Published online Jun 7, 2015. doi: 10.3748/wjg.v21.i21.6479
Peer-review started: December 11, 2014
First decision: January 8, 2015
Revised: January 31, 2015
Accepted: April 28, 2015
Article in press: April 28, 2015
Published online: June 7, 2015
Processing time: 183 Days and 9.7 Hours
The burden of illness from esophageal adenocarcinoma continues to rise in the Western world, and overall prognosis is poor. Given that Barrett’s esophagus (BE), a metaplastic change in the esophageal lining is a known cancer precursor, an opportunity to decrease disease development by screening and surveillance might exist. This review examines recent updates in the pathogenesis of BE and comprehensively discusses known risk factors. Diagnostic definitions and challenges are outlined, coupled with an in-depth review of management. Current challenges and potential solutions related to screening and surveillance are discussed. The effectiveness of currently available endoscopic treatment techniques, particularly with regards to recurrence following successful endotherapy and potential chemopreventative agents are also highlighted. The field of BE is rapidly evolving and improved understanding of pathophysiology, combined with emerging methods for screening and surveillance offer hope for future disease burden reduction.
Core tip: This review highlights recent updates in the pathogenesis, diagnosis and therapy for Barrett’s esophagus (BE), the pre-malignant lesion for esophageal adenocarcinoma (EAC). The incidence of EAC continues to rise, and prognosis once diagnosed is poor. In this paper we critically reviewed the diagnostic criteria as well as new understanding of risk factors. Comparative recommendations from gastrointestinal societies are presented, and approaches to BE therapy, and management of recurrent BE after ablation is discussed.
- Citation: Halland M, Katzka D, Iyer PG. Recent developments in pathogenesis, diagnosis and therapy of Barrett's esophagus. World J Gastroenterol 2015; 21(21): 6479-6490
- URL: https://www.wjgnet.com/1007-9327/full/v21/i21/6479.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i21.6479
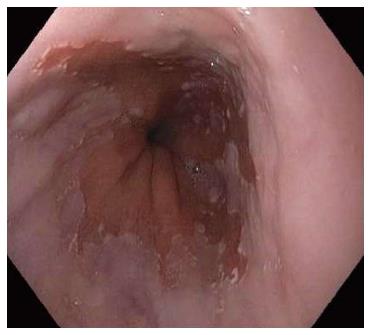
Barrett’s esophagus (BE), a metaplastic change of the esophageal mucosa from squamous to columnar mucosa with intestinal metaplasia, is the dominant pre-malignant lesion of esophageal adenocarcinoma (EAC) (Figure 1). The incidence of EAC continues to rise in the western world[1,2] and thus efforts to prevent EAC by screening and surveillance for BE, coupled with endoscopic therapy for BE related dysplasia/neoplasia is practiced in many clinical settings (Table 1). Despite this, the effectiveness of current strategies in preventing EAC is not well proven[3,4] as only a small fraction of patients with BE will develop cancer[1]. Furthermore, up to 95% of EAC develops in patients with no prior diagnosis of BE despite its presence[5,6]. New developments in the understanding of BE pathogenesis, screening, the neoplastic potential of BE, along with improvements in endoscopic therapeutic options, imaging techniques and molecular markers may impact future EAC mortality. In 2015, however, the management of the individual patient with BE remains challenging and the optimal approach to EAC prevention on a population level remains uncertain[7].
| Screening | Surveillance NDBE | Surveillance LGD | Surveillance HGD | |
| AGA[104] | Screening for patients with multiple risk factors | Recommend surveillance | Low-grade dysplasia: 6-12 mo | High-grade dysplasia in the absence of eradication therapy: 3 mo |
| Age 50 yr or older | No dysplasia: | |||
| Male sex | three to five years | |||
| White race | ||||
| Chronic GERD | ||||
| Hiatal hernia | ||||
| Elevated body mass index | ||||
| Intra-abdominal distribution of body fat | ||||
| Screening in the general population is not recommended | ||||
| BSG[48] | Endoscopic screening can be considered in patients with chronic GERD symptoms and multiple risk factors (at least three of age 50 yr or older, white race, male sex, obesity) | Patients with Barrett’s oesophagus shorter than 3 cm, with IM, should receive endoscopic surveillance every 3-5 yr | High resolution endoscopy every 6 mo | Not recommended |
| The threshold of multiple risk factors should be lowered in the presence of family history including at least one first-degree relative with Barrett’s or OAC | Patients with segments of 3 cm or longer should receive surveillance every 2-3 yr | For HGD and Barrett’s-related adenocarcinoma confined to the mucosa, endoscopic therapy is preferred over esophagectomy or endoscopic surveillance | ||
| ASGE[33] | Endoscopic screening for BE can be | Consider no surveillance | Confirm with expert GI pathologist | Confirm with expert GI pathologist |
| considered in select patients with multiple risk factors for BE and EAC, but patients should be informed that there is insufficient evidence to affirm that this practice prevents cancer or prolongs life | If surveillance is elected, perform EGD every 3 to 5 years with 4-quadrant biopsies every 2 cm | Repeat EGD in 6 mo to confirm LGD | Consider surveillance EGD every 3 mo in select patients, 4-quadrant biopsies every 1 cm | |
| Consider endoscopic ablation in select cases | Surveillance EGD every year, 4-quadrant biopsies every 1 to 2 cm | Consider endoscopic resection or RFA ablation | ||
| Consider endoscopic resection or ablation | Consider EUS for local staging and lymphadenopathy | |||
| Consider surgical consultation | ||||
| ACP[34] | Upper endoscopy may be indicated among men older than 50 yr with chronic GERD symptoms (symptoms for more than 5 yr) and additional risk factors (nocturnal reflux symptoms, hiatal hernia, elevated body mass index, tobacco use, and intra-abdominal distribution of fat) to detect esophageal adenocarcinoma and be | For surveillance evaluation in men and women with a history of be. In men and women with be and no dysplasia, surveillance examinations should occur at intervals no more frequently than 3 to 5 yr. More frequent intervals are indicated in patients with Barrett esophagus and dysplasia | ||
Correctly defining the presence of BE in patients is crucial before committing patients to lifelong surveillance endoscopy. BE can be found in up to 10%-15% of patients with gastroesophageal reflux (GERD) symptoms who undergo upper endoscopy[8,9]. In a recent study three gastroenterologists underwent intensive didactic and practical training in accurate identification of gastroesophageal junction (GEJ) landmarks and BE diagnosis which lead to a third of previously diagnosed BE patients being re-classified to have minor squamocolumnar junction abnormalities, but no longer to have BE[8]. This study demonstrates that misdiagnosis of BE is common in clinical practice. This could likely lead to underestimation of the protective effect of a surveillance program by including patients with intestinal metaplasia of the GEJ or cardia (IMGEJ) frequently misclassified as short segment BE[10], which is found in up to 15% of the normal population. Furthermore, the risk of progression to EAC in IMGEJ is vastly different when compared to Barrett’s as demonstrated by Jung et al[10] who followed 86 patients with IMGEJ for 8 years and no patient progressed to dysplasia or adenocarcinoma. A diagnosis of BE also adds significant financial and psychological burden to patients. Therefore improved diagnostic skills of endoscopists will be crucial to avoid misclassification between intestinal metaplasia at the cardia and BE.
The need for intestinal metaplasia for a diagnosis of BE continues to be a point of controversy, particularly between American and European gastroenterology societies. Evidence of an increased risk of progression to EAC in subjects exhibiting columnar metaplasia with goblet cells (a marker of intestinal differentiation) compared to those without goblet cells is robust, with large population based cohort studies showing substantially different progression risks amongst these two histologic types[11,12]. Nevertheless, there is some evidence of comparable molecular abnormalities found in columnar esophageal metaplasia with and without goblet cells associated with neoplastic risk in cross sectional studies of Barrett’s patients[13]. Moreover, sampling error and/or a patchy distribution of these cell types may account for a variable appearance of goblet cells on biopsy as repeated biopsies may demonstrate goblet cells in those without goblet cells at baseline. A recent study longitudinally followed 107 patients with columnar lined epithelium (CLE) without metaplasia. At repeat endoscopy after 2 years, 71% had suspected CLE confirmed at repeat endoscopy of which 29% had IM consistent with a BE diagnosis. These data suggest that surveillance may be discontinued in those without goblet cells at a second endoscopic examination after ensuring adequate sampling to detect intestinal metaplasia[14].
In addition to the classic risk factors of gastroesophageal reflux, male gender and Caucasian race, other BE risk factors recently identified include obesity[15], specifically central obesity[16], metabolic syndrome[17], and obstructive sleep apnea[18]. These factors may contribute to BE risk independent of their additive effects on gastroesophageal reflux. More recently it has become clear that the distribution of excess body fat appears important, with central obesity confirmed as an independent predictor of BE[19] although the effect on progression of BE is less clear[20]. A recent meta-analysis summarized the findings of 40 studies which examined the association of body mass index (BMI) and central obesity (waist circumference, waist to hip ratio and quantitative measures of visceral fat by cross sectional body imaging) and the risk of erosive esophagitis, BE and EAC, and a reflux and BMI independent effect of central adiposity was again noted[16].
The exact molecular mechanisms by which central obesity promotes replacement of injured squamous epithelium with columnar metaplasia are the focus of intense research. While increased gastroesophageal reflux disease due to mechanical effects has been proposed as the mediator of this association[21], a recent study by Lagergren et al[22] support the existence of additional mechanisms. Lagergren et al[22] analyzed data from a population-based Swedish nationwide study of patients with a new diagnosis of EAC or GEJ adenocarcinoma and matched controls and found no evidence that the increased risk of EAC in obese subjects is mediated by symptomatic reflux alone. However, it is clear that symptoms alone underestimate the severity of reflux in Barrett’s patients[23]. Several studies have assessed the association of smoking and alcohol with BE and EAC[24-27]. In a recent meta-analysis which included thirty-nine studies comprising 7069 BE patients[28], having ever-smoked was associated with an increased risk of BE compared with non-gastroesophageal reflux disease controls (OR = 1.44; 95%CI: 1.20-1.74), population-based controls (OR = 1.42; 95%CI: 1.15-1.76), but not GERD controls (OR = 1.18; 95%CI: 0.75-1.86). With regards to alcohol consumption, a recent meta-analysis of population based case-control studies found that there was a no significant association (any vs none, summary OR = 0.77, 95%CI: 0.60-1.00) between any alcohol consumption and the risk of Barrett ’s esophagus[29].
Genetic factors which contribute to BE have also been discovered. Two recent genome wide association studies have identified polymorphisms which are associated with an increased risk of BE and EAC[30,31]. Associations have been found in 19p13 in CRTC1, whose aberrant activation has been associated with oncogenic activity, as well as 9q22 in BARX1 which encodes a transcription factor which is important in esophageal specification[31]. Furthermore, polymorphisms near TBX5 and GDF7 which encode for a bone morphogentic protein and a transcription factors which regulates esophageal development respectively, are associated with an increased risk of BE[30].
An inverse relationship between Helicobacter pylori (H. pylori) and BE has been noted in observational studies, but significant heterogeneity between studies exists. In a meta-analysis which focused on only four methodologically robust studies found a relative risk of 0.46 (95%CI: 0.35-0.60) for the development of BE among persons infected with H. pylori[32]. A subgroup analysis of seven studies showed that this effect was stronger for infection with CagA-positive strains (OR = 0.38; 95%CI: 0.19-0.78). Despite this association the increased risk of gastric cancer outweights the protection against GERD offered by H. pylori induced gastric atrophy.
The role of screening is controversial. Even the leading United States gastroenterology societies differ in recommendations on whether screening should be performed. For example, the American Gastroenterological Association states that screening may be considered in patients 50 years and older with multiple risk factors whereas the American Society for Gastrointestinal endoscopy states that screening should be offered after the pros and cons are discussed. In general, however, most societies agree that the high risk group for BE includes Caucasian men aged 50 and above with chronic reflux symptoms and with other coexisting risk factors such as central obesity and history of smoking[9,33,34]. Conventional endoscopy for screening is expensive, not widely applicable and is usually performed by a physician. A possible alternative is un-sedated ultrathin endoscopy which avoids ancillary costs of sedation, personnel, recovery time and requirement for time off work and a patient escort[35,36]. Peery et al[37] assessed the use of office based trans-nasal endoscopy using a 4.5 mm scope (Vision Sciences, Orangeburg, NY) with a disposable sheath. 426 participants were scoped and 99% completed the examination and no serious adverse effects were reported. The examination was well-tolerated based on post-procedure surveys. Trans-nasal endoscopy was also compared with standard endoscopy in a randomized cross-over study[38]. In this study of 95 patients TNE correctly diagnosed 48 of 49 BE cases and thus had a sensitivity and specificity of 0.98 and 1.00, respectively. Furthermore, physician extenders have also been shown to be able to accurately recognize esophago-gastric landmarks and reliably perform BE screening using transnasal endoscopy after a short training program[39]. A recent study demonstrated that the majority of adults in a population based survey was willing to undergo screening for BE, and that unsedated techniques were preferred by 64% vs 36% for sedated endoscopy[40].
Another recently described non-invasive screening method has been described using an ingestible sampling device, (Cytosponge)[41]. This device consists of an ingestible gelatin capsule containing a compressed mesh attached to a string. The brushings obtained by the device are analyzed with an immunological assay for trefoil factor 3, a marker for columnar epithelium with intestinal metaplasia. In the largest study of this device, 501 of 504 patients were able to swallow the capsule with a sensitivity and specificity of 73% and 94%, respectively for detection of BE[41]. The test also appears cost-effective compared to no screening assuming increased participation when compared to conventional endoscopy[42]. Trials of other office based devices such as the EG II Scan (Intromedic, Seoul, South Korea), an ultrathin transnasal operator-controllable video capsule based esophagoscope with a disposable delivery system are underway (NCT02066233). Data on patient tolerance and diagnostic accuracy from these trials are awaited.
Novel biomarkers that examine chromosomal alterations, epigenetic markers as well as gene expression markers and microRNAs are all being evaluated[31,43]. Kendall et al[44] demonstrated that a high serum leptin was associated with BE among men. Furthermore, Alashkar et al[39] found that patients with the high serum insulin levels had an increased risk (OR = 2.02, 95%CI: 1.15-3.54) of having BE, indicating a potential role for insulin or insulin-like growth factor in BE pathogenesis. These results were echoed in a case-control study where levels of circulating cytokines including adipokines had a modest association with BE[45]. A biomarker panel was also recently evaluated in a case-control study of predominantly white male veterans[45]. A risk prediction model including a multi-biomarker score, derived from serum levels of cytokines and leptin, as well as GERD frequency and duration, age, sex, race, waist-to-hip ratio, and H. pylori infection, achieved an area under the curve of 0.85, thus more accurately identifying persons in this population with BE than in previous non-invasive methods[45-47].
Currently surveillance is recommended for all patients with BE at intervals depending on the grade of dysplasia. In those without dysplasia, intervals of 3-5 years are suggested[9,33,48]. In patients with low grade dysplasia (LGD) the interval in shortened to 6-12 mo, and high grade dysplasia (HGD) is generally accepted as a reason to intervene endoscopically or surgically. The rationale behind surveillance is based on estimated risk of progression from non-dysplastic BE to dysplasia and EAC. However, recent large studies have consistently demonstrated a lower than previously estimated annual risk of progression to EAC, and a recent meta-analysis estimated the incidence of EAC in non-dysplastic BE (NDBE) to be 0.33% per year[11,49,50]. Furthermore, Gaddam et al[51] recently demonstrated that patients who have had persistence of nondysplastic BE after several surveillance endoscopies may have an even lower risk of progressing to EAC. In this study of predominantly white men, the annual risk of EAC was decreased by 50% and 70% after 3 and 5 consecutive surveillance endoscopies respectively, without dysplasia. These data support the need for revised surveillance intervals for such patients and perhaps even development of “exit-rules” for those as lowest risk as risk stratification evolves.
The risk of progression to EAC in patients with BE with LGD is less well defined[52,53]. Recently, a meta-analysis found that the annual rate of progression to EAC among patients with LGD is 0.54%, but the authors commented on the wide variability that was observed across studies[54]. This may be related to the low interobserver agreement for the diagnosis of LGD among pathologists[55]. However, when LGD is confirmed by three expert gastrointestinal pathologists progression is significantly higher[56,57]. Staining for aberrant p53 over-expression may help corroborate the presence of dysplasia and has now been recommended as an adjunct to standard histopathological examination in some guidelines[48]. Because of the variable progression rate of LGD and diagnostic uncertainty, surveillance has been favored over endoscopic intervention until recently. Recently, a randomized trial compared radiofrequency ablation (RFA) vs endoscopic surveillance for patients with LGD[58]. 136 subjects were randomized to receive RFA or endoscopic surveillance. During a median follow up period of 36 mo 1.5% of patients in the ablation group developed HGD or EAC compared to 26.5% in the control group (P < 0.001). The trial was terminated early due to the superiority of ablation, but the external validity of these trial results warrant consideration. First, the progression rate of 11.8% per person-year is higher than that what is observed in community based studies, and second, 28% of patients in the control group had no dysplasia detected during follow up despite expert pathologists confirming the diagnosis at study entry. This suggests that not all patients with LDG will necessarily benefit from ablation, but that in a small subgroup of patients with persistent and perhaps multifocal confirmed LGD endoscopic ablation may be considered.
Ideally, the ability to non-invasively determine who is at risk of progression would make both ablation and surveillance programs more cost effective. This is particularly prudent as one of the most rigorous studies on the impact of endoscopic surveillance on mortality from EAC failed to find a significant benefit[59], supporting findings from previous studies[60,61]. These sobering data urge a review of current clinical practice, and the need for novel approaches in preventing deaths from EAC.
Detection of dysplasia during routine endoscopy relies on obtaining random biopsies per Seattle protocol[62] in addition to targeting any visible abnormality. In a study which compared inspection times of less than 1 minute vs longer per 1 centimeter of BE, more patients with endoscopically suspicious lesions (54.2% vs 13.3% P = 0.04) and HGD/EAC (40.2% vs 6.7%, P = 0.06) were detected with longer inspection time[63]. Also, some data suggest that neoplastic lesions in BE are more commonly found in the right half of the esophagus compared to the left (84.9% vs 15.1%, P = 0.001), with the highest rate in the 12 to 3 o’clock quadrant[64], and thus particular attention to this anatomical location is important. This observation is not surprising as this is the area of greatest acid exposure and erosive esophagitis[65]. Newer developments in endoscopic techniques, such as image magnification, chromoendoscopy (dye or filtering techniques which highlight dysplasia) and use of autofluorescence imaging have been evaluated, but have failed to become standard of care either due to lack of efficacy or practicality[66-70]. For example, a recent study found only a 2% extra yield of autofluorescence compared to high definition white light endoscopy with random biopsies[68]. The use of autofluorescence combined with magnification narrow band imaging was found[71] to not be a useful technique for detection of dysplasia. More promise was observed with the first human data of targeted imaging of esophageal neoplasia using a fluorescently labeled peptide[72]. In this study, a novel peptide which binds to areas of HGD and neoplasia provided 3.8 greater fold fluorescence intensity, and demonstrated 75% sensitivity and 97% specificity for neoplasia. Canto et al[73] recently evaluated in-vivo endoscope based confocal laser endomicroscopy (eCLE, a probe based technique which has resolution to yield close to a histologic view of the epithelium) in a randomized design. Among the 192 patients studied, the addition of eCLE to high definition white light endoscopy and target biopsies led to a lower number of mucosal biopsies and higher diagnosis yield for neoplasia (34% vs 7%, P = 0.001), compared with compared high definition white light endoscopy with random biopsies, with but comparable accuracy. The use of such techniques may allow for targeted rather than random biopsies but experience is currently limited to tertiary centers with expert endoscopists. More recently techniques using optical coherence tomography are being developed to allow comprehensive assessment of the BE segment with assessment of the subepithelial layers making this an intriguing technique to study sub-squamous BE[74].
An increasing number of patients now undergo endoscopic ablative therapy for BE. Techniques include thermal ablation with radiofreqency ablation (RFA), freezing of BE tissue with liquid nitrogen or endoscopic mucosal resection (EMR). In a recent study where patients with non-dysplastic BE were presented a simulated scenario which compared endoscopic ablation with chemoprevention of EAC, 78% vs 53% (P≤ 0.1) preferred ablation[65]. Table 2 presents an estimate of efficacy and durability of current endotherapies for BE. RFA, liquid nitrogen spray cryotherapy and EMR all have acceptable success rates for eliminating HGD and IM in the short to medium term. A systematic review of studies assessing efficacy and durability of RFA found that complete eradication of dysplasia (CE-D) and complete remission from intestinal metaplasia was achieved in 91% and 76% respectively[75]. However, IM recurred in 13% over an average follow up of 18 mo. EAC developed in 9 of 3802 patients during 20.5 mo of treatment (0.1% per year). More recently, Orman et al[76] reported a lower recurrence rate of 5.2% per year, but this study was limited by relying on a single, negative endoscopy utilizing Seattle -protocol biopsies. Several larger studies which assessed durability of remission of intestinal metaplasia of longer time periods are now delivering more sobering results[77,78] reporting higher recurrence rates. A recurrence rate of 33% at 24 mo following complete remission has been found. 25% of the recurrences were dysplastic and 50% occurred at the GEJ. All except one were treated endoscopically. These data highlight the need for careful endoscopic surveillance following successful BE eradication. Less data exist for the long term outcomes of liquid nitrogen spray cryotherapy after successful ablation, but in one such study of 32 patients a rigorous surveillance program was employed[79]. 37.5% of patients needed treatment for recurrence during surveillance.
| Radiofrequency ablation with or without EMR[75,77,78,105] | 1Cryotherap[79] | Endoscopic mucosal resection[80] | Photo-dynamic therapy[106,107] | |
| Initial eradication of HGD | 90%-95% | 100% | 90% | 81% |
| Initial CRIM | 70%-86% | 100% | 90% | 72% |
| Recurrence of non-dysplastic Barrett’s | 13%-33% at 2-3 yr | 19% at 36 mo1 | 39.5% at 5 yr | Unknown |
| Recurrence of dysplasia or cancer | 1.6%-11% at 1.5-2.5 yr | 3% at 36 mo | 6.2% at 5 yr | 16%-20% at 2-5 yr |
| Adverse events | Stricture 4%-11.9% | Stricture 9% | Stricture 47% (widespread EMR) | Stricture 37% |
Outcomes of endoscopic mucosal resection of BE and early esophageal cancer are also encouraging, although recurrence of BE is common. Almond et al[80] reported outcomes of 90 patients who underwent widespread EMR with the aim of eradicating BE, with ablative procedures used as an addition during follow up. CE-IM was achieved in 90% of patients, but during follow 5 years of follow up NDBE recurred in 39.5% and neoplastic BE was found in 6.2%. In another study of 81 patients who underwent EMR for esophageal lesions (50% HGD and 50% EAC) the complete eradication rate of HGD was 84% at a median follow up of 3.25 years[81]. The cancer specific survival was 100%. More recently, a meta-analysis compared the safety and efficacy of endotherapy vs surgery for early neoplasia in BE in 7 retrospective studies which included a total of 870 patients[82]. No difference between endotherapy and esophagectomy was seen with regards to neoplasia remission rate (RR = 0.96, 95%CI: 0.91-1.01) and overall survival rate at 1, 3 and 5 years (5 years survival RR = 1.00, 95%CI: 0.93-1.06). Endotherapy was associated with a higher neoplasia recurrence rate (RR = 9.50, 95%CI: 3.26-27.75), but fewer major adverse events (RR = 0.38; 95%CI: 0.20-0.73). Furthermore, a recent randomized trial examined the use of argon plasma coagulation therapy to residual non-neoplastic BE with PPI compared with standard surveillance and PPI[83]. The number of secondary lesions was 1 in the ablation group (3%), and 11 in the surveillance group (36.7%), leading to significantly higher recurrence-free survival for the patients undergoing ablation (P = 0.005). The most significant problem with circumferential EMR is the occurrence of esophageal strictures in up to 40% of patients[75].
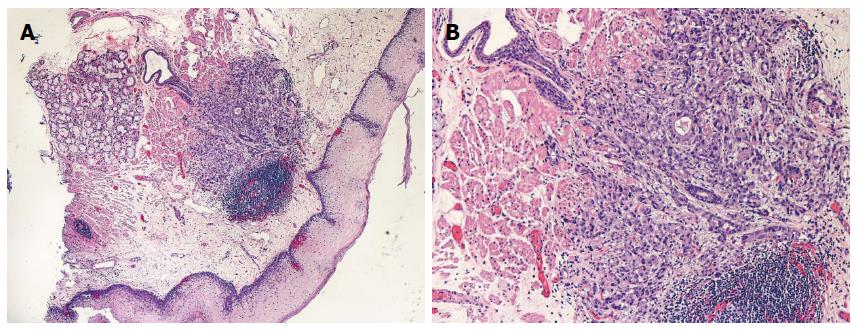
All eradicative therapies rely of the replacement of BE with neosquamous epithelium but the durability and functional characteristics of this tissue are less clear. In a recent study, biopsies of the neosquamous epithelium exhibited dilated intracellular spaces and defective barrier function[84]. The molecular profile on FISH analysis is also different in neosquamous epithelium when compared to native type[85]. Finally, it is also important to note that the existence of “sub squamous” intestinal metaplasia occurs in up to 5%-30% of patients undergoing ablation which may lead to neoplasia[86] (Figure 2). For all these reasons, ongoing surveillance despite successful eradication is recommended until the long term behavior and durability of the neosquamous epithelium has been further delineated.
The role of chemoprevention in BE remains controversial. Non-steroidal anti-inflammatory agents, statins and metformin have all been studied as potential chemopreventative agents in BE[87-89]. Previous studies have produced conflicting results with regards to a potential role of NSAIDs in preventing neoplastic progression of BE[88,90,91], and no protective role against the development of BE itself has been observed[92]. No interventional trials till date have examined the role of NSAIDs in chemoprevention, and thus currently the overall risk-benefit ratio is estimated from observations studies, and routine use is not currently recommended[93]. Metformin has shown in vitro effects on esophageal cancer cells[94], but clinical data on adenocarcinoma appear negative[95]. In a recent trial of 74 patients with BE, twelve week administration of metformin for 12 wk did have an effect on cell proliferation as measured by levels of pS6K1, compared with placebo[96]. Statins, which have a proven role in primary and secondary prevention for cardiovascular disease, may also prevent development of EAC through inhibition of proliferation and induction of apoptosis among esophageal cancer cells. Previous association studies have also found an inverse relationship between statin use and EAC[87,88]. A recent nested case-control study found that in addition to the inverse association between statin prescription and EAC (OR = 0.58, 95%CI: 0.39-0.87, P < 0.01) a robust dose and duration response was observed[97]. Furthermore, a recent meta-analysis of observational study found an 28%) reduction in the risk of esophageal cancer among patients who took statins (adjusted OR = 0.72; 95%CI: 0.60-0.86)[98]. This adds further weight to the potential chemo protective role of statins, although no data from a randomized controlled trial are currently available. Proton pump inhibitors are frequently prescribed to patients with BE to control reflux symptoms, but given the role of acid damage in BE pathogenesis a potential protective role for progression has been postulated[99]. Conversely, given the rise in serum gastrin produced by PPIs a potential for oncogenesis could also be present. Epidemiological studies of a potential association between acid-suppressive therapy and progression to EAC have produced conflicting results[100,101]. A recent meta-analysis pooled data from seven observational studies and found that PPI use associated with a 71% reduction in risk of EAC or high grade dysplasia (adjusted OR = 0.29; 95%CI: 0.19-0.79)[102]. Thus, PPIs, which is already universally prescribed to BE patients, may have a significant chemo protective effect and further randomized trials are warranted. A recent study also suggests that the use of PPIs might be a cost-effective approach assuming a minimum risk reduction in EAC of 19%[103].
Current efforts at preventing deaths from esophageal adenocarcinoma by screening for and surveying BE come at a great cost to society. New insights into BE pathogenesis, coupled with the development of non-invasive risk assessment tools will hopefully lead to more focused and effective non-invasive screening programs in a well-defined population. The currently available endotherapies for HGD and early cancer appear to be effective and are less morbid than surgery, but identifying patients who are most likely to benefit from these therapies is currently challenging. The ability to predict which patients are less likely to progress to cancer, and thus may not need ongoing surveillance is emerging, and will be crucial for cost-effectiveness of current surveillance strategies. Chemoprevention, including use of PPI’s, statins and anti-inflammatory medications may become important public health strategies to help hamper the overall disease burden from esophageal cancer on a population basis.
P- Reviewer: Brusselaers N, Castilho FM, Fassan M, Iovino P, Kim GH, Lorenzo-Zuniga V, Namikawa T S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH
| 1. | Cook MB, Chow WH, Devesa SS. Oesophageal cancer incidence in the United States by race, sex, and histologic type, 1977-2005. Br J Cancer. 2009;101:855-859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 288] [Cited by in RCA: 286] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 2. | Botterweck AA, Schouten LJ, Volovics A, Dorant E, van Den Brandt PA. Trends in incidence of adenocarcinoma of the oesophagus and gastric cardia in ten European countries. Int J Epidemiol. 2000;29:645-654. [PubMed] |
| 3. | Shaheen NJ, Hur C. Garlic, silver bullets, and surveillance upper endoscopy for Barrett’s esophagus. Gastroenterology. 2013;145:273-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Gordon LG, Mayne GC. Cost-effectiveness of Barrett’s oesophagus screening and surveillance. Best Pract Res Clin Gastroenterol. 2013;27:893-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Dulai GS, Guha S, Kahn KL, Gornbein J, Weinstein WM. Preoperative prevalence of Barrett’s esophagus in esophageal adenocarcinoma: a systematic review. Gastroenterology. 2002;122:26-33. [PubMed] |
| 6. | Verbeek RE, Leenders M, Ten Kate FJ, van Hillegersberg R, Vleggaar FP, van Baal JW, van Oijen MG, Siersema PD. Surveillance of Barrett’s esophagus and mortality from esophageal adenocarcinoma: a population-based cohort study. Am J Gastroenterol. 2014;109:1215-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 111] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 7. | Reid BJ, Li X, Galipeau PC, Vaughan TL. Barrett’s oesophagus and oesophageal adenocarcinoma: time for a new synthesis. Nat Rev Cancer. 2010;10:87-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 293] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 8. | Ganz RA, Allen JI, Leon S, Batts KP. Barrett’s esophagus is frequently overdiagnosed in clinical practice: results of the Barrett’s Esophagus Endoscopic Revision (BEER) study. Gastrointest Endosc. 2014;79:565-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Wang KK, Sampliner RE. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett’s esophagus. Am J Gastroenterol. 2008;103:788-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 850] [Cited by in RCA: 786] [Article Influence: 46.2] [Reference Citation Analysis (1)] |
| 10. | Jung KW, Talley NJ, Romero Y, Katzka DA, Schleck CD, Zinsmeister AR, Dunagan KT, Lutzke LS, Wu TT, Wang KK. Epidemiology and natural history of intestinal metaplasia of the gastroesophageal junction and Barrett’s esophagus: a population-based study. Am J Gastroenterol. 2011;106:1447-1455; quiz 1456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 151] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 11. | Dias Pereira A, Chaves P. Columnar-lined oesophagus without intestinal metaplasia: results from a cohort with a mean follow-up of 7 years. Aliment Pharmacol Ther. 2012;36:282-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Bhat S, Coleman HG, Yousef F, Johnston BT, McManus DT, Gavin AT, Murray LJ. Risk of malignant progression in Barrett’s esophagus patients: results from a large population-based study. J Natl Cancer Inst. 2011;103:1049-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 573] [Cited by in RCA: 517] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 13. | Liu W, Hahn H, Odze RD, Goyal RK. Metaplastic esophageal columnar epithelium without goblet cells shows DNA content abnormalities similar to goblet cell-containing epithelium. Am J Gastroenterol. 2009;104:816-824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 136] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 14. | Khandwalla HE, Graham DY, Kramer JR, Ramsey DJ, Duong N, Green LK, El-Serag HB. Barrett’s esophagus suspected at endoscopy but no specialized intestinal metaplasia on biopsy, what’s next? Am J Gastroenterol. 2014;109:178-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Kubo A, Corley DA. Body mass index and adenocarcinomas of the esophagus or gastric cardia: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:872-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 273] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 16. | Singh S, Sharma AN, Murad MH, Buttar NS, El-Serag HB, Katzka DA, Iyer PG. Central adiposity is associated with increased risk of esophageal inflammation, metaplasia, and adenocarcinoma: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2013;11:1399-1412.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 249] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 17. | Leggett CL, Nelsen EM, Tian J, Schleck CB, Zinsmeister AR, Dunagan KT, Locke GR, Wang KK, Talley NJ, Iyer PG. Metabolic syndrome as a risk factor for Barrett esophagus: a population-based case-control study. Mayo Clin Proc. 2013;88:157-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Leggett CL, Gorospe EC, Calvin AD, Harmsen WS, Zinsmeister AR, Caples S, Somers VK, Dunagan K, Lutzke L, Wang KK. Obstructive sleep apnea is a risk factor for Barrett’s esophagus. Clin Gastroenterol Hepatol. 2014;12:583-588.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Edelstein ZR, Bronner MP, Rosen SN, Vaughan TL. Risk factors for Barrett‘s esophagus among patients with gastroesophageal reflux disease: a community clinic-based case-control study. Am J Gastroenterol. 2009;104:834-842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 20. | Prasad GA, Bansal A, Sharma P, Wang KK. Predictors of progression in Barrett’s esophagus: current knowledge and future directions. Am J Gastroenterol. 2010;105:1490-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 21. | Lagergren J. Influence of obesity on the risk of esophageal disorders. Nat Rev Gastroenterol Hepatol. 2011;8:340-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 22. | Lagergren J, Mattsson F, Nyrén O. Gastroesophageal reflux does not alter effects of body mass index on risk of esophageal adenocarcinoma. Clin Gastroenterol Hepatol. 2014;12:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Katzka DA, Castell DO. Successful elimination of reflux symptoms does not insure adequate control of acid reflux in patients with Barrett’s esophagus. Am J Gastroenterol. 1994;89:989-991. [PubMed] |
| 24. | Kubo A, Levin TR, Block G, Rumore G, Quesenberry CP, Buffler P, Corley DA. Cigarette smoking and the risk of Barrett’s esophagus. Cancer Causes Control. 2009;20:303-311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Smith KJ, O’Brien SM, Green AC, Webb PM, Whiteman DC. Current and past smoking significantly increase risk for Barrett’s esophagus. Clin Gastroenterol Hepatol. 2009;7:840-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Steevens J, Schouten LJ, Driessen AL, Huysentruyt CJ, Keulemans YC, Goldbohm RA, van den Brandt PA. A prospective cohort study on overweight, smoking, alcohol consumption, and risk of Barrett’s esophagus. Cancer Epidemiol Biomarkers Prev. 2011;20:345-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 27. | Thrift AP, Kramer JR, Richardson PA, El-Serag HB. No significant effects of smoking or alcohol consumption on risk of Barrett’s esophagus. Dig Dis Sci. 2014;59:108-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 28. | Andrici J, Cox MR, Eslick GD. Cigarette smoking and the risk of Barrett’s esophagus: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2013;28:1258-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 29. | Thrift AP, Cook MB, Vaughan TL, Anderson LA, Murray LJ, Whiteman DC, Shaheen NJ, Corley DA. Alcohol and the risk of Barrett’s esophagus: a pooled analysis from the International BEACON Consortium. Am J Gastroenterol. 2014;109:1586-1594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 30. | Palles C, Chegwidden L, Li X, Findlay JM, Farnham G, Castro Giner F, Peppelenbosch MP, Kovac M, Adams CL, Prenen H. Polymorphisms near TBX5 and GDF7 are associated with increased risk for Barrett’s esophagus. Gastroenterology. 2015;148:367-378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 31. | Levine DM, Ek WE, Zhang R, Liu X, Onstad L, Sather C, Lao-Sirieix P, Gammon MD, Corley DA, Shaheen NJ. A genome-wide association study identifies new susceptibility loci for esophageal adenocarcinoma and Barrett’s esophagus. Nat Genet. 2013;45:1487-1493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 155] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 32. | Fischbach LA, Nordenstedt H, Kramer JR, Gandhi S, Dick-Onuoha S, Lewis A, El-Serag HB. The association between Barrett’s esophagus and Helicobacter pylori infection: a meta-analysis. Helicobacter. 2012;17:163-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 33. | Evans JA, Early DS, Fukami N, Ben-Menachem T, Chandrasekhara V, Chathadi KV, Decker GA, Fanelli RD, Fisher DA, Foley KQ. The role of endoscopy in Barrett’s esophagus and other premalignant conditions of the esophagus. Gastrointest Endosc. 2012;76:1087-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 241] [Article Influence: 18.5] [Reference Citation Analysis (1)] |
| 34. | Shaheen NJ, Weinberg DS, Denberg TD, Chou R, Qaseem A, Shekelle P. Upper endoscopy for gastroesophageal reflux disease: best practice advice from the clinical guidelines committee of the American College of Physicians. Ann Intern Med. 2012;157:808-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 110] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 35. | Saeian K, Staff DM, Vasilopoulos S, Townsend WF, Almagro UA, Komorowski RA, Choi H, Shaker R. Unsedated transnasal endoscopy accurately detects Barrett’s metaplasia and dysplasia. Gastrointest Endosc. 2002;56:472-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Jobe BA, Hunter JG, Chang EY, Kim CY, Eisen GM, Robinson JD, Diggs BS, O’Rourke RW, Rader AE, Schipper P. Office-based unsedated small-caliber endoscopy is equivalent to conventional sedated endoscopy in screening and surveillance for Barrett’s esophagus: a randomized and blinded comparison. Am J Gastroenterol. 2006;101:2693-2703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 92] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 37. | Peery AF, Hoppo T, Garman KS, Dellon ES, Daugherty N, Bream S, Sanz AF, Davison J, Spacek M, Connors D. Feasibility, safety, acceptability, and yield of office-based, screening transnasal esophagoscopy (with video). Gastrointest Endosc. 2012;75:945-953.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 38. | Shariff MK, Bird-Lieberman EL, O’Donovan M, Abdullahi Z, Liu X, Blazeby J, Fitzgerald R. Randomized crossover study comparing efficacy of transnasal endoscopy with that of standard endoscopy to detect Barrett’s esophagus. Gastrointest Endosc. 2012;75:954-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 39. | Alashkar B, Faulx AL, Hepner A, Pulice R, Vemana S, Greer KB, Isenberg GA, Falck-Ytter Y, Chak A. Development of a program to train physician extenders to perform transnasal esophagoscopy and screen for Barrett’s esophagus. Clin Gastroenterol Hepatol. 2014;12:785-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 40. | Gupta M, Beebe TJ, Dunagan KT, Schleck CD, Zinsmeister AR, Talley NJ, Locke GR, Iyer PG. Screening for Barrett’s esophagus: results from a population-based survey. Dig Dis Sci. 2014;59:1831-1850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 41. | Kadri SR, Lao-Sirieix P, O’Donovan M, Debiram I, Das M, Blazeby JM, Emery J, Boussioutas A, Morris H, Walter FM. Acceptability and accuracy of a non-endoscopic screening test for Barrett’s oesophagus in primary care: cohort study. BMJ. 2010;341:c4372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 247] [Cited by in RCA: 235] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 42. | Benaglia T, Sharples LD, Fitzgerald RC, Lyratzopoulos G. Health benefits and cost effectiveness of endoscopic and nonendoscopic cytosponge screening for Barrett’s esophagus. Gastroenterology. 2013;144:62-73.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 124] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 43. | Zhang S, Wang XI. SIRT1 is a useful biomarker for high-grade dysplasia and carcinoma in Barrett’s esophagus. Ann Clin Lab Sci. 2013;43:373-377. [PubMed] |
| 44. | Kendall BJ, Macdonald GA, Hayward NK, Prins JB, Brown I, Walker N, Pandeya N, Green AC, Webb PM, Whiteman DC. Leptin and the risk of Barrett’s oesophagus. Gut. 2008;57:448-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 45. | Garcia JM, Splenser AE, Kramer J, Alsarraj A, Fitzgerald S, Ramsey D, El-Serag HB. Circulating inflammatory cytokines and adipokines are associated with increased risk of Barrett’s esophagus: a case-control study. Clin Gastroenterol Hepatol. 2014;12:229-238.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 46. | Rubenstein JH, Morgenstern H, Appelman H, Scheiman J, Schoenfeld P, McMahon LF, Metko V, Near E, Kellenberg J, Kalish T. Prediction of Barrett’s esophagus among men. Am J Gastroenterol. 2013;108:353-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 135] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 47. | Thrift AP, Garcia JM, El-Serag HB. A multibiomarker risk score helps predict risk for Barrett’s esophagus. Clin Gastroenterol Hepatol. 2014;12:1267-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 48. | Fitzgerald RC, di Pietro M, Ragunath K, Ang Y, Kang JY, Watson P, Trudgill N, Patel P, Kaye PV, Sanders S. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett’s oesophagus. Gut. 2014;63:7-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1016] [Cited by in RCA: 878] [Article Influence: 79.8] [Reference Citation Analysis (0)] |
| 49. | Hvid-Jensen F, Pedersen L, Drewes AM, Sørensen HT, Funch-Jensen P. Incidence of adenocarcinoma among patients with Barrett’s esophagus. N Engl J Med. 2011;365:1375-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 985] [Cited by in RCA: 980] [Article Influence: 70.0] [Reference Citation Analysis (1)] |
| 50. | de Jonge PJ, van Blankenstein M, Looman CW, Casparie MK, Meijer GA, Kuipers EJ. Risk of malignant progression in patients with Barrett’s oesophagus: a Dutch nationwide cohort study. Gut. 2010;59:1030-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 211] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 51. | Gaddam S, Singh M, Balasubramanian G, Thota P, Gupta N, Wani S, Higbee AD, Mathur SC, Horwhat JD, Rastogi A. Persistence of nondysplastic Barrett’s esophagus identifies patients at lower risk for esophageal adenocarcinoma: results from a large multicenter cohort. Gastroenterology. 2013;145:548-553.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 52. | Lim CH, Treanor D, Dixon MF, Axon AT. Low-grade dysplasia in Barrett’s esophagus has a high risk of progression. Endoscopy. 2007;39:581-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 79] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 53. | Schnell TG, Sontag SJ, Chejfec G, Aranha G, Metz A, O’Connell S, Seidel UJ, Sonnenberg A. Long-term nonsurgical management of Barrett’s esophagus with high-grade dysplasia. Gastroenterology. 2001;120:1607-1619. [PubMed] |
| 54. | Singh S, Manickam P, Amin AV, Samala N, Schouten LJ, Iyer PG, Desai TK. Incidence of esophageal adenocarcinoma in Barrett’s esophagus with low-grade dysplasia: a systematic review and meta-analysis. Gastrointest Endosc. 2014;79:897-909.e4; quiz 983.e1, 983.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 160] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 55. | Skacel M, Petras RE, Gramlich TL, Sigel JE, Richter JE, Goldblum JR. The diagnosis of low-grade dysplasia in Barrett‘s esophagus and its implications for disease progression. Am J Gastroenterol. 2000;95:3383-3387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 252] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 56. | Curvers WL, ten Kate FJ, Krishnadath KK, Visser M, Elzer B, Baak LC, Bohmer C, Mallant-Hent RC, van Oijen A, Naber AH. Low-grade dysplasia in Barrett’s esophagus: overdiagnosed and underestimated. Am J Gastroenterol. 2010;105:1523-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 332] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 57. | Duits LC, Phoa KN, Curvers WL, Ten Kate FJ, Meijer GA, Seldenrijk CA, Offerhaus GJ, Visser M, Meijer SL, Krishnadath KK. Barrett’s oesophagus patients with low-grade dysplasia can be accurately risk-stratified after histological review by an expert pathology panel. Gut. 2015;64:700-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 180] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 58. | Phoa KN, van Vilsteren FG, Weusten BL, Bisschops R, Schoon EJ, Ragunath K, Fullarton G, Di Pietro M, Ravi N, Visser M. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. JAMA. 2014;311:1209-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 509] [Cited by in RCA: 440] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 59. | Corley DA, Mehtani K, Quesenberry C, Zhao W, de Boer J, Weiss NS. Impact of endoscopic surveillance on mortality from Barrett’s esophagus-associated esophageal adenocarcinomas. Gastroenterology. 2013;145:312-9.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 179] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 60. | Macdonald CE, Wicks AC, Playford RJ. Final results from 10 year cohort of patients undergoing surveillance for Barrett’s oesophagus: observational study. BMJ. 2000;321:1252-1255. [PubMed] |
| 61. | Garside R, Pitt M, Somerville M, Stein K, Price A, Gilbert N. Surveillance of Barrett’s oesophagus: exploring the uncertainty through systematic review, expert workshop and economic modelling. Health Technol Assess. 2006;10:1-142, iii-iv. [PubMed] |
| 62. | Levine DS, Haggitt RC, Blount PL, Rabinovitch PS, Rusch VW, Reid BJ. An endoscopic biopsy protocol can differentiate high-grade dysplasia from early adenocarcinoma in Barrett’s esophagus. Gastroenterology. 1993;105:40-50. [PubMed] |
| 63. | Gupta N, Gaddam S, Wani SB, Bansal A, Rastogi A, Sharma P. Longer inspection time is associated with increased detection of high-grade dysplasia and esophageal adenocarcinoma in Barrett’s esophagus. Gastrointest Endosc. 2012;76:531-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 152] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 64. | Enestvedt BK, Lugo R, Guarner-Argente C, Shah P, Falk GW, Furth E, Ginsberg GG. Location, location, location: does early cancer in Barrett’s esophagus have a preference? Gastrointest Endosc. 2013;78:462-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 65. | Yachimski P, Wani S, Givens T, Howard E, Higginbotham T, Price A, Berman K, Hosford L, Katcher PM, Ozanne E. Preference of endoscopic ablation over medical prevention of esophageal adenocarcinoma by patients with Barrett’s esophagus. Clin Gastroenterol Hepatol. 2015;13:84-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 66. | Lim CH, Rotimi O, Dexter SP, Axon AT. Randomized crossover study that used methylene blue or random 4-quadrant biopsy for the diagnosis of dysplasia in Barrett’s esophagus. Gastrointest Endosc. 2006;64:195-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 67. | Singh M, Bansal A, Curvers WL, Kara MA, Wani SB, Alvarez Herrero L, Lynch CR, van Kouwen MC, Peters FT, Keighley JD. Observer agreement in the assessment of narrowband imaging system surface patterns in Barrett’s esophagus: a multicenter study. Endoscopy. 2011;43:745-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 68. | Boerwinkel DF, Holz JA, Kara MA, Meijer SL, Wallace MB, Wong Kee Song LM, Ragunath K, Wolfsen HC, Iyer PG, Wang KK. Effects of autofluorescence imaging on detection and treatment of early neoplasia in patients with Barrett’s esophagus. Clin Gastroenterol Hepatol. 2014;12:774-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 69. | Kara MA, Peters FP, Ten Kate FJ, Van Deventer SJ, Fockens P, Bergman JJ. Endoscopic video autofluorescence imaging may improve the detection of early neoplasia in patients with Barrett’s esophagus. Gastrointest Endosc. 2005;61:679-685. [PubMed] |
| 70. | Ham NS, Jang JY, Ryu SW, Kim JH, Park EJ, Lee WC, Shim KY, Jeong SW, Kim HG, Lee TH. Magnifying endoscopy for the diagnosis of specialized intestinal metaplasia in short-segment Barrett’s esophagus. World J Gastroenterol. 2013;19:7089-7096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 71. | Giacchino M, Bansal A, Kim RE, Singh V, Hall SB, Singh M, Rastogi A, Moloney B, Wani SB, Gaddam S. Clinical utility and interobserver agreement of autofluorescence imaging and magnification narrow-band imaging for the evaluation of Barrett’s esophagus: a prospective tandem study. Gastrointest Endosc. 2013;77:711-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 72. | Sturm MB, Joshi BP, Lu S, Piraka C, Khondee S, Elmunzer BJ, Kwon RS, Beer DG, Appelman HD, Turgeon DK. Targeted imaging of esophageal neoplasia with a fluorescently labeled peptide: first-in-human results. Sci Transl Med. 2013;5:184ra61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 132] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 73. | Canto MI, Anandasabapathy S, Brugge W, Falk GW, Dunbar KB, Zhang Z, Woods K, Almario JA, Schell U, Goldblum J. In vivo endomicroscopy improves detection of Barrett’s esophagus-related neoplasia: a multicenter international randomized controlled trial (with video). Gastrointest Endosc. 2014;79:211-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 139] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 74. | Leggett CL, Gorospe E, Owens VL, Anderson M, Lutzke L, Wang KK. Volumetric laser endomicroscopy detects subsquamous Barrett’s adenocarcinoma. Am J Gastroenterol. 2014;109:298-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 75. | Orman ES, Li N, Shaheen NJ. Efficacy and durability of radiofrequency ablation for Barrett‘s Esophagus: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2013;11:1245-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 213] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 76. | Orman ES, Kim HP, Bulsiewicz WJ, Cotton CC, Dellon ES, Spacek MB, Chen X, Madanick RD, Pasricha S, Shaheen NJ. Intestinal metaplasia recurs infrequently in patients successfully treated for Barrett’s esophagus with radiofrequency ablation. Am J Gastroenterol. 2013;108:187-195; quiz 196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 77. | Gupta M, Iyer PG, Lutzke L, Gorospe EC, Abrams JA, Falk GW, Ginsberg GG, Rustgi AK, Lightdale CJ, Wang TC. Recurrence of esophageal intestinal metaplasia after endoscopic mucosal resection and radiofrequency ablation of Barrett’s esophagus: results from a US Multicenter Consortium. Gastroenterology. 2013;145:79-86.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 172] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 78. | Phoa KN, Pouw RE, van Vilsteren FG, Sondermeijer CM, Ten Kate FJ, Visser M, Meijer SL, van Berge Henegouwen MI, Weusten BL, Schoon EJ. Remission of Barrett’s esophagus with early neoplasia 5 years after radiofrequency ablation with endoscopic resection: a Netherlands cohort study. Gastroenterology. 2013;145:96-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 169] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 79. | Gosain S, Mercer K, Twaddell WS, Uradomo L, Greenwald BD. Liquid nitrogen spray cryotherapy in Barrett’s esophagus with high-grade dysplasia: long-term results. Gastrointest Endosc. 2013;78:260-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 80. | Almond LM, Hutchings J, Lloyd G, Barr H, Shepherd N, Day J, Stevens O, Sanders S, Wadley M, Stone N. Endoscopic Raman spectroscopy enables objective diagnosis of dysplasia in Barrett’s esophagus. Gastrointest Endosc. 2014;79:37-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 81. | Nurkin SJ, Nava HR, Yendamuri S, LeVea CM, Nwogu CE, Groman A, Wilding G, Bain AJ, Hochwald SN, Khushalani NI. Outcomes of endoscopic resection for high-grade dysplasia and esophageal cancer. Surg Endosc. 2014;28:1090-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 82. | Wu J, Pan YM, Wang TT, Gao DJ, Hu B. Endotherapy versus surgery for early neoplasia in Barrett’s esophagus: a meta-analysis. Gastrointest Endosc. 2014;79:233-241.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 83. | Manner H, Rabenstein T, Pech O, Braun K, May A, Pohl J, Behrens A, Vieth M, Ell C. Ablation of residual Barrett’s epithelium after endoscopic resection: a randomized long-term follow-up study of argon plasma coagulation vs. surveillance (APE study). Endoscopy. 2014;46:6-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 84. | Orlando RC. How good is the neosquamous epithelium? Dig Dis. 2014;32:164-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 85. | Krishnadath KK, Wang KK, Taniguchi K, Sebo TJ, Buttar NS, Anderson MA, Lutzke LS, Liu W. Persistent genetic abnormalities in Barrett’s esophagus after photodynamic therapy. Gastroenterology. 2000;119:624-630. [PubMed] |
| 86. | Mashimo H. Subsquamous intestinal metaplasia after ablation of Barrett’s esophagus: frequency and importance. Curr Opin Gastroenterol. 2013;29:454-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 87. | Kastelein F, Spaander MC, Biermann K, Steyerberg EW, Kuipers EJ, Bruno MJ. Nonsteroidal anti-inflammatory drugs and statins have chemopreventative effects in patients with Barrett‘s esophagus. Gastroenterology. 2011;141:2000-2008; quiz e13-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 88. | Nguyen DM, Richardson P, El-Serag HB. Medications (NSAIDs, statins, proton pump inhibitors) and the risk of esophageal adenocarcinoma in patients with Barrett’s esophagus. Gastroenterology. 2010;138:2260-2266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 140] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 89. | Thrift AP, Pandeya N, Smith KJ, Green AC, Webb PM, Whiteman DC. The use of nonsteroidal anti-inflammatory drugs and the risk of Barrett’s oesophagus. Aliment Pharmacol Ther. 2011;34:1235-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 90. | Falk GW, Buttar NS, Foster NR, Ziegler KL, Demars CJ, Romero Y, Marcon NE, Schnell T, Corley DA, Sharma P. A combination of esomeprazole and aspirin reduces tissue concentrations of prostaglandin E(2) in patients with Barrett’s esophagus. Gastroenterology. 2012;143:917-26.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 91. | Heath EI, Canto MI, Piantadosi S, Montgomery E, Weinstein WM, Herman JG, Dannenberg AJ, Yang VW, Shar AO, Hawk E. Secondary chemoprevention of Barrett’s esophagus with celecoxib: results of a randomized trial. J Natl Cancer Inst. 2007;99:545-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 133] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 92. | Khalaf N, Nguyen T, Ramsey D, El-Serag HB. Nonsteroidal anti-inflammatory drugs and the risk of Barrett’s esophagus. Clin Gastroenterol Hepatol. 2014;12:1832-1839.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 93. | Tsibouris P, Vlachou E, Isaacs PE. Role of chemoprophylaxis with either NSAIDs or statins in patients with Barrett’s esophagus. World J Gastrointest Pharmacol Ther. 2014;5:27-39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 94. | Xu Y, Lu S. Metformin inhibits esophagus cancer proliferation through upregulation of USP7. Cell Physiol Biochem. 2013;32:1178-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 95. | Becker C, Meier CR, Jick SS, Bodmer M. Case-control analysis on metformin and cancer of the esophagus. Cancer Causes Control. 2013;24:1763-1770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 96. | Chak A, Buttar NS, Foster NR, Seisler DK, Marcon NE, Schoen R, Cruz-Correa MR, Falk GW, Sharma P, Hur C. Metformin does not reduce markers of cell proliferation in esophageal tissues of patients with Barrett’s esophagus. Clin Gastroenterol Hepatol. 2015;13:665-672.e1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 97. | Alexandre L, Clark AB, Bhutta HY, Holt S, Lewis MP, Hart AR. Statin use is associated with reduced risk of histologic subtypes of esophageal cancer: a nested case-control analysis. Gastroenterology. 2014;146:661-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 98. | Singh S, Singh AG, Singh PP, Murad MH, Iyer PG. Statins are associated with reduced risk of esophageal cancer, particularly in patients with Barrett’s esophagus: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2013;11:620-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 136] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 99. | Hillman LC, Chiragakis L, Shadbolt B, Kaye GL, Clarke AC. Effect of proton pump inhibitors on markers of risk for high-grade dysplasia and oesophageal cancer in Barrett’s oesophagus. Aliment Pharmacol Ther. 2008;27:321-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 100. | García Rodríguez LA, Lagergren J, Lindblad M. Gastric acid suppression and risk of oesophageal and gastric adenocarcinoma: a nested case control study in the UK. Gut. 2006;55:1538-1544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 150] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 101. | Kastelein F, Spaander MC, Steyerberg EW, Biermann K, Valkhoff VE, Kuipers EJ, Bruno MJ. Proton pump inhibitors reduce the risk of neoplastic progression in patients with Barrett’s esophagus. Clin Gastroenterol Hepatol. 2013;11:382-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 140] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 102. | Singh S, Garg SK, Singh PP, Iyer PG, El-Serag HB. Acid-suppressive medications and risk of oesophageal adenocarcinoma in patients with Barrett’s oesophagus: a systematic review and meta-analysis. Gut. 2014;63:1229-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 199] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 103. | Sharaiha RZ, Freedberg DE, Abrams JA, Wang YC. Cost-effectiveness of chemoprevention with proton pump inhibitors in Barrett’s esophagus. Dig Dis Sci. 2014;59:1222-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 104. | Spechler SJ, Sharma P, Souza RF, Inadomi JM, Shaheen NJ. American Gastroenterological Association technical review on the management of Barrett’s esophagus. Gastroenterology. 2011;140:e18-52; quiz e13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 901] [Cited by in RCA: 804] [Article Influence: 57.4] [Reference Citation Analysis (0)] |
| 105. | Fudman DI, Lightdale CJ, Poneros JM, Ginsberg GG, Falk GW, Demarshall M, Gupta M, Iyer PG, Lutzke L, Wang KK. Positive correlation between endoscopist radiofrequency ablation volume and response rates in Barrett’s esophagus. Gastrointest Endosc. 2014;80:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 106. | Overholt BF, Lightdale CJ, Wang KK, Canto MI, Burdick S, Haggitt RC, Bronner MP, Taylor SL, Grace MG, Depot M. Photodynamic therapy with porfimer sodium for ablation of high-grade dysplasia in Barrett’s esophagus: international, partially blinded, randomized phase III trial. Gastrointest Endosc. 2005;62:488-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 348] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 107. | Gray J, Fullarton GM. Long term efficacy of Photodynamic Therapy (PDT) as an ablative therapy of high grade dysplasia in Barrett’s oesophagus. Photodiagnosis Photodyn Ther. 2013;10:561-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |