Published online May 28, 2015. doi: 10.3748/wjg.v21.i20.6398
Peer-review started: October 31, 2014
First decision: November 26, 2014
Revised: December 10, 2014
Accepted: January 30, 2015
Article in press: January 30, 2015
Published online: May 28, 2015
Processing time: 211 Days and 9 Hours
Gastric abscess is a localized pyogenic inflammation of the gastric wall, which is a rare form of suppurative gastritis. The rarity of gastric abscess may be associated with the difficulty of early diagnosis and high mortality as a result. In general, subepithelial lesions (SELs) of the stomach are incidentally detected during the course of upper endoscopy without specific clinical symptoms and signs. However, some gastric SELs present rarely as a form of hemorrhage, obstruction, perforation, and abscess. Here we report a 45-year-old man with gastric SEL presenting as a gastric abscess, which was diagnosed as an ectopic pancreas of the stomach, along with a review of the literature. Although gastric SEL presenting as an abscess is known as a serious and life-threatening lesion, the patient made a complete recovery through surgical resection as well as medical treatment.
Core tip: Suppurative gastritis is a rare disorder characterized by a purulent, exudative inflammatory process involving the submucosa that may extend to involve the entire gastric wall and may be divided into two categories: phlegmonous, or diffuse type; and localized, gastric abscess type. Predisposing conditions of phlegmonous gastritis or gastric abscess include alcoholism, diabetes mellitus, decreased gastric acidity, and immunosuppression, such as HIV infection. High mortality rate of suppurative gastritis, between 37% and 84%, has been reported. Medical treatment, such as endoscopic drainage and antibiotics, and surgical resection of the mass should be considered.
- Citation: Kim SB, Oh MJ, Lee SH. Gastric subepithelial lesion complicated with abscess: Case report and literature review. World J Gastroenterol 2015; 21(20): 6398-6403
- URL: https://www.wjgnet.com/1007-9327/full/v21/i20/6398.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i20.6398
Gastric abscess is a localized pyogenic inflammation of the gastric wall, which is a rare form of suppurative gastritis, and is known to be life-threatening. The rarity of gastric abscess may be associated with sufficient blood supply to the stomach and the bactericidal effect of gastric acid[1]. In addition, the infrequency of gastric abscess makes early diagnosis difficult. As a result, gastric abscess has high mortality.
Subepithelial lesions (SELs) of the stomach are incidentally detected during performance of routine upper endoscopy. Although a previous study reported that gastric SEL was found in about 0.36% of cases by upper gastrointestinal endoscopy, the prevalence of SELs of the stomach is increasing as a result of the high frequency of endoscopy for health examination[2]. Most gastric SELs are regarded as benign lesions, however, an estimated 13% are likely to be malignant[3]. Most SELs of the stomach are observed without specific clinical symptoms and signs. However, a few gastric SELs may rarely present as gastric abscess accompanied by fever and chill[4-9].
Here, we report a 45-year-old man with gastric SEL presenting as an acute gastric abscess, which was completely cured through surgical resection after medical treatment. We also provide a short review of the literature on the rare condition of gastric SEL presenting as a gastric abscess.
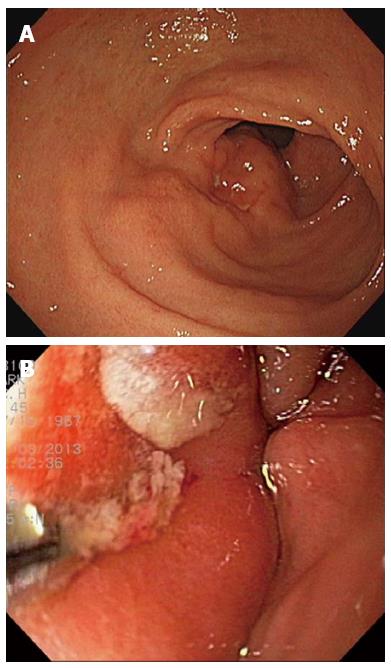
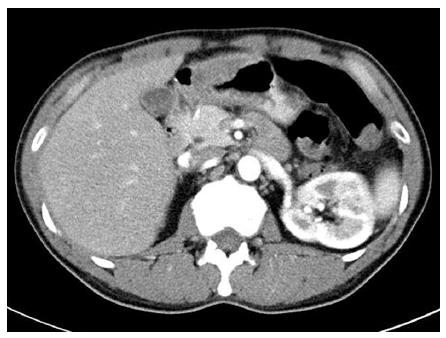
A 45-year-old man visited a local private clinic because of intermittent febrile or chilling sensation, indigestion, and vague abdominal pain for 5 d. Upper endoscopy, abdominal ultrasonography, and contrast-enhanced computed tomography (CT) were performed to investigate the cause of fever and abdominal pain. On endoscopic examination, a large mass measuring about 5 cm, with superficial ulcers and a fistula of the gastric lumen was noted at the anterior wall of the distal antrum of the stomach. A small amount of milky discharge was observed to flow from the fistula. The endoscopist at the local clinic strongly pushed on the wall of the mass using a biopsy forceps, and more pus-like yellowish discharge spewed out from the opening of the fistula (Figure 1). An inhomogeneous, hypoechoic mass with vague contours was also observed at the anterior wall of the stomach through ultrasonographic examination. In addition, on abdominal CT, a heterogeneously enhanced mass measuring approximately 5 cm × 4 cm with concentric elevation and an overhanging edge was detected on the anterior wall of gastric antrum (Figure 2). Therefore, the patient was referred to the Gastroenterology Center of Yeungnam University Hospital for further evaluation and treatment of a bizarre-shaped mass of the stomach.
The patient, who did not have a significant past medical history, presented with a 5-d history of low-grade fever and intermittent abdominal pain. The abdominal pain worsened after eating meals and was accompanied by indigestion. Past medical history was only remarkable in that he had received dental implantation due to traumatic dental injury during the previous few days, and he already knew that SEL of the stomach through upper endoscopy had been detected 2 years ago. Because there were no specific clinical symptoms of the gastric SEL, no further evaluation or treatment was performed. The patient was not taking any specific medication, including proton-pump inhibitors and nonsteroidal anti-inflammatory drugs. Regarding family history, his father died of lung cancer. He was an ex-smoker with 15 pack-years, and was not an alcohol drinker.
Relative appearance of well-being and alertness of mental status were noted. Physical examination of the abdomen revealed a soft and non-tender point with no palpable mass and normoactive bowel sounds. Initial vital signs included blood pressure 110/60 mmHg, heart rate 89 beats/min, respiration rate 20 breaths/min, and body temperature 38.1 °C. The initial laboratory evaluation showed white blood cell count of 11580 cells/μL (neutrophils: 74.5%), hemoglobin 15.4 g/dL, platelet count 1.70 × 105 cells/μL, total bilirubin 3.36 mg/dL, total protein 6.31 g/dL, albumin 3.47 g/dL, aspartate aminotransferase 116 IU/L, alanine aminotransferase 136 IU/L, alkaline phosphatase 618 IU/L, γ-glutamyl transpeptidase 289 IU/L, lactate dehydrogenase 457 IU/L, prothrombin time 10.2 s (international normalized ratio: 0.94), amylase 58 U/L, lipase 18U/L, blood urea nitrogen 10.09 mg/dL, creatinine 1.1 mg/dL, Na+ 135 mEq/L, K+ 3.6 mEq/L, Cl- 100 mEq/L, erythrocyte sedimentation rate (ESR) 42 mm/H, and C-reactive protein (CRP) 18.866 mg/dL. Viral markers for chronic hepatitis B and C were unremarkable. Anti-HIV antibody test was negative. Of tumor markers, only carbohydrate antigen (CA) 19-9 level was elevated to 145.42 U/mL (reference range: 0-37 U/mL). On urine analysis, pyuria (WBC: > 10/HPF) was detected.
Plain chest and abdominal X-rays were non-specific. On follow-up abdominal contrast-enhanced CT, shrinkage of the heterogeneously enhanced mass of the stomach with central necrosis and air-fluid level was observed. Upper endoscopy and endosonography for the unusual mass were performed. On follow-up endoscopy, a bulging mucosa measuring about 5 cm, with multiple external ulcers and erosions, was still noted on the anterior wall of the gastric antrum, however, no pus-like discharge or fistula was observed.
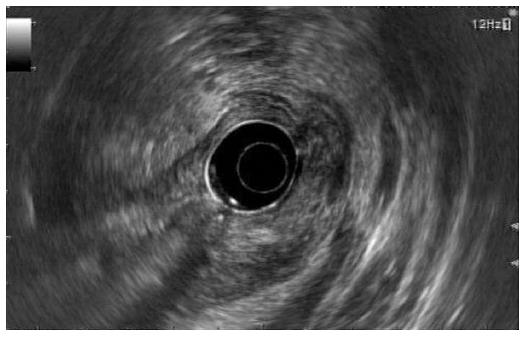
On endoscopic ultrasonography (EUS), a heterogeneous, loculated mass measuring about 5 cm, with mixed echogenicity (partial hypoechogenicity as well as iso- and hyperechogenicity) and an irregular margin, was observed at the antrum of the stomach. In addition, the mass originated from the third layer or submucosal layer of the stomach with air shadows and fluid densities within the mass (Figure 3). According to endosonography of the mass, the lesion was comparable to ectopic pancreas. Considering the irregular contours and mixture of air shadows and fluid densities, internal inflammatory change, such as an abscess or necrosis, might have been combined with ectopic pancreas.
Our initial impression for symptoms and signs of the patient could be summarized as a systemic inflammatory response syndrome due to gastric abscess overlapping with an SEL or urinary tract infection. First, the patient was empirically started on intravenous antibiotics, including third-generation cephalosporin and metronidazole for severe infection. However, body temperature fluctuated to as high as 38.6 °C for 3 d. Due to high mortality of gastric abscess, surgical resection for the gastric SEL combined with abscess was considered in the case of no alleviation of fever. Fortunately, on admission day 4, the fever gradually subsided and his general condition also showed improvement. No growth was observed on the initial blood and sputum culture. Only Klebsiella pneumoniae with extended-spectrum β lactamase negativity was cultured in the initial urine culture test. Empirical intravenous antibiotics were administered continuously for 7 d. After replacement of intravenous with oral antibiotics, the patient showed good tolerance, and was in good condition at discharge. Oral antibiotics were administered over 1 wk in the outpatient clinic. The white cell count and liver function tests returned to normal after endoscopic drainage of the abscess through the fistula and intravenous administration of antibiotics. ESR and CRP level declined, but remained over the upper normal limit in spite of antibiotic therapy.
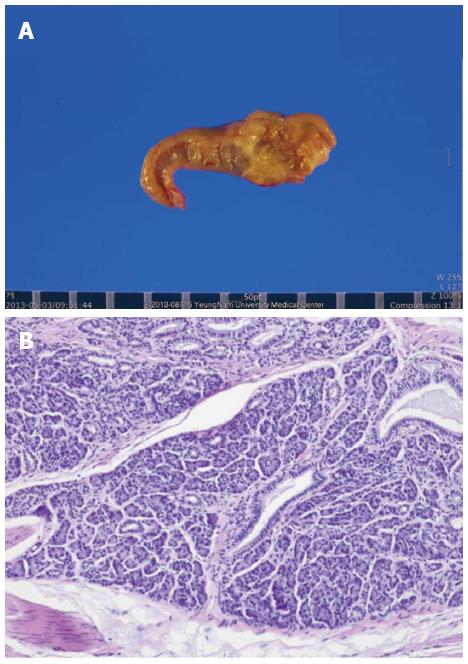
Three weeks after discharge, the patient was admitted again, and underwent laparoscopic wedge resection for the gastric SEL at the Department of Surgery because of concern over relapse of the gastric abscess and lack of complete remission of ESR and CRP level. The size of the specimen removed from the stomach by wedge resection was 5.6 cm × 3.5 cm × 2.4 cm. On opening, a submucosal tumor measuring 2.3 cm × 1.2 cm around necrotic tissue was identified. On microscopic view, multifocal benign acini around ductal structures were observed (Figure 4). In addition, hypertrophied muscularis mucosa, Brunner’s gland hyperplasia, and gastritis cystica profunda were noted throughout certain parts of the resected specimen. The final pathological result of the gastric SEL was confirmed as an ectopic pancreas of Heinrich type III observed only in the acinus and duct. The mass in the stomach was finally diagnosed as an ectopic pancreas accompanied by a gastric abscess. After surgical resection, the patient recovered without specific complications in 10 d. In addition, on postoperative follow-up laboratory tests, ESR was 3 mm/h, CRP 0.053 mg/dL, and CA 19-9 6.03 U/mL, which were normalized.
Suppurative gastritis is a rare disorder characterized by a purulent, exudative inflammatory process involving the submucosa that may extend to involve the entire gastric wall. The bactericidal effect of gastric acid is partially responsible for the rarity of this condition[1]. In general, suppurative gastritis may be divided into two categories based on the extension of pathology: the phlegmonous, or diffuse type; and the localized, gastric abscess type. The localized form of suppurative gastritis or gastric abscess occurs less frequently. The pylorus and antrum are the most frequently involved areas for gastric abscess, however, abscess may develop anywhere in the stomach. Although the pathogen of gastric abscess is unknown, it most commonly involves infection with oral microflora. Although streptococci are the most common pathogens isolated in culture from gastric abscess, accounting for about 70% of cases, a variety of aerobic and anaerobic bacteria, including Escherichia coli, Proteus vulgaris, Clostridium perfringens, Clostridium welchi, Pseudomonas aeruginosa, Proteus mirabilis, Staphylococcus and Bacillus subtilis, as well as various fungi, have been implicated[7,9,10-14]. In this case, the gastric abscess was located at the antrum. The culture from the mass or pus could not be obtained directly, and no growth was observed on the blood culture test.
Pathogenic mechanisms of gastric abscess include either direct invasion by microorganisms secondary to gastric mucosal trauma/injury or hematogenous spreading by metastasis of distant infection[7,14]. In our case, the patient had undergone dental implantation several days before. Through more detailed history taking to investigate the cause of gastric abscess, it was revealed that abdominal pain and febrile sensation occurred after eating fried chicken. Poor oral hygiene associated with the dental implantation process and gastric mucosal injury by chicken bone intake related to abnormal masticatory movement might lead to formation of an inflammatory mass in the stomach. In addition, urinary tract infection by K. pneumoniae might be associated with a hematogenous origin of the gastric abscess. Thus, in our case, involvement of both the pathogenic mechanisms of gastric abscess might be a possibility.
Predisposing conditions of phlegmonous gastritis or gastric abscess include alcoholism, diabetes mellitus, decreased gastric acidity, and immunosuppression, such as HIV infection[15,16]. In our case, there were no predisposing conditions of gastric abscess. The patient had no significant past medical history, including diabetes mellitus. He did not drink alcohol, and took no medication such as proton pump inhibitors and antacids. Anti-HIV antibody test was also negative. In addition, according to a review of English-language case reports, it was confirmed that all patients with gastric SELs presenting as gastric abscesses had no predisposing conditions (Table 1). Most patients developed gastric abscesses subacutely over an average of 6-8 wk. However, rarely, as in our case, formation of a gastric abscess took only 5 d. As shown in Table 1, Nozawa et al[8] reported that abscess formation in a gastrointestinal stromal tumor following endoscopic biopsy occurred within 1 wk. Thus, in spite of no predisposing conditions and acute manifestations, if the patient with gastric SEL complains of vague epigastric pain concomitant with fever, attention should be paid to development of gastric abscess accompanied with SEL.
| Ref. | Age/sex | Predisposing conditions | Onset | ESR or CRP | Site/Layer | Pathogens of gastric abscess | Size of mass (Max.) | Treatment | Pathological diagnosis |
| Kaneda et al[5] | 29/female | None | Subacute (8 wk) | Elevated | Antrum (GC)/SM | Unknown | 3 cm | Partial gastrectomy | Ectopic pancreas |
| Honda et al[6] | 78/female | None | Subacute (8 wk) | Elevated | Antrum(LC)/MP | Alpha Streptococci, Gram (-) anaerobes | 9 cm | Partial gastrectomy | GIST (GI autonomic nerve tumor) |
| Seidel et al[7] | 50/female | None | Subacute (6 wk) | Unknown | Angle (LC)/MP | Unknown | 6 cm | Endoscopic drainage and wedge resection of the mass | Leiomyosarcoma |
| Nozawa et al[8] | 74/male | None | Acute (7 d) | Unknown | Fundus to body (AW)/NA | Clostridium perfringens | 13 cm | Proximal partial gastrectomy | GIST |
| Osada et al[9] | 74/male | None | Subacute (8 wk) | Elevated | Fundus/NA | Streptococcus intermedius | 12 cm | Endoscopic drainage, antibiotics and proximal partial gastrectomy | GIST |
| Present case | 45/male | None | Acute (5 d) | Elevated | Antrum(AW)/SM | Unknown | 5.6 cm | Endoscopic drainage, antibiotics and wedge resection of the mass | Ectopic pancreas |
A high mortality rate of suppurative gastritis, between 37% and 84%, has been reported[11,13,17]. Miller et al[11] reported a mortality rate of 100% in patients treated medically, compared with 18% mortality in patients treated with gastric resection and antibiotics. Thus, in all of the case reports, including our present case, surgical resection and medical treatment, such as endoscopic drainage and intravenous antibiotics, were performed on all patients with gastric SEL combined with gastric abscess (Table 1). In our case, although clinical signs and symptoms were improved through medical therapy, ESR and CRP as serological markers indicated that inflammatory processes were not normalized. For complete remission of gastric inflammation accompanied with SEL, gastric wedge resection was performed, and ESR and CRP level showed a normal range after resection.
Most patients with an ectopic pancreas have no clinical symptoms, and, if present, the symptoms are non-specific. However, specific clinical symptoms of heterotopic pancreas are presented by either mass effect or underlying pathology[5]. Clinical symptoms revealed due to mass effect depend on the site of an ectopic pancreas: intussusception (small bowel), obstructive jaundice (biliary tract), and pyloric obstruction (prepyloric region). Clinical manifestations according to underlying pathology are related to almost all of the changes arising from the pancreas itself. Underlying pathology includes exocrine parts such as pancreatic cancer, cystic formation, acute pancreatitis, and abscess formation, as well as endocrine parts such as insulinoma, gastrinoma, and growth hormone-producing tumor. In the current case, among many tumor markers, CA 19-9 was exclusively elevated to approximately four times the upper limit of normal. CA 19-9 is used as a screening test for cancer, particularly pancreatic cancer. In general, elevated CA 19-9 can occur in many types of gastrointestinal cancer, including colorectal cancer, esophageal cancer, and hepatocellular carcinoma. Apart from cancer, elevated level of CA 19-9 may also occur in pancreatitis, cirrhosis, and many diseases of the bile ducts, including obstruction of the bile ducts[18,19]. In other words, CA 19-9 can be elevated in the case of occurrence of inflammation of the pancreatobiliary tract. Although the initial levels of amylase and lipase were within the normal range, elevation of CA 19-9 in our case may be associated with pancreatic inflammation such as pancreatitis of the ectopic pancreas arising from gastric abscess or pancreatic tissue itself, and not pancreatic cancer. This hypothesis is supported by the fact that CA 19-9 was normalized at 6.03 U/mL after wedge resection of the gastric SEL, and leukocytes were mildly aggregated around the pancreatic tissue on microscopic examination of the resected specimen.
In conclusion, despite its rarity, SEL of the stomach can present with gastric abscess. Therefore, attention should be paid to patients with gastric SEL who develop vague abdominal pain, fever, and elevation of ESR or CRP. Further and thorough evaluation, including abdominal CT and EUS should be performed carefully due to the potential risk of infection. Medical treatment, such as endoscopic drainage and antibiotics, and surgical resection of the mass should be considered.
A 45-year-old man presented with intermittent febrile or chilling sensation, indigestion, and vague abdominal pain for 5 d.
Physical examination on the abdomen revealed unremarkable findings and initial body temperature was 38.1 °C.
Systemic inflammatory response syndrome secondary to gastric abscess overlapping with a subepithelial lesion (SEL) or other causes of infection, such as urinary tract infection.
Initial laboratory findings were elevated for neutrophil count (11580 cells/μL), erythrocyte sedimentation rate (42 mm/h), C-reactive protein (18.866 mg/dL), aspartate aminotransferase (116 IU/L), alanine aminotransferase (136 IU/L), and on urinalysis, pyuria (WBC: > 10/HPF) was detected.
Endoscopic ultrasonography showed a heterogeneous, loculated mass measuring about 5 cm, with mixed echogenicity and an irregular margin at the antrum of the stomach. Abdominal computed tomography showed a heterogeneously enhanced mass measuring about 5 cm × 4 cm with concentric elevation and an overhanging edge on the anterior wall of the gastric antrum.
Histological examination showed multifocal benign acini around ductal structures and the final pathological diagnosis of the gastric SEL was confirmed as ectopic pancreas of Heinrich type III, observed only in the acinus and duct.
The patient received intravenous antibiotics and underwent laparoscopic wedge resection for the gastric SEL.
Only five cases of gastric SEL complicated with abscess have been reported in the literature.
Suppurative gastritis is a rare disorder characterized by a purulent, exudative inflammatory process involving the submucosa that may extend to involve the entire gastric wall and may be divided into two categories: phlegmonous, or diffuse type; and localized, gastric abscess type.
This case report presents the clinical characteristics and treatment of gastric SEL complicated with abscess. We recommend that medical and surgical treatment should be considered according to response.
This is a well written case report concerning a diagnosis and possibility of surgical resection of gastric subepithelial lesion of the stomach presenting as an acute gastric abscess by a 45-year-old male patient.
P- Reviewer: Vorobjova T S- Editor: Ma YJ L- Editor: Kerr C E- Editor: Zhang DN
| 1. | Farman J, Dallemand S, Rosen Y. Gastric abscess--a complication of pancreatitis. Am J Dig Dis. 1974;19:751-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 2. | Hedenbro JL, Ekelund M, Wetterberg P. Endoscopic diagnosis of submucosal gastric lesions. The results after routine endoscopy. Surg Endosc. 1991;5:20-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 207] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 3. | Polkowski M. Endoscopic ultrasound and endoscopic ultrasound-guided fine-needle biopsy for the diagnosis of malignant submucosal tumors. Endoscopy. 2005;37:635-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 128] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 4. | Uhrenholt L, Stimpel H. [Abscess formation in ectopic pancreatic tissue in the stomach]. Ugeskr Laeger. 1984;146:883-884. [PubMed] |
| 5. | Kaneda M, Yano T, Yamamoto T, Suzuki T, Fujimori K, Itoh H, Mizumoto R. Ectopic pancreas in the stomach presenting as an inflammatory abdominal mass. Am J Gastroenterol. 1989;84:663-666. [PubMed] |
| 6. | Honda K, Mikami T, Ohkusa T, Takashimizu I, Fujiki K, Araki A, Shimoi K, Enomoto Y, Ariake K, Miyasaka N. Gastrointestinal autonomic nerve tumor with giant abscess. A case report and literature review. J Clin Gastroenterol. 1997;24:280-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Seidel RH, Burdick JS. Gastric leiomyosarcoma presenting as a gastric wall abscess. Am J Gastroenterol. 1998;93:2241-2244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Nozawa S, Bando T, Nagata T, Tsukada K. Abscess formation in a giant gastrointestinal stromal tumor of the stomach following endoscopic biopsy. Endoscopy. 2006;38:955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Osada T, Nagahara A, Kodani T, Namihisa A, Kawabe M, Yoshizawa T, Ohkusa T, Watanabe S. Gastrointestinal stromal tumor of the stomach with a giant abscess penetrating the gastric lumen. World J Gastroenterol. 2007;13:2385-2387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Miller AI, Smith B, Rogers AI. Phlegmonous gastritis. Gastroenterology. 1975;68:231-238. [PubMed] |
| 11. | Nevin NC, Eakins D, Clarke SD, Carson DJ. Acute phlegmonous gastritis. Br J Surg. 1969;56:268-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Starr A, Wilson JM. Phlegmonous gastritis. Ann Surg. 1957;145:88-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Smith GE. Subacute phlegmonous gastritis simulating intramural neoplasm: case report and review. Gastrointest Endosc. 1972;19:23-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Chahal P, Levy MJ. Potential Utility of EUS in the Diagnosis and Management of Intramural Gastric Abscess. Gastroenterol Hepatol (N Y). 2008;4:644-645. [PubMed] |
| 15. | Avilés JF, Fernández-Seara J, Bárcena R, Domíinguez F, Fernández C, Ledo L. Localized phlegmonous gastritis: endoscopic view. Endoscopy. 1988;20:38-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Zazzo JF, Troché G, Millat B, Aubert A, Bedossa P, Kéros L. Phlegmonous gastritis associated with HIV-1 seroconversion. Endoscopic and microscopic evolution. Dig Dis Sci. 1992;37:1454-1459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Stephenson SE, Yasrebi H, Rhatigan R, Woodward ER. Acute phlegmasia of the stomach. Am Surg. 1970;36:225-231. [PubMed] |
| 18. | Perkins GL, Slater ED, Sanders GK, Prichard JG. Serum tumor markers. Am Fam Physician. 2003;68:1075-1082. [PubMed] |
| 19. | Goonetilleke KS, Siriwardena AK. Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur J Surg Oncol. 2007;33:266-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 606] [Article Influence: 31.9] [Reference Citation Analysis (1)] |