0.316 | |||
| Male | 106 | 7.1% | 23.6% | ||
| Female | 26 | 21.7% | 38.5% | ||
| Tumor size | 0.122 | 0.116 | |||
| ≥ 5 cm | 90 | 11.8% | 28.9% | ||
| < 5 cm | 42 | 5.6% | 21.4% | ||
| TNM stage | 0.001 | 0.003 | |||
| I, II | 52 | 10.2% | 34.6% | ||
| III, IV | 80 | 9.7% | 21.3% | ||
| Differentiation | 0.108 | 0.119 | |||
| Well | 33 | 23.3% | 33.3% | ||
| Moderate | 52 | 2.0% | 9.6% | ||
| Poor | 47 | 9.5% | 12.8% | ||
| Serum AFP | 0.044 | 0.099 | |||
| ≥ 200 ng/dL | 42 | 2.3% | 18.0% | ||
| < 200 ng/dL | 90 | 13.6% | 14.0% | ||
| Invasion | 0.000 | 0.000 | |||
| Yes | 70 | 7.7% | 12.9% | ||
| No | 62 | 12.5% | 21.0% | ||
| Number | 0.041 | 0.057 | |||
| Multiple | 31 | 10.7% | 16.1% | ||
| Single | 101 | 9.7% | 16.8% | ||
| Capsule | 0.064 | 0.055 | |||
| Yes | 58 | 14.3% | 21.6% | ||
| No | 74 | 6.2% | 32.8% |
To further evaluate the prognostic value of NG2 expression, a Cox multivariate proportional hazards regression analysis was performed with all the variables to derive risk estimates related to disease-free and overall survival and to control for confounders. Multivariate analysis (Table 4) of the above-mentioned significant indicators of OS and DFS showed that NG2 expression (P = 0.002), serum AFP (P = 0.003), TNM stage (P = 0.001), and portal vein invasion (P = 0.002) were independent prognostic indicators for OS in HCC patients. Furthermore, NG2 expression (P = 0.003), serum AFP (P = 0.008), TNM stage (P = 0.001), tumor capsule (P = 0.045), and portal vein invasion (P = 0.002) were independent prognostic indicators for DFS in HCC patients (Table 4). NG2 expression in HCC was identified as a powerful prognostic indicator of disease-free and overall survival.
| Variable | Overall survival | Disease-free survival | ||||
| HR | (95%CI) | P value | HR | (95%CI) | P value | |
| TNM stage | 2.039 | (1.329-3.130) | 0.001 | 2.078 | (1.347-3.206) | 0.001 |
| Serum AFP | 1.903 | (1.247-2.903) | 0.003 | 1.767 | (1.163-2.685) | 0.008 |
| Tumor capsule | - | - | - | 0.652 | (0.429-0.991) | 0.045 |
| Invasion | 1.938 | (1.273-2.950) | 0.002 | 1.941 | (1.265-2.980) | 0.002 |
| NG2 | 2.035 | (1.302-3.179) | 0.002 | 1.974 | (1.258-3.097) | 0.003 |
This study is the first report to establish NG2 as an independent and powerful prognostic marker for disease progression and overall survival for patients with surgically resected primary HCC. In this study, we evaluated NG2 expression at both the transcriptional and translational levels in numerous hepatocellular carcinoma tissue samples and corresponding matched adjacent non-tumor tissue samples using qRT-PCR and Western blot. We found that NG2 mRNA and protein were significantly overexpressed in tumor tissue compared with matched adjacent non-tumorous tissue, which was consistent with the IHC analysis. These results suggested that NG2 might play an important role in HCC tumorigenesis. Indeed, NG2 expression has been correlated with the progression of melanoma[7], glioma[9], astrocytoma[10], and myeloid leukemia[18].
We also found that high NG2 expression was significantly correlated with poor tumor differentiation, the presence of vascular invasion, TNM stage and recurrence. In HCC, the correlations between NG2 expression and poor tumor differentiation, TNM stage and the presence of vascular invasion are intriguing. Kaplan-Meier survival analysis revealed a significant correlation between NG2 expression and overall and relapse-free survival. A more comprehensive analysis of the survival data revealed that among patients with TNM stage III/IV disease or the presence of vascular invasion, most patients also had NG2-positive tumors and an unfavorable outcome. However, a large proportion of the patients with TNM stage I/II disease without vascular invasion but with NG2-positive tumors still had an unfavorable outcome. These results indicated that the combination of NG2 expression with current clinicopathological prognostic markers may accurately predict HCC patient prognosis after hepatic resection. The Cox regression analysis demonstrated that NG2 expression was a significant independent predictor of OS and DFS in HCC patients after hepatectomy, increasing its promise as a predictive biomarker.
NG2 might play a role in promoting HCC progression and metastasis. Many different signaling mechanisms have been proposed by which NG2 could contribute to malignant transformation and subsequently promote tumor formation and metastasis. The complex mechanisms by which NG2 affects melanoma progression have started to be defined, in particular the association with other cell surface proteins and receptor tyrosine kinases (RTKs) and its central role in modulating the function of these proteins[19]. NG2 is essential to the growth of melanoma tumors through its modulation of integrin function and enhanced growth factor receptor-regulated pathways including sustained activation of ERK 1/2. The activation of integrin, RTK, and ERK 1/2 function by NG2 modulates numerous aspects of tumor progression[20,21]. Expression of a full-length NG2 in RGP human melanoma cells lacking endogenous NG2 expression results in significantly enhanced integrin mediated spreading and FAK activation[22]. As NG2 can activate MMP complexes on melanoma cell surface, the collective results implicate NG2 as an important contributing factor to localized invasion at the leading edge of invasive primary tumors[23-25]. Finally, NG2-mediated activation of integrin facilitates enhanced survival of human glioblastoma cells when they were exposed to clinically used chemotherapeutic agents by enhancing sustained activation of AKT via PI3K[22].
NG2 overexpression may induce highly aggressive tumors characterized by increased angiogenesis and a moderately invasive phenotype[26,27]. Indeed, NG2-positive tumors grow more rapidly, have disrupted blood-brain barrier integrity, and exhibit an increased vascular volume fraction[26,28]. The NG2 proteoglycan, whose extended central D2 domain binds to type VI collagen, acts as a link between the cell surface and the extracellular matrix[13]. Similar results have been achieved on laminin 2-coated surfaces[29,30]. The roles of collagen VI and laminin 2 in the brain vasculature and their association with axonal processes enable the migration of NG2-positive glioma cells along blood vessels and nerve fiber tracts[31,32].
This study is limited by its retrospective nature and the inclusion of only patients with resectable tumors. It is possible that these findings pertain only to those patients who have undergone resection. Larger prospective studies examining NG2 are necessary to further validate the usefulness of this system. Suppressing NG2 overexpression may prevent invasive progression and metastatic relapse, which would improve the prognosis and quality of life for HCC patients after hepatic resection. In the near future, targeting the NG2 pathway may be a therapeutic strategy for HCC that assists conventional chemotherapy or radiotherapy. NG2 might play a role in promoting HCC progression and metastasis. However, current studies on the functional roles of NG2 are superficial, and future studies should concentrate on the molecular mechanisms by which NG2 contributes to HCC development and progression.
Neuron-glial antigen 2 (NG2)/chondroitin sulfate proteoglycan is an integral membrane proteoglycan located on the surface of many types of progenitor cells. NG2 is a phylogenetically conserved protein composed of a 280-kDa N-linked glycoprotein and a 450-kDa proteoglycan that plays numerous mediatory roles in the proliferation, migration, and metastasis of melanoma cells. NG2 is expressed on the surface of differentiated malignant cells, progenitor cells, and cancer stem cells in various tumors. Furthermore, NG2 plays a critical role in the growth, migration, and metastasis of tumor cells. Whether NG2 is overexpressed in hepatocellular carcinoma (HCC), whether it is associated with HCC progression, invasion, metastasis, and recurrence, and whether it could be an effective prognostic marker remain unknown.
This study utilized immunohistochemistry (IHC) to gauge NG2 expression semi-quantitatively based on the number of positive cells and the staining intensity. To confirm the NG2 expression levels observed by IHC, we measured NG2 expression in 30 randomly selected tumor and adjacent noncancerous tissue samples by qRT-PCR and Western blot. Those techniques are highly specific and efficient, easy to control and manipulate, versatile, and time saving.
This study demonstrates that NG2 expression in primary tumor samples was significantly more than that in the adjacent noncancerous tissue samples. In addition, it demonstrated a significant difference in the overall survival of HCC patients with high NG2 expression compared with those with low NG2 expression. Furthermore, NG2 expression was an independent prognostic indicator for DFS in HCC patients.
The results of the present study suggest that NG2 expression in HCC was identified as a powerful prognostic indicator of disease-free and overall survival.
Overall survival was defined as the period between surgery and death or the last contact. Survivor data at the last follow-up were abridged. Relapse-free survival was defined as the period between surgery and any form of recurrence.
The study “NG2 overexpression in HCC predicts poor prognosis” is an attractive and interesting study, although, as noted by the author, it remains retrospective and is focused only on subjects who underwent surgical resection.
P- Reviewer: Berkane S, Tomizawa M, Zhang ZM S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Liu XM
| 1. | Yang ZQ, Yang ZY, Zhang LD, Ping-Bie SG, Ma KS, Li XW, Dong JH. Increased liver-infiltrating CD8+FoxP3+ regulatory T cells are associated with tumor stage in hepatocellular carcinoma patients. Hum Immunol. 2010;71:1180-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 2. | Kondo K, Chijiiwa K, Makino I, Kai M, Maehara N, Ohuchida J, Naganuma S. Risk factors for early death after liver resection in patients with solitary hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2005;12:399-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Khan AS, Fowler KJ, Chapman WC. Current surgical treatment strategies for hepatocellular carcinoma in North America. World J Gastroenterol. 2014;20:15007-15017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Trotter J, Karram K, Nishiyama A. NG2 cells: Properties, progeny and origin. Brain Res Rev. 2010;63:72-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 196] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 5. | Sakry D, Karram K, Trotter J. Synapses between NG2 glia and neurons. J Anat. 2011;219:2-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | O’Brien CA, Kreso A, Jamieson CH. Cancer stem cells and self-renewal. Clin Cancer Res. 2010;16:3113-3120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 350] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 7. | Russell KC, Tucker HA, Bunnell BA, Andreeff M, Schober W, Gaynor AS, Strickler KL, Lin S, Lacey MR, O’Connor KC. Cell-surface expression of neuron-glial antigen 2 (NG2) and melanoma cell adhesion molecule (CD146) in heterogeneous cultures of marrow-derived mesenchymal stem cells. Tissue Eng Part A. 2013;19:2253-2266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Benassi MS, Pazzaglia L, Chiechi A, Alberghini M, Conti A, Cattaruzza S, Wassermann B, Picci P, Perris R. NG2 expression predicts the metastasis formation in soft-tissue sarcoma patients. J Orthop Res. 2009;27:135-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Stallcup WB, Huang FJ. A role for the NG2 proteoglycan in glioma progression. Cell Adh Migr. 2008;2:192-201. [PubMed] |
| 10. | Seyfried NT, Huysentruyt LC, Atwood JA, Xia Q, Seyfried TN, Orlando R. Up-regulation of NG2 proteoglycan and interferon-induced transmembrane proteins 1 and 3 in mouse astrocytoma: a membrane proteomics approach. Cancer Lett. 2008;263:243-252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Mauvieux L, Delabesse E, Bourquelot P, Radford-Weiss I, Bennaceur A, Flandrin G, Valensi F, MacIntyre EA. NG2 expression in MLL rearranged acute myeloid leukaemia is restricted to monoblastic cases. Br J Haematol. 1999;107:674-676. [PubMed] |
| 12. | Petrini S, Tessa A, Stallcup WB, Sabatelli P, Pescatori M, Giusti B, Carrozzo R, Verardo M, Bergamin N, Columbaro M. Altered expression of the MCSP/NG2 chondroitin sulfate proteoglycan in collagen VI deficiency. Mol Cell Neurosci. 2005;30:408-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Xiong J, Wang Y, Zhu Z, Liu J, Wang Y, Zhang C, Hammes HP, Lang F, Feng Y. NG2 proteoglycan increases mesangial cell proliferation and extracellular matrix production. Biochem Biophys Res Commun. 2007;361:960-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Cattaruzza S, Nicolosi PA, Braghetta P, Pazzaglia L, Benassi MS, Picci P, Lacrima K, Zanocco D, Rizzo E, Stallcup WB. NG2/CSPG4-collagen type VI interplays putatively involved in the microenvironmental control of tumour engraftment and local expansion. J Mol Cell Biol. 2013;5:176-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O. Skeletal muscle neural progenitor cells exhibit properties of NG2-glia. Exp Cell Res. 2013;319:45-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 16. | Dede K, Salamon F, Landherr L, Jakab F, Bursics A. Pathologic assessment of response to chemotherapy in colorectal cancer liver metastases after hepatic resection: which method to use? Pathol Oncol Res. 2015;21:173-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Schnabel CL, Wagner S, Wagner B, Durán MC, Babasyan S, Nolte I, Pfarrer C, Feige K, Murua Escobar H, Cavalleri JM. Evaluation of the reactivity of commercially available monoclonal antibodies with equine cytokines. Vet Immunol Immunopathol. 2013;156:1-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Bueno C, Montes R, Martín L, Prat I, Hernandez MC, Orfao A, Menendez P. NG2 antigen is expressed in CD34+ HPCs and plasmacytoid dendritic cell precursors: is NG2 expression in leukemia dependent on the target cell where leukemogenesis is triggered? Leukemia. 2008;22:1475-1478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Price MA, Colvin Wanshura LE, Yang J, Carlson J, Xiang B, Li G, Ferrone S, Dudek AZ, Turley EA, McCarthy JB. CSPG4, a potential therapeutic target, facilitates malignant progression of melanoma. Pigment Cell Melanoma Res. 2011;24:1148-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 151] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 20. | Ishii A, Fyffe-Maricich SL, Furusho M, Miller RH, Bansal R. ERK1/ERK2 MAPK signaling is required to increase myelin thickness independent of oligodendrocyte differentiation and initiation of myelination. J Neurosci. 2012;32:8855-8864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 166] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 21. | You WK, Yotsumoto F, Sakimura K, Adams RH, Stallcup WB. NG2 proteoglycan promotes tumor vascularization via integrin-dependent effects on pericyte function. Angiogenesis. 2014;17:61-76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 22. | Chekenya M, Krakstad C, Svendsen A, Netland IA, Staalesen V, Tysnes BB, Selheim F, Wang J, Sakariassen PØ, Sandal T. The progenitor cell marker NG2/MPG promotes chemoresistance by activation of integrin-dependent PI3K/Akt signaling. Oncogene. 2008;27:5182-5194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 123] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 23. | Liu H, Shubayev VI. Matrix metalloproteinase-9 controls proliferation of NG2+ progenitor cells immediately after spinal cord injury. Exp Neurol. 2011;231:236-246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Joo NE, Miao D, Bermúdez M, Stallcup WB, Kapila YL. Shedding of NG2 by MMP-13 attenuates anoikis. DNA Cell Biol. 2014;33:854-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Schultz N, Nielsen HM, Minthon L, Wennström M. Involvement of matrix metalloproteinase-9 in amyloid-β 1-42-induced shedding of the pericyte proteoglycan NG2. J Neuropathol Exp Neurol. 2014;73:684-692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Wang J, Svendsen A, Kmiecik J, Immervoll H, Skaftnesmo KO, Planagumà J, Reed RK, Bjerkvig R, Miletic H, Enger PØ. Targeting the NG2/CSPG4 proteoglycan retards tumour growth and angiogenesis in preclinical models of GBM and melanoma. PLoS One. 2011;6:e23062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 27. | Van Sinderen M, Cuman C, Winship A, Menkhorst E, Dimitriadis E. The chrondroitin sulfate proteoglycan (CSPG4) regulates human trophoblast function. Placenta. 2013;34:907-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Girolamo F, Dallatomasina A, Rizzi M, Errede M, Wälchli T, Mucignat MT, Frei K, Roncali L, Perris R, Virgintino D. Diversified expression of NG2/CSPG4 isoforms in glioblastoma and human foetal brain identifies pericyte subsets. PLoS One. 2013;8:e84883. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Doane KJ, Howell SJ, Birk DE. Identification and functional characterization of two type VI collagen receptors, alpha 3 beta 1 integrin and NG2, during avian corneal stromal development. Invest Ophthalmol Vis Sci. 1998;39:263-275. [PubMed] |
| 30. | Iida J, Meijne AM, Spiro RC, Roos E, Furcht LT, McCarthy JB. Spreading and focal contact formation of human melanoma cells in response to the stimulation of both melanoma-associated proteoglycan (NG2) and alpha 4 beta 1 integrin. Cancer Res. 1995;55:2177-2185. [PubMed] |
| 31. | Aya-ay J, Mayer J, Eakin AK, Muffly BG, Anello M, Sandy JD, Gottschall PE. The effect of hypoxic-ischemic brain injury in perinatal rats on the abundance and proteolysis of brevican and NG2. Exp Neurol. 2005;193:149-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 32. | Morita S, Hourai A, Miyata S. Changes in pericytic expression of NG2 and PDGFRB and vascular permeability in the sensory circumventricular organs of adult mouse by osmotic stimulation. Cell Biochem Funct. 2014;32:51-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |