Published online Jun 7, 2015. doi: 10.3748/wjg.v21.i21.6591
Peer-review started: November 8, 2014
First decision: December 11, 2014
Revised: December 22, 2014
Accepted: January 8, 2015
Article in press: January 8, 2015
Published online: June 7, 2015
Processing time: 215 Days and 20.5 Hours
AIM: To investigate the continuous hepatic histopathological processes which occur in response to the loss of Dicer1.
METHODS: We generated a hepatocyte-selective Dicer1 knockout mouse and observed the gradual hepatic histopathological changes in the mutant liver. Immunohistochemistry and Western blotting were performed to detect Dicer1 expression. We performed hematoxylin and eosin staining, Periodic acid-Schiff staining, Oil Red O staining, and Masson’s trichrome staining to detect histological changes in Dicer1-deficient livers. Ki67 immunohistochemistry, terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling assay, and Western blotting were used to determine hepatocyte proliferation and apoptosis. Serum biochemistry, cytokine assays, and flow cytometric analysis were performed to quantity liver necrosis and inflammation. Fibrogenic markers were determined by Western blotting and qPCR. CK19, CD133, and OV6 immunofluorescence were used to observe liver progenitor cells. Immunofluorescence and qPCR were performed to reveal embryonic gene expression. We also performed histological staining and Western blotting to analyze hepatocellular carcinoma (HCC) development.
RESULTS: Dicer1 inactivation resulted in significant architecture disorganization and metabolism disruption in the liver. Dicer1 disruption impaired hepatocyte survival and resulted in profound cell apoptosis and continuous necrosis. In contrast to previous reports, the mutant liver exhibited chronic inflammation and progressive fibrosis, and could not be repopulated by Dicer1-positive cells. In addition, extensive activation of hepatic progenitor cells was observed. Primary HCC was observed as early as 4 mo after birth.
CONCLUSION: Hepatic loss of Dicer1 results in complex chronic pathological processes, including hepatocyte death, inflammatory infiltration, chronic fibrosis, compensatory proliferation, progenitor activation, and spontaneous hepatocarcinogenesis.
Core tip: MicroRNAs (miRNAs) play a critical role in the regulation of multiple biological genes in the liver. The liver-specific loss of Dicer1, an endoribonuclease essential for precursor miRNAs maturation, results in liver injury and spontaneous development of hepatocellular carcinoma (HCC); however, the gradual histological changes involved in these processes have not been completely characterized. In contrast to previous reports, we found that Dicer1 inactivation causes typical chronic liver injury characterized by profound hepatocyte apoptosis, continuous liver necrosis, active compensatory proliferation, noticeable triad inflammation, progressive liver fibrosis, significant progenitor activation, and spontaneous hepatocarcinogenesis. Our work provides new insights into the role of miRNA in liver injury and HCC development. These results may also aid in the development of a miRNA therapeutic strategy for the prevention and treatment of HCC.
- Citation: Lu XF, Zhou YJ, Zhang L, Ji HJ, Li L, Shi YJ, Bu H. Loss of Dicer1 impairs hepatocyte survival and leads to chronic inflammation and progenitor cell activation. World J Gastroenterol 2015; 21(21): 6591-6603
- URL: https://www.wjgnet.com/1007-9327/full/v21/i21/6591.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i21.6591
MicroRNAs (miRNAs) are a large family of small non-coding endogenous RNAs (19-25 nucleotides in length) that negatively mediate gene expression at the post-transcriptional level through processes such as transcript degradation or translational repression by binding to the 3’ untranslated region (3’UTR) of target messenger RNAs (mRNAs)[1,2]. Dicer1 is a highly conserved ribonuclease III enzyme that is critical for further cleavage of about 70-nucleotide hairpin precursor RNAs (pre-miRNAs) to generate mature miRNAs[3,4]. Dicer1 depletion essentially causes a global loss of mature Dicer1-dependent miRNAs, resulting in a lethal phenotype at approximately embryonic day 7.5 (E7.5) due to loss of pluripotency and embryonic stem cell proliferation defects[5]. Thus, approaches involving the conditional deletion of the Dicer1 allele have been used to evaluate miRNA function in a range of tissues and organs. Accumulating reports have revealed the critical role of miRNAs in diverse fundamental biological processes, such as proliferation, differentiation, apoptosis, genomic stability, and signaling pathways[1,6-9]. Moreover, hundreds of miRNAs have previously been identified in mammalian genomes, and these miRNAs are estimated to regulate more than 50% of human protein-coding genes[7]. In addition, each miRNA targets numerous mRNAs[10].
Given that miRNAs are key players associated with various diseases or cancers, growing evidence suggests that miRNAs function as either tumor suppressors or oncogenes in tumorigenesis[11,12]. Specific deletion of hepatocyte Dicer1 results in increased fetal gene expression in the mature liver, defective liver zonation, preserved hepatic function, and the spontaneous development of hepatocellular carcinoma (HCC)[13-15]. Understanding the progression of HCC will contribute to the development of better diagnosis and therapies; however, the functions of Dicer1 and miRNAs in liver cancer remain unclear. miR-122 is a product of Dicer1 and the most abundant hepatocyte-specific miRNA, accounting for approximately 70% of the total miRNA population in the liver. miR-122 plays a fundamental role in fatty acid and cholesterol metabolism, as well as in hepatitis C viral replication[16,17]. miR-122 loss in murine liver results in the development of nonalcoholic steatohepatitis, liver fibrosis, and HCC[18]. Decreased miR-122 levels in liver cancer promote metastasis[19]. miR-199a/b-3p suppresses HCC growth through the PAK4/Raf/MEK/ERK pathway[20], whereas miR-26a targets cyclin D2 and cyclin E2 mRNA to induce G1 arrest and promote tumor development[21]. The downregulation of certain miRNAs, such as miR-24a, miR-15a/b, miR-150, and miR-195, has also been noted in human liver cancer through the use of a c-Myc-induced mouse model of HCC[22,23]. miRNA-deficient hepatocytes exhibit a delay in cell cycle progression via a G1 to S phase block during liver regeneration[24,25].
Despite the important role of miRNAs in the liver, their function in the development of HCC remains unclear. Here, we developed a hepatocyte-specific Dicer1-deficient mouse and comprehensively elucidated the gradual hepatic pathological changes that occur over time.
The animal protocol was designed to minimize pain and discomfort for the animals used in the study. The animals were acclimatized to laboratory conditions (23 °C, 12 h/12 h light/dark, 50% humidity, ad libitum access to food and water) for two weeks prior to experimentation. All animals were euthanized by barbiturate overdose (intravenous injection, 150 mg/kg pentobarbital sodium) for tissue collection.
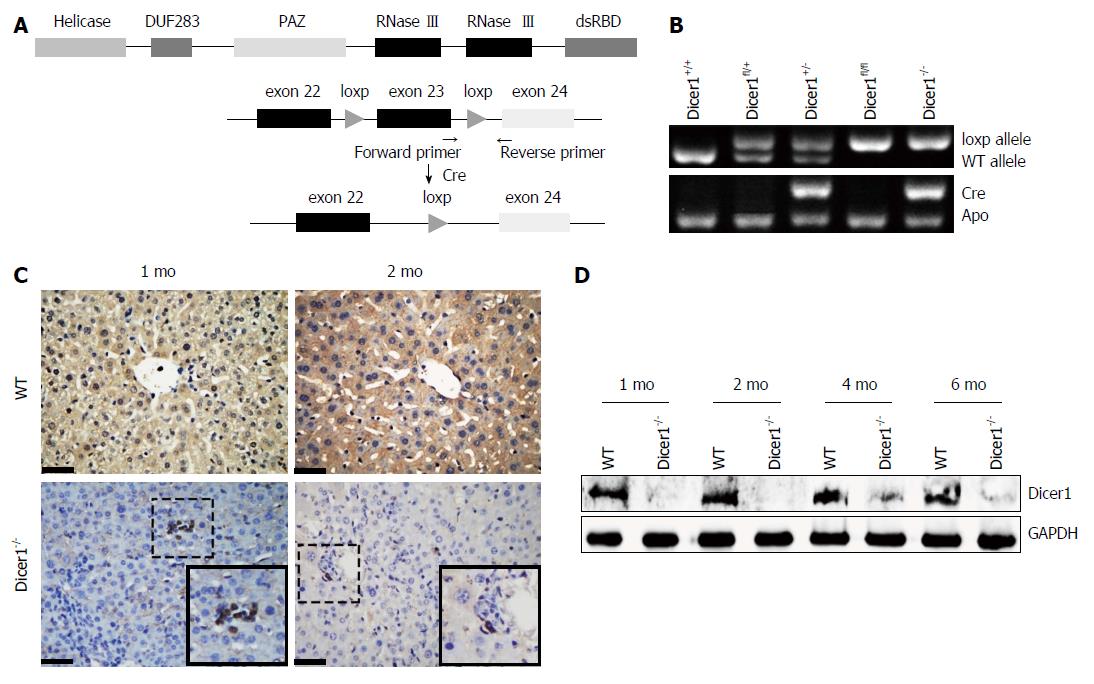
Alb-Cre mice were mated with Dicer1fl/fl mice to generate hepatocyte-specific Dicer1 knockout mice (Alb-Cre; Dicer1fl/fl) as described previously[13,26]. Genotyping was performed by PCR of tail DNA to distinguish wild-type mice and knockout mice. Liver samples were obtained at indicated time points for gross observation and to create frozen or paraffin sections. All animal experiments were approved by the Ethics Committee of the West China Hospital of Sichuan University.
Liver tissues were fixed in 10% formaldehyde and the 4-μm sections were subjected to hematoxylin and eosin staining, Periodic acid-Schiff staining, and Masson’s trichrome staining. All staining was performed using standard procedures. For Oil Red O staining, fresh liver samples were cut into 6-μm thick cryosections. Cryosections were incubated with Oil Red O solution (0.5% w/v Oil Red O in isopropyl alcohol) for 20 min and counterstained with hematoxylin.
Paraffin-embedded liver sections were incubated with appropriately diluted antibodies, including Dicer1 (Novus Biologicals, United States), Ki67 (Thermo Scientific, United States), CK19 (Abcam, United Kingdom), SOX2 (Abcam, United Kingdom), OCT4 (Abcam, United Kingdom), MIC1 (OV6) (Novus Biologicals, United States), and CD133 (Millipore, United States). The results were developed by the 3,3’-diaminobenzidine (DAB) method or directly observed under a fluorescent microscope.
The terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay was used to detect apoptotic hepatocytes according to the manufacturer’s instructions (Roche Applied Science, Swiss).
Protein samples were separated using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto activated-polyvinylidene difluoride membrane (Bio-Rad, United States). Immunoblotting was performed using standard protocol. Signals were detected via chemiluminescence using an ECL kit (Millipore, United States) on a ChemiDoc XRS gel documentation system (Bio-Rad, United States). The primary antibodies used for immunoblotting include cyclin D1 (Abcam, United Kingdom), CDK1 (Abcam, United Kingdom), Bax (Cell Signaling Technology, United States), Bcl-2 (Cell Signaling Technology, United States), cleaved Caspase-3 (Cell Signaling Technology, United States), α-smooth muscle actin (α-SMA) (Abcam, United Kingdom), β-actin (Cell Signaling Technology, United States), and GAPDH (Kangchen, China).
Total RNA was extracted from liver samples using Trizol reagent (Invitrogen, United States). Total RNA was used as a template to synthesize cDNA using the iScript cDNA synthesis kit (Bio-Rad, United States). q-PCR reactions were performed using a Bio-Rad CFX96TM Real-Time PCR system with SsoFastTM EvaGreen Supermix (Bio-Rad, United States). The relative expression of target genes was normalized to an internal β-actin control. Relative gene expression with a greater than 2-fold change was considered statistically significant.
Blood samples were obtained from the orbital vascular plexus of the mice. Aspartate amino-transferase (AST) and alanine amino-transferase (ALT) serum levels were determined. Serum cytokine levels were analyzed on a Luminex 200 system using a Millipore Map Mouse Cytokine/Chemokine Magnetic Bead Panel kit (Millipore, United States) according to the manufacturer’s instructions.
Liver leukocytes were isolated and subjected to flow cytometric analysis as described previously[27]. The monoclonal antibody CD45-APC (BD, United States) was used to detect total liver leukocytes. The population of intrahepatic inflammation cells was normalized to the amount of total live liver cells. Flow cytometric analysis was performed on a Navios flow cytometer (Beckman Coulter, United States) and analyzed with Kaluza (Beckman Coulter, United States).
All results were expressed as the mean ± SD. For statistical analysis, P-values were calculated using an unpaired Student’s t-test (GraphPad Prism 5 software). P < 0.05 was considered statistically significant.
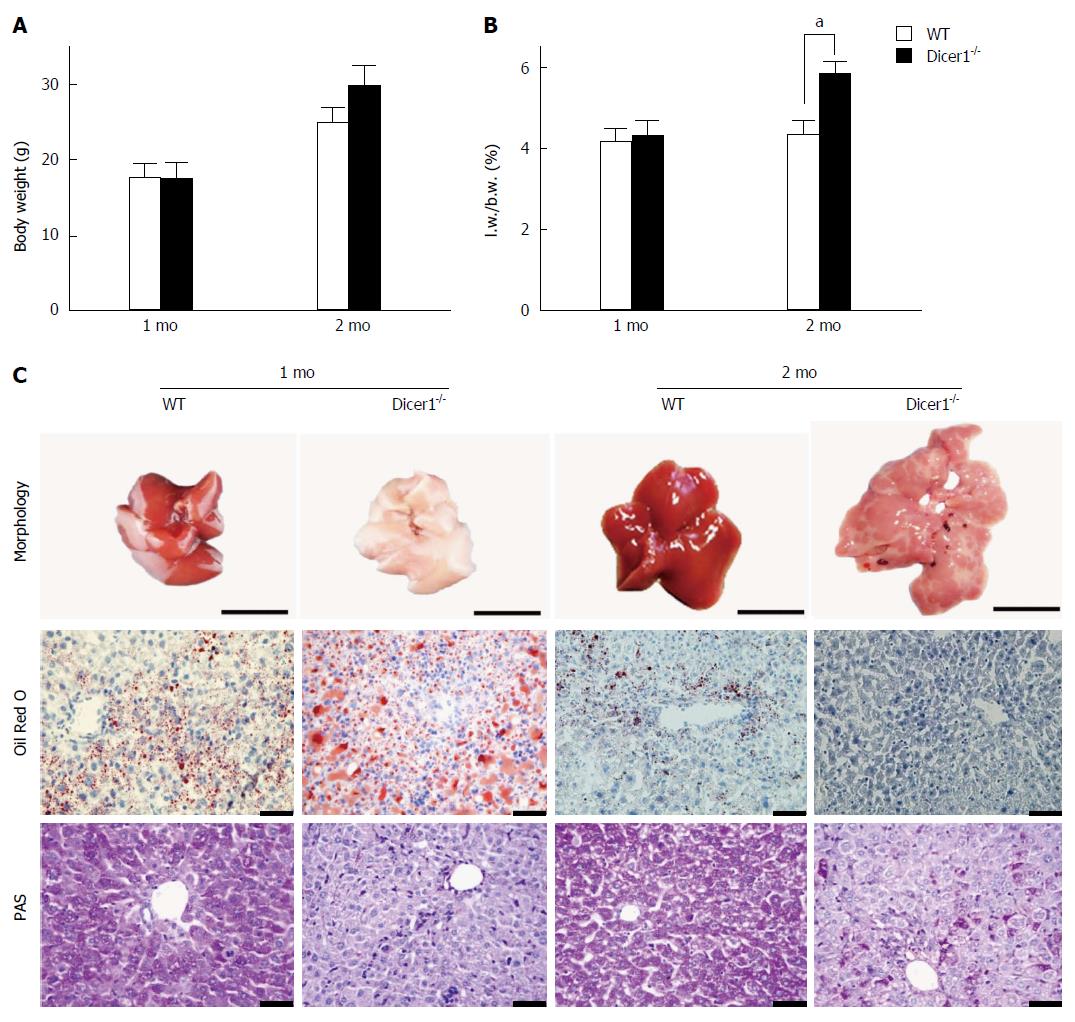
To address the overall loss of miRNAs in the liver, we generated hepatocyte-specific Dicer1-deficient mice and examined Dicer1 protein expression in the liver. As shown in Figure 1C and D, Dicer1 protein was robustly depleted in the livers of both young and adult mutant mice, which suggested that Dicer1 disruption was persistent with the development and growth of the mutant liver. The mutant mice were born alive at the expected Mendelian ratio; no obvious abnormalities regarding appearance, body weight, or weaning behavior were noted compared with the littermate controls. All mutant mice matured normally and were fertile. Notably, the livers of the adult Alb-Cre; Dicer1fl/fl mice exhibited an approximately 25% increase in the ratio of liver weight/body weight (L.W./B.W.) compared with their littermates (Figure 2A and B). Similar to the previous study, the Dicer1-deficient liver exhibited noticeable appearance defects. The 1-mo-old mutant liver was pale in color and gradually returned to normal liver color at 3 mo of age.
HE staining revealed distinctive features of the mutant livers, including a disrupted architecture due to enlarged hepatocytes, disorganized hepatic trabeculae, fibrosis surrounding portal tracts, and obliterated hepatic sinuses. Dicer1 loss in liver impairs lipid and glucose metabolism. To further address metabolic disorders in the mutant liver, we examined lipid and glycogen deposition in hepatocytes. Consistent with previous reports, increased accumulation of lipid droplets was noted in 1-mo-old mutant livers compared with wild-type livers as determined by Oil Red O staining (Figure 2C). However, the lipid droplets were significantly reduced in 2-mo-old mice, which might contribute to alterations in liver color ranging from pale to dark red. PAS staining indicated that the mutant liver exhibited significantly reduced glycogen storage after overnight fasting (Figure 2C). In contrast to steatosis, which gradually disappears as the liver matures, the glycogen deficiency was not corrected throughout the lifespan.
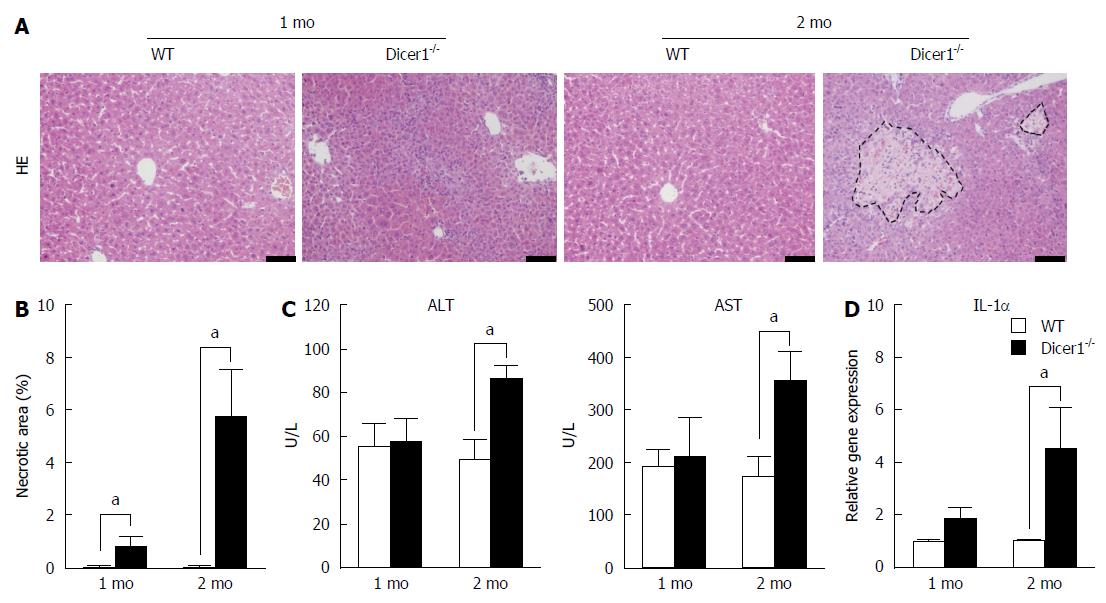
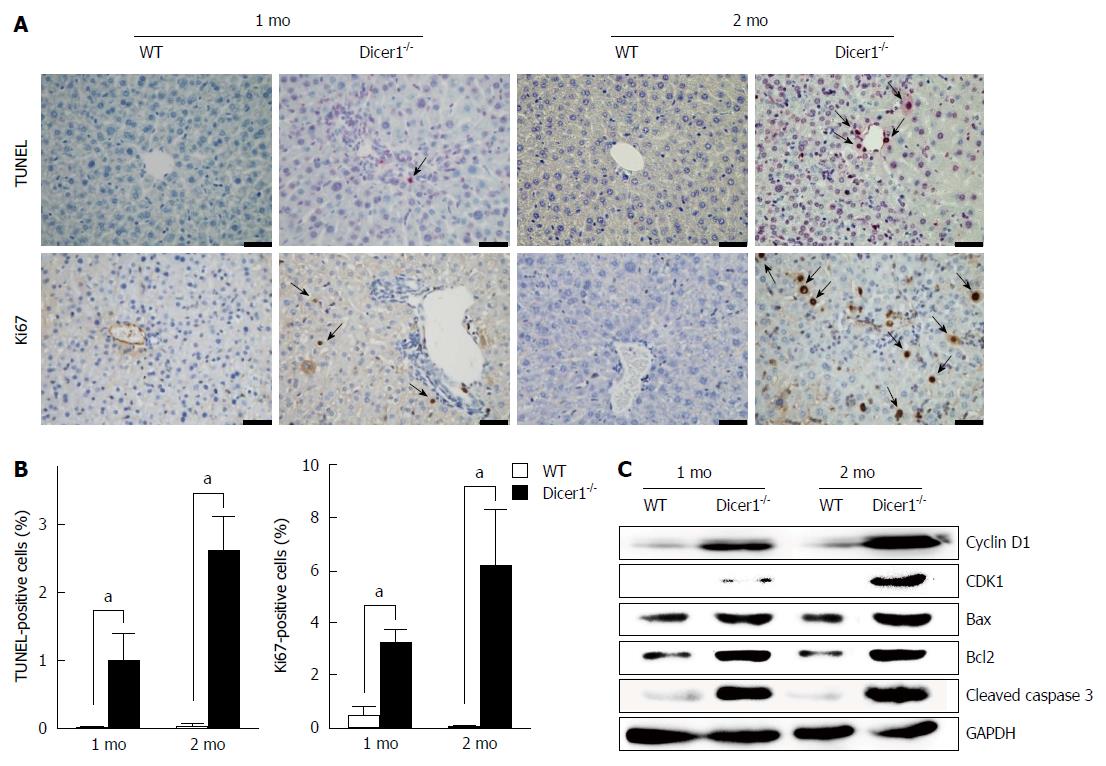
We examined hepatocyte necrosis, apoptosis, and proliferation in the Dicer1-inactive liver. Noticeable necrosis was observed in 2-mo-old Dicer1-deficient mice (Figure 3A and B). Tissue damage in the Dicer1-deficient livers was also confirmed by the examination of ALT and AST, both of which exhibited a statistical increase at two months after birth (Figure 3C). Hepatocyte necrosis induced the release of interleukin-1α (IL-1α), which triggers compensatory proliferation and HCC development (Figure 3D). Moreover, as observed in TUNEL staining, Dicer1-deficient livers exhibited approximately 1% TUNEL-positive hepatocytes in 1-mo-old mice, and this number increased to greater than 2% in 2-mo-old mice (Figure 4A and B). Hepatocyte death prompted us to examine regeneration in the diseased livers. We performed Ki67 immunohistochemistry and determined the number of the proliferating hepatocytes. Dicer1-deficient livers exhibited an increased number of Ki67-positive cells (Figure 4A and B), indicating compensatory proliferation in response to the cell death. The proportion of Ki67 positive cells increased with age. To obtain further insight into hepatocyte proliferation and apoptosis, we performed immunoblotting to analyze the expression of mitotic markers (e.g., cyclin D1 and CDK1) and apoptotic markers (e.g., including Bax, Bcl-2, and Cleaved Caspase-3). This analysis revealed that the Dicer1-deficient hepatocytes exhibited survival defects, resulting in continuous hepatocyte death and the triggering of long-lasting compensatory proliferation (Figure 4C).
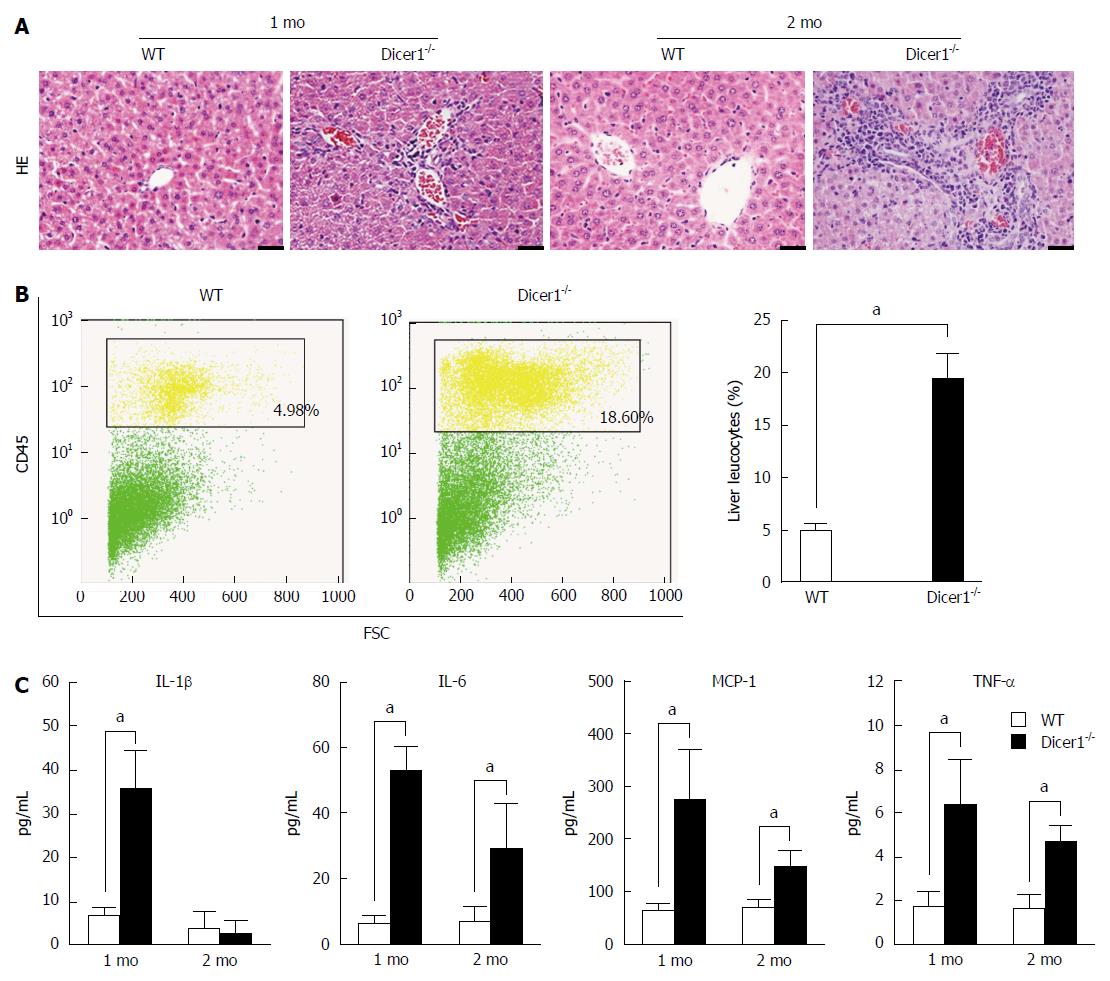
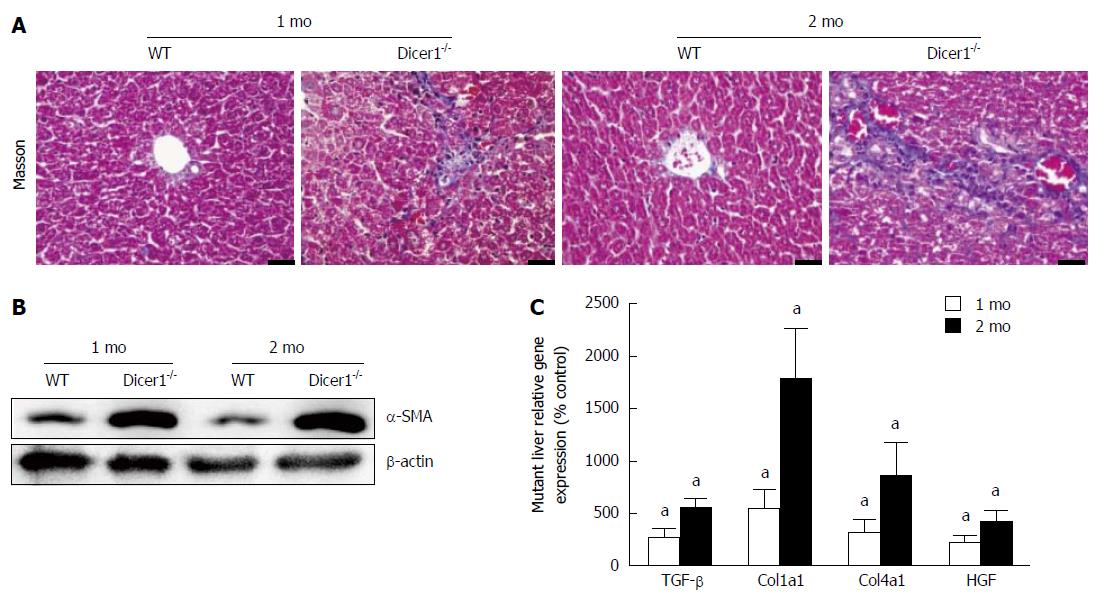
Hepatocyte death is always associated with inflammation; thus, we explored whether liver injury activated inflammatory infiltration in the mutant livers. At 1 mo, Dicer1-deficient livers exhibited focal necrosis with a mild infiltration of inflammatory cells that was concentrated around the portal triads and extended into the parenchyma (Figure 5A). Moderate to severe inflammation consistent with increased necrosis was observed in the 2-mo-old Dicer1-deficient livers. Consistent with the histologic appearance of mutant livers, flow cytometric analysis revealed that infiltrating inflammatory cells were increased 4-fold in the Dicer1-deficient livers (Figure 5B). We further investigated whether serum cytokines increased in response to chronic inflammation. Interleukin-1β (IL-1β), interleukin-6 (IL-6), monocyte chemoattractant protein-1 (MCP-1), and tumor necrosis factor-α (TNF-α) serum levels were significantly increased in Dicer1-deficient mice compared with control mice at the indicated time points (Figure 5C). Remarkably, a low degree of fibrosis at the portal triads was observed in young Dicer1-deficient livers, as revealed by Masson’s trichrome staining. Enlarging fibrosis, as well as mild fibrotic septa around the portal triads, was observed thereafter (Figure 6A). Portal fibrosis was accompanied by a number of activated hepatic stellate cells (HSCs) that expressed α-SMA and produced hepatocyte growth factor (HGF) (Figure 6B and C). Accordingly, the expression of fibrogenic factors, such as transforming growth factor-β (TGF-β), collagen type 1a1 (Col1a1), and collagen type 4a1 (Col4a1), were significantly elevated in mutant livers (Figure 6C).
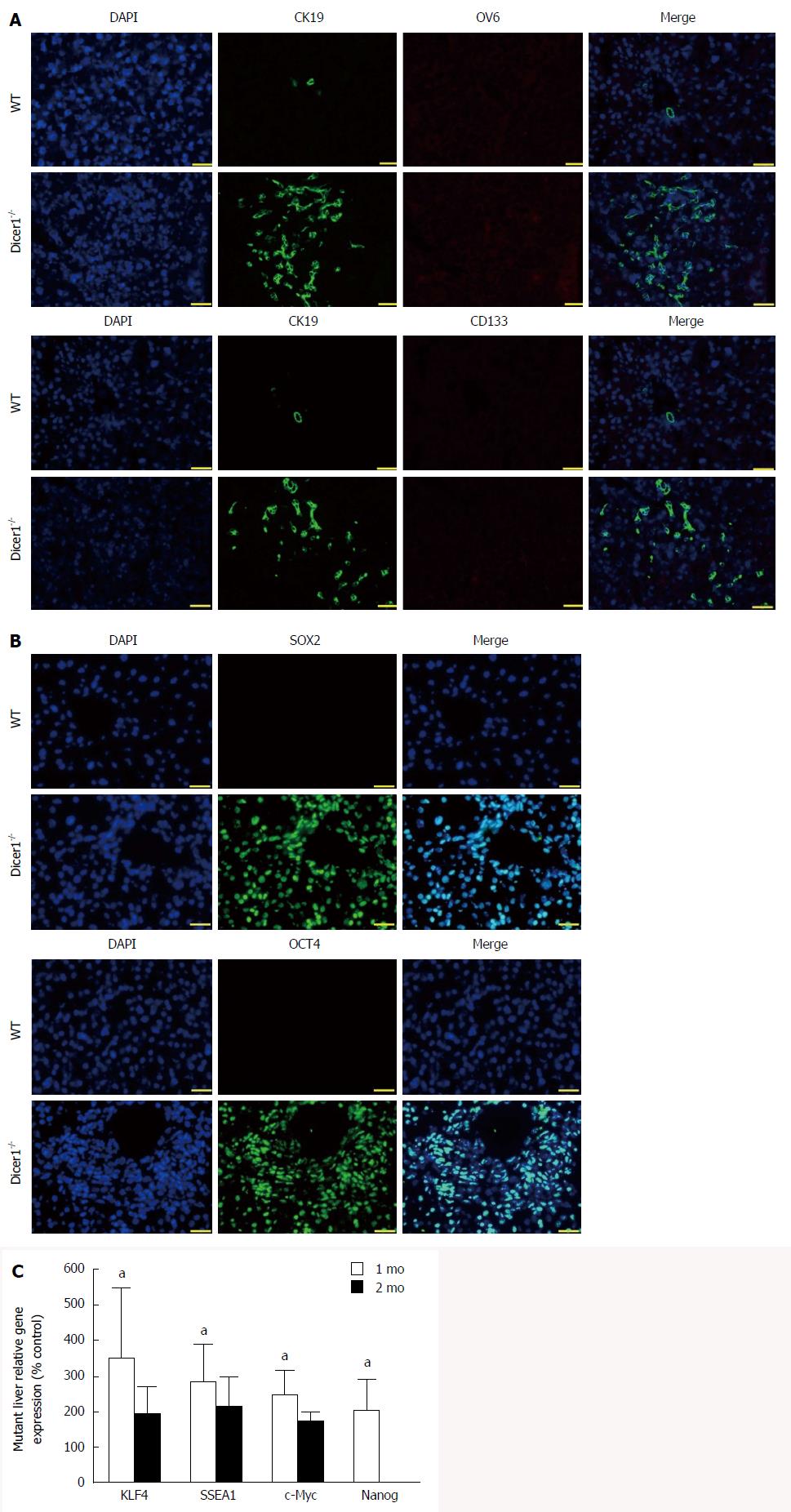
Active proliferation of hepatic progenitor cells marked by CK19, also known as ductular reaction, was evident in the mutant liver, and the progenitors appeared to extensively extend into the lobules (Figure 7A). We next examined stem cell marker and embryonic protein expression in the liver. Unexpectedly, neither CD133, a widely-expressed mesenchymal stem cell marker, nor MIC1 (OV6), a characteristic hepatic progenitor cell marker, was observed in Dicer1-deficient livers (Figure 7A). Sparse hematopoietic cells were observed throughout Dicer1-deficient livers until 7 wk, and these cells were not observed in the control livers shortly after birth (data not shown). Instead, positive expression of OCT4 and SOX2, both of which are embryonic and multifunctional gene products[28], was consistently observed in hepatocytes at least two months after birth (Figure 7B). We next analyzed the expression of other core pluripotency transcription factors, such as KLF4, SSEA1, c-Myc, and Nanog. As shown in Figure 7C, the expression of these genes was noticeably increased in mutant livers.
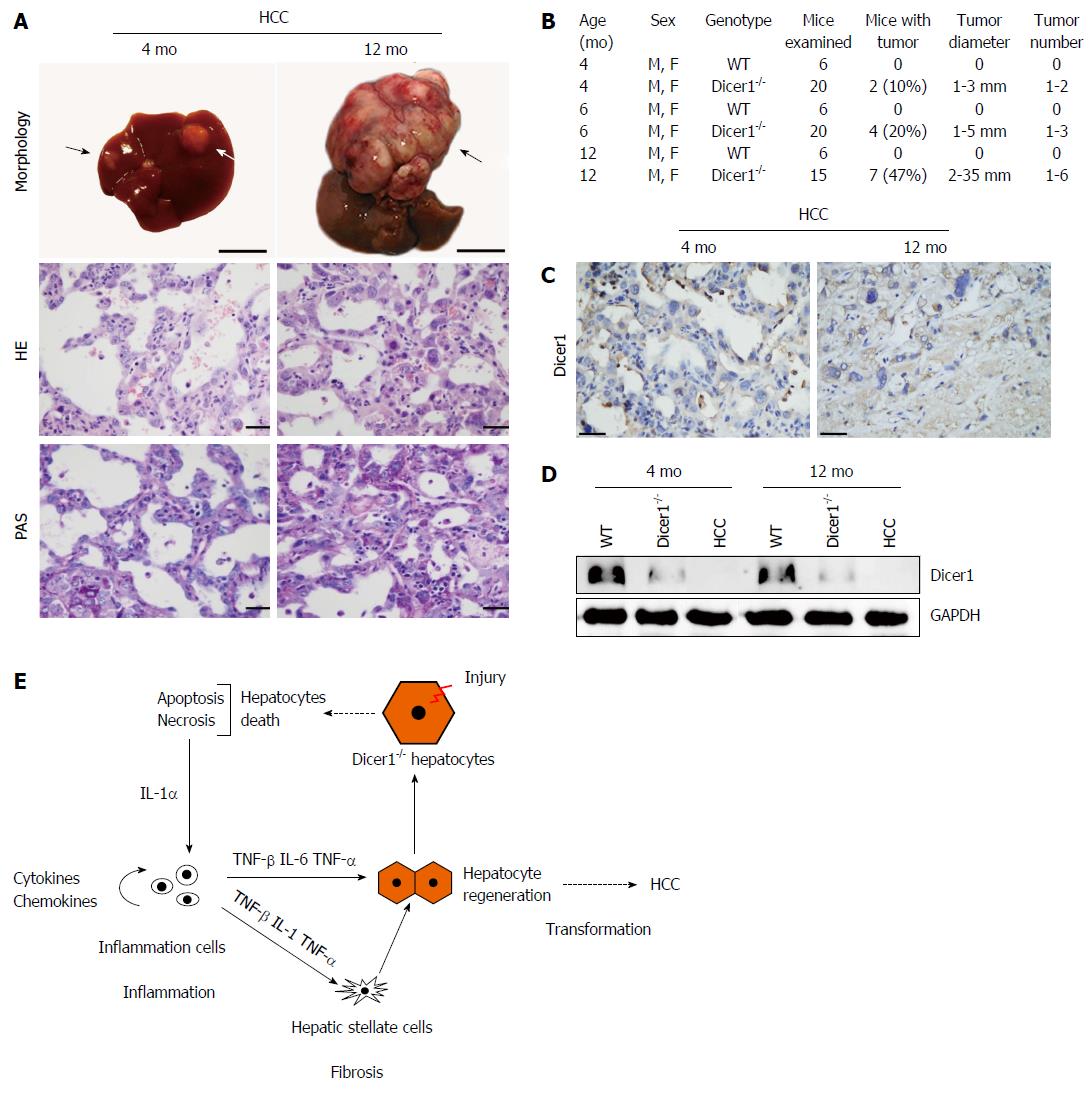
Spontaneous HCC in both male and female Dicer1-deficient mice was observed as early as 4 mo after birth. By 6 mo, 4 of the 20 mutant mice developed HCC with 1 to 3 separate macroscopic tumor foci in each liver (Figure 8A and B). The tumor cells exhibited remarkable features of smoothly demarcated edges surrounded by fibrous encapsulation, pseudoglandular, and nuclear pleomorphism as assessed by microscopy (Figure 8A). The later tumor stages were well-differentiated with an invasive appearance (data not shown). Most noticeably, Dicer1 expression was negative in the HCC lesion and non-tumor tissues (Figure 8C and D).
A number of new insights into the role of miRNAs in the coordination of diverse hepatic biological processes were revealed using a mouse model with hepatocyte-specific deletion of the Dicer1 gene. Similar to previous reports, we observed that loss of Dicer1 led to significant hepatic architecture disorganization and metabolic disorders. Most notably, in contrast to previous reports, we found that Dicer1 inactivation caused typical chronic liver injury characterized by profound hepatocyte apoptosis, continuous liver necrosis, active compensatory proliferation, noticeable triad inflammation, progressive liver fibrosis, significant progenitor activation, and spontaneous hepatocarcinogenesis.
In agreement with a previous study, Dicer1 is required to sustain proper lipid and glucose metabolism. miR-122, the most abundant liver-specific miRNA, plays a critical role in cholesterol biosynthesis[29]. miR-122 loss secondary to Dicer1 deficiency leads to marked steatosis. Which miRNA is responsible for glucose metabolism dysregulation remains unclear. It is also unclear why glycogen deficiency remained evident when steatosis was resolved in the adult mice.
The most intriguing finding in our work involves the continuous hepatocyte necrosis and progressive fibrosis noted in the Dicer1-deficient mice. Our findings are quite different from a previous report by Sekine et al[13], which suggested that Dicer1 loss resulted in robust apoptosis, not necrosis, in mutant hepatocytes. Noticeable apoptosis was similarly shown in our mutant mouse liver. In addition, we observed continuous parenchymal necrosis in the liver as early as only one month after birth. It is widely known that necrosis causes inflammation, whereas apoptosis does not[30,31]. Prominent chronic inflammatory infiltration and progressive fibrosis in the liver tissues, together with significantly increased serum hepatic enzyme and inflammatory cytokine levels, provides solid evidence of parenchymal necrosis in the mutant livers. The continuous death of hepatocytes triggers compensatory liver regeneration[32]. A previous report by Song et al[24] demonstrated that global loss of miRNAs secondary to the knockout of DGCR8 (an enzyme upstream of Dicer1 in RNA biogenesis) caused a delay in cell cycle progression that involved the G1 to S phase transition after a two-thirds hepatectomy. It remains unclear whether the mutant cells completely lose regenerative properties in response to Dicer1 ablation. We observed a few scattered proliferating hepatocytes in the mutant liver parenchyma. Sekine et al[13] reported that the hepatocytes that escaped from Cre recombinase and regained Dicer1 expression repopulated the liver when the mutant cells underwent apoptosis. However, we confirmed that the liver remains Dicer1 negative throughout life. It would be interesting to determine whether the mutant liver is repopulated by the hepatocytes that escaped from Cre recombinase rather than the continuous proliferation of mutant hepatocyte. Sekine et al[13] did not observe hepatic progenitor cell activation. In our Dicer1-inactive liver, robust expansion of liver progenitors marked by CK19 was observed. Most intriguingly, these CK19 cells were Dicer1 negative, and it appears that these progenitors are potentially activated for parenchymal compensation. It is reasonable to assume that if the mutant liver is completely repopulated by Dicer1-positive hepatocytes, it will eventually exhibit a normal architecture with normal metabolism in the absence of inflammation, fibrosis, or tumorigenesis; this notion has been well elucidated in Fah-deficient mice[33]. In Fah-/- mice, the lack of fumarylacetoacetate hydrolase (FAH) causes the mice to rely on a lifelong dietary supplement of 2-[2-nitro-4-(trifluoromethyl)benzoyl]-1, 3-cyclohexanedione (NTBC). The transplantation of hepatocytes or hepatic progenitors typically repopulates the Fah-/- liver by replacing dead hepatocytes caused by the withdrawal of NTBC. In this case, the Fah mutant liver is completely and properly reconstituted by FAH-positive cells[34,35]. The results were quite different in our Dicer1-deficient mouse liver, given that Dicer1 was not expressed in adult mutant mouse liver or tumor tissue. Other evidence also confirmed that the hepatocytes that escaped from Cre-mediated recombination did not repopulate the Dicer1-deficient livers. First, constant glycogen deficiency was noted in the mutant liver, whereas lipid metabolism appeared to normalize. Second, embryonic gene products, such as OCT4 and SOX2, were extensively expressed in hepatocytes and progenitors even at 2 mo after birth. Third, extramedullary hematopoiesis cells, a marker of the immature state of the mutant liver, were often observed until adulthood. Taken together, we hypothesize that the continuous proliferation of mutant hepatocytes and active expansion of the progenitors, but not the hepatocytes that regained normal Dicer1 expression, were primarily responsible for the histological recovery of the mutant liver. Our study presents a different phenotype of Dicer1-inactive livers. Our observation also differs from the report using hepatic-selective DGCR8 inactive mice, wherein no histological or developmental defects were observed. The cause of the different phenotypes of these miRNA-deficient mice requires further intensive study.
In our Dicer1-inactive mice, HCC was observed as early as 4 mo after birth, suggesting that a great number of oncogenic factors are regulated by miRNAs. Chronic hepatic injury and active progenitor expansion are considered to be crucial cellular events in the development of HCC[36]. In addition, embryonic gene expression secondary to Dicer1 inactivation in both hepatocytes and progenitors might also be required for tumorigenesis given that embryonic protein expression significantly increases the risk of tumorigenicity[37,38]. In addition, increased levels of chronic pro-tumorigenic cytokines, such as IL-6 and TNF-α, are also considered risk factors for hepatocarcinogenesis[39]. These pathways are initiated by hepatocyte death and cause inflammation, fibrosis, compensatory proliferation, and HCC promotion, as summarized in Figure 8E.
Our study has revealed that hepatic loss of Dicer1 results in complex pathological processes, including hepatocyte immaturity, metabolic disorders, hepatocyte death, inflammatory infiltration, chronic fibrosis, compensatory proliferation, progenitor activation, and ultimately spontaneous hepatocarcinogenesis. miRNAs dysregulation is essential for HCC carcinogenesis. However, it is still difficult to confirm the specific miRNA that plays the dominate role in the development of HCC using Dicer1-inactive mice. Intensive studies should be performed to clarify the distinct role of each individual miRNA in HCC. An improved understanding of the pathogenesis of liver cancer would provide cues for liver cancer diagnostics and prognosis. These results may also provide further insights for the development of a miRNA therapeutic strategy for the prevention and treatment of HCC.
We thank Dr. Hua-Shun Li for kindly providing the Dicer1 flox mice.
Hepatocellular carcinoma (HCC) is associated with numerous liver diseases, such as hepatitis B virus, nonalcoholic steatohepatitis, and miRNAs loss. miRNAs play critical roles in diverse liver fundamental biological processes, but the role of miRNAs in the development of HCC remains unclear.
Dicer1 is an endoribonuclease essential for the maturation of precursor miRNAs. Specific deletion of Dicer1 in hepatocytes has revealed liver injury and spontaneous development of HCC. The hotspot of current research is the gradual histological changes involved in HCC processes in the Dicer1-deficient liver.
The liver-specific loss of Dicer1 results in liver injury, hepatocyte death, and progenitor activation. Recent studies show that hepatocyte death causes inflammation, fibrosis, compensatory proliferation, and HCC promotion.
The study results suggest that the ablation of miRNA accelerates liver injury, progenitor activation, and HCC development, and may be useful for the prevention and treatment of HCC in the future.
Dicer1 is a highly conserved ribonuclease III enzyme that is critical for further cleavage of pre-miRNAs to generate mature miRNAs. Dicer1 depletion essentially causes a global loss of mature Dicer1-dependent miRNAs, resulting in a lethal phenotype due to the loss of pluripotency and the defects of embryonic stem cell proliferation. miRNAs dysregulation is essential for HCC carcinogenesis.
The authors generated a hepatocyte-selective Dicer1 knockout mouse and further characterized the gradual hepatic histopathological changes in the mutant liver, including hepatocyte proliferation and apoptosis, liver necrosis, inflammation, fibrosis, and HCC development. The authors did a great job providing further detail on the continuous hepatic histopathological processes that occur in response to the loss of Dicer1. Experiments were properly performed and data interpretation is mostly appropriate. This is a good complement to previous research.
P- Reviewer: Cheng J, Helle F, Tanimizu N S- Editor: Qi Y L- Editor: Rutherford A E- Editor: Wang CH
| 1. | Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11:597-610. [PubMed] |
| 2. | Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14460] [Cited by in RCA: 16067] [Article Influence: 1004.2] [Reference Citation Analysis (2)] |
| 3. | Kim VN. MicroRNA biogenesis: coordinated cropping and dicing. Nat Rev Mol Cell Biol. 2005;6:376-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1784] [Cited by in RCA: 1823] [Article Influence: 91.2] [Reference Citation Analysis (0)] |
| 4. | Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25833] [Cited by in RCA: 27819] [Article Influence: 1324.7] [Reference Citation Analysis (0)] |
| 5. | Bernstein E, Kim SY, Carmell MA, Murchison EP, Alcorn H, Li MZ, Mills AA, Elledge SJ, Anderson KV, Hannon GJ. Dicer is essential for mouse development. Nat Genet. 2003;35:215-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1408] [Cited by in RCA: 1435] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 6. | Pritchard CC, Cheng HH, Tewari M. MicroRNA profiling: approaches and considerations. Nat Rev Genet. 2012;13:358-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1165] [Cited by in RCA: 1297] [Article Influence: 99.8] [Reference Citation Analysis (0)] |
| 7. | Pasquinelli AE. MicroRNAs and their targets: recognition, regulation and an emerging reciprocal relationship. Nat Rev Genet. 2012;13:271-282. [PubMed] |
| 8. | Inui M, Martello G, Piccolo S. MicroRNA control of signal transduction. Nat Rev Mol Cell Biol. 2010;11:252-263. [PubMed] |
| 9. | Malone CD, Hannon GJ. Small RNAs as guardians of the genome. Cell. 2009;136:656-668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 715] [Cited by in RCA: 630] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 10. | Carthew RW, Sontheimer EJ. Origins and Mechanisms of miRNAs and siRNAs. Cell. 2009;136:642-655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4017] [Cited by in RCA: 3696] [Article Influence: 231.0] [Reference Citation Analysis (0)] |
| 11. | Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5384] [Cited by in RCA: 5605] [Article Influence: 295.0] [Reference Citation Analysis (0)] |
| 12. | Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5705] [Cited by in RCA: 6024] [Article Influence: 317.1] [Reference Citation Analysis (0)] |
| 13. | Sekine S, Ogawa R, Ito R, Hiraoka N, McManus MT, Kanai Y, Hebrok M. Disruption of Dicer1 induces dysregulated fetal gene expression and promotes hepatocarcinogenesis. Gastroenterology. 2009;136:2304-2315.e1-4. [PubMed] |
| 14. | Sekine S, Ogawa R, Mcmanus MT, Kanai Y, Hebrok M. Dicer is required for proper liver zonation. J Pathol. 2009;219:365-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 15. | Hand NJ, Master ZR, Le Lay J, Friedman JR. Hepatic function is preserved in the absence of mature microRNAs. Hepatology. 2009;49:618-626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 86] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 16. | Hsu SH, Wang B, Kota J, Yu J, Costinean S, Kutay H, Yu L, Bai S, La Perle K, Chivukula RR. Essential metabolic, anti-inflammatory, and anti-tumorigenic functions of miR-122 in liver. J Clin Invest. 2012;122:2871-2883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 583] [Cited by in RCA: 628] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 17. | García-Sastre A, Evans MJ. miR-122 is more than a shield for the hepatitis C virus genome. Proc Natl Acad Sci USA. 2013;110:1571-1572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Tsai WC, Hsu SD, Hsu CS, Lai TC, Chen SJ, Shen R, Huang Y, Chen HC, Lee CH, Tsai TF. MicroRNA-122 plays a critical role in liver homeostasis and hepatocarcinogenesis. J Clin Invest. 2012;122:2884-2897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 669] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 19. | Coulouarn C, Factor VM, Andersen JB, Durkin ME, Thorgeirsson SS. Loss of miR-122 expression in liver cancer correlates with suppression of the hepatic phenotype and gain of metastatic properties. Oncogene. 2009;28:3526-3536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 585] [Cited by in RCA: 590] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 20. | Hou J, Lin L, Zhou W, Wang Z, Ding G, Dong Q, Qin L, Wu X, Zheng Y, Yang Y. Identification of miRNomes in human liver and hepatocellular carcinoma reveals miR-199a/b-3p as therapeutic target for hepatocellular carcinoma. Cancer Cell. 2011;19:232-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 567] [Cited by in RCA: 587] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 21. | Kota J, Chivukula RR, O’Donnell KA, Wentzel EA, Montgomery CL, Hwang HW, Chang TC, Vivekanandan P, Torbenson M, Clark KR. Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell. 2009;137:1005-1017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1388] [Cited by in RCA: 1362] [Article Influence: 85.1] [Reference Citation Analysis (0)] |
| 22. | Szabo G, Bala S. MicroRNAs in liver disease. Nat Rev Gastroenterol Hepatol. 2013;10:542-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 489] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 23. | Huang S, He X. The role of microRNAs in liver cancer progression. Br J Cancer. 2011;104:235-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 189] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 24. | Song G, Sharma AD, Roll GR, Ng R, Lee AY, Blelloch RH, Frandsen NM, Willenbring H. MicroRNAs control hepatocyte proliferation during liver regeneration. Hepatology. 2010;51:1735-1743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 168] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 25. | Shu J, Kren BT, Xia Z, Wong PY, Li L, Hanse EA, Min MX, Li B, Albrecht JH, Zeng Y. Genomewide microRNA down-regulation as a negative feedback mechanism in the early phases of liver regeneration. Hepatology. 2011;54:609-619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 26. | Zhao X, He X, Han X, Yu Y, Ye F, Chen Y, Hoang T, Xu X, Mi QS, Xin M. MicroRNA-mediated control of oligodendrocyte differentiation. Neuron. 2010;65:612-626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 407] [Cited by in RCA: 396] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 27. | Schneider C, Teufel A, Yevsa T, Staib F, Hohmeyer A, Walenda G, Zimmermann HW, Vucur M, Huss S, Gassler N. Adaptive immunity suppresses formation and progression of diethylnitrosamine-induced liver cancer. Gut. 2012;61:1733-1743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 154] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 28. | Tay Y, Zhang J, Thomson AM, Lim B, Rigoutsos I. MicroRNAs to Nanog, Oct4 and Sox2 coding regions modulate embryonic stem cell differentiation. Nature. 2008;455:1124-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1052] [Cited by in RCA: 1064] [Article Influence: 62.6] [Reference Citation Analysis (0)] |
| 29. | Esau C, Davis S, Murray SF, Yu XX, Pandey SK, Pear M, Watts L, Booten SL, Graham M, McKay R. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. 2006;3:87-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1612] [Cited by in RCA: 1636] [Article Influence: 86.1] [Reference Citation Analysis (0)] |
| 30. | Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3079] [Cited by in RCA: 3283] [Article Influence: 142.7] [Reference Citation Analysis (0)] |
| 31. | Wang K. Molecular mechanisms of hepatic apoptosis. Cell Death Dis. 2014;5:e996. [PubMed] |
| 32. | Maeda S, Kamata H, Luo JL, Leffert H, Karin M. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121:977-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 910] [Cited by in RCA: 954] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 33. | Azuma H, Paulk N, Ranade A, Dorrell C, Al-Dhalimy M, Ellis E, Strom S, Kay MA, Finegold M, Grompe M. Robust expansion of human hepatocytes in Fah-/-/Rag2-/-/Il2rg-/- mice. Nat Biotechnol. 2007;25:903-910. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 712] [Cited by in RCA: 647] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 34. | Vassilopoulos G, Wang PR, Russell DW. Transplanted bone marrow regenerates liver by cell fusion. Nature. 2003;422:901-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1005] [Cited by in RCA: 897] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 35. | Wang X, Willenbring H, Akkari Y, Torimaru Y, Foster M, Al-Dhalimy M, Lagasse E, Finegold M, Olson S, Grompe M. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature. 2003;422:897-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1230] [Cited by in RCA: 1114] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 36. | Blechacz B, Mishra L. Hepatocellular carcinoma biology. Recent Results Cancer Res. 2013;190:1-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 37. | Ben-David U, Benvenisty N. The tumorigenicity of human embryonic and induced pluripotent stem cells. Nat Rev Cancer. 2011;11:268-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 653] [Cited by in RCA: 649] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 38. | Visvader JE, Lindeman GJ. Cancer stem cells: current status and evolving complexities. Cell Stem Cell. 2012;10:717-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 916] [Cited by in RCA: 985] [Article Influence: 75.8] [Reference Citation Analysis (0)] |
| 39. | Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer RG, Osterreicher CH, Takahashi H, Karin M. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197-208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1403] [Cited by in RCA: 1365] [Article Influence: 91.0] [Reference Citation Analysis (1)] |