INTRODUCTION
Schistosomiasis mansoni, a chronic parasitic disease, is the most prevalent tropical liver disease in northeastern Brazil. Approximately 200 million individuals are affected by Schistosoma mansoni (S. mansoni) worldwide, with 600 million exposed to the infection. Approximately 5%-7% of patients infected by S. mansoni progress to hepatosplenic schistosomiasis, which is the most severe form of the disease. These patients exhibit periportal fibrosis, portal hypertension, splenomegaly and cytopenia and have a risk of developing upper digestive tract bleeding[1-6].
Hypersplenism is a consequence of massive splenomegaly and commonly occurs in chronic liver diseases. In schistosomiasis, hypersplenism results from hyperplasia of the reticuloendothelial system and the subsequent venous congestion caused by portal hypertension. Studies have reported a correlation between increased splenic size and drops in blood cell count, mainly platelets. These findings depend on the severity of portal hypertension, as some studies have shown that thrombocytopenia is more common in hepatosplenic schistosomiasis, especially after episodes of digestive tract bleeding[7-9].
There are many treatment modalities for hypersplenism secondary to portal hypertension, including splenectomy and embolization of the splenic artery[10-12]. These treatments are effective in the prevention of bleeding and in the correction of thrombocytopenia, but they are associated with high morbidity[13,14], as portal vein thrombosis, pain, splenic abscess and even rupture can occur in cases of splenic embolization[14-17].
Splenectomy remains a popular choice for the treatment of patients with spleen diseases and hypersplenism. However, it has been shown that the preservation of at least 25% of the splenic parenchyma ensures the maintenance of organ function in the short and long term[18], thereby avoiding post-splenectomy infection[19,20] and reducing immunologic induction of thrombocytopenia[21]. Therefore, preservation of splenic function by less invasive therapies has gained a place in the current therapeutic context.
Thermal ablation represents an important technological advance, with radiofrequency ablation (RFA) being the most commonly used technique. RFA is a minimally invasive and well-accepted method used mainly in the treatment of solid tumors of various organs such as the kidneys, liver and lungs[22,23]. The literature on splenic RFA is sparse, and the main indication is local control of neoplasms, but this technique has also been used for the treatment of infected hydatid cysts, hypersplenism and hemostasis in trauma[15,22-27].
There are no standardized criteria for splenic RFA in patients with hypersplenism. In some studies, this minimally invasive treatment has been shown to be safe and less expensive than conventional therapies; however, more studies are needed to determine the effectiveness of splenic ablation in patients with this condition[15,18,23,28].
Here, we report a case of a patient with hypersplenism and severe thrombocytopenia secondary to schistosomiasis. To receive systemic chemotherapy, splenic RFA is indicated to treat the resulting thrombocytopenia, as every other therapeutic possibility was contraindicated.
CASE REPORT
A 60-year-old male patient with a diagnosis of hepatosplenic schistosomiasis since 1989 presented with portal hypertension, esophageal varices, persistent thrombocytopenia secondary to hypersplenism (between 37000 and 44000/mm³ platelets), and chest pain. A CT scan showed a mass in the mediastinum, and a biopsy led to the diagnosis of a moderately differentiated thymoma (type B3) (Figure 1). The patient also suffered from comorbidities (i.e., severe hypertension, dyslipidemia and diabetes), which were difficult to control. Chemotherapy was proposed to treat the patient´s thymoma; however, it was necessary to improve the thrombocytopenia before the treatment could commence.
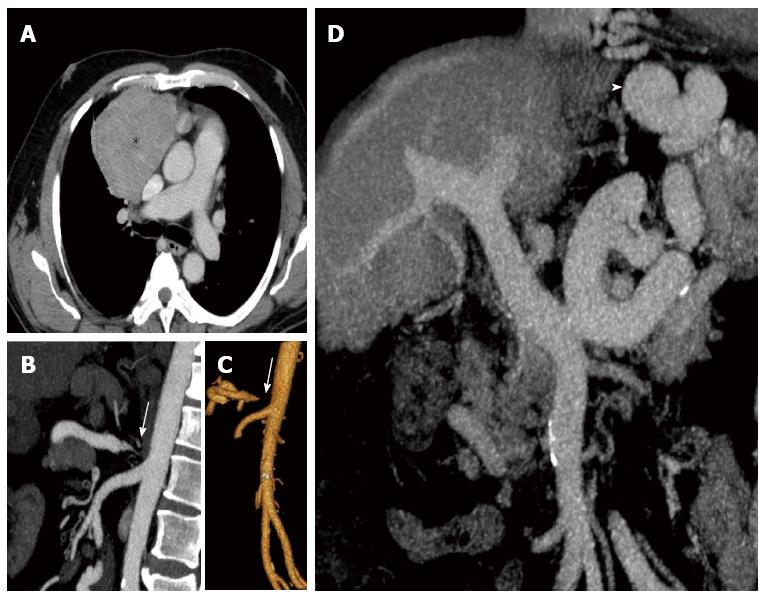
Figure 1 Axial, coronal and sagittal reconstructions of enhanced-computed tomography scan of the patient.
A: Shows the anterior mediastinal mass (Asterisk) compressing and displacing the adjacent structures (after biopsy, the patient was diagnosed with thymoma). To reverse the thrombocytopenia to initiate chemotherapy treatment, the first option was splenic artery embolization, but this was contraindicated due to severe stenosis of the celiac trunk [arrows in (B) and (C)]; D: Cirrhotic liver, and the large portal trunk caliber, all characteristics of severe portal hypertension secondary to schistosomiasis. Also of note, the large sized collateral circulation (arrowhead in D) common to this profile.
Because of the high morbidity associated with splenectomy, splenic artery embolization was the first choice of treatment. However, the CT scan showed a critical stenosis of the celiac trunk, which precluded the use of this procedure (Figure 1). Therefore, once informed consent was obtained from the patient, we chose to perform splenic RFA, which we present here as an alternative treatment option for hypersplenism.
The patient was positioned obliquely, with his right side down on the CT table, and the ablation was conducted under general anesthesia with prophylactic antibiotics. For treatment planning, a non-enhanced CT scan (Philips Brilliance 40) was acquired. Images in axial, sagittal, and coronal planes were reconstructed, and an experienced interventional radiologist chose the optimal puncture approaches and sequences. A single 3 cm internally cooled electrode (Cool-Tip; Covidien, Mansfield, Massachusetts) was percutaneously introduced into the spleen under CT fluoroscopic guidance and was repositioned multiple times to cover most of the parenchyma (mainly the middle and lower thirds, avoiding central and subcapsular regions) to ablation of more than 50% of the splenic parenchyma (Figure 2). Using an impedance control algorithm, RF energy was applied during internal cooling of the electrode, with a maximum power of 150 W for 6-12 min in each punctured site, for a total procedure time of 4 h.
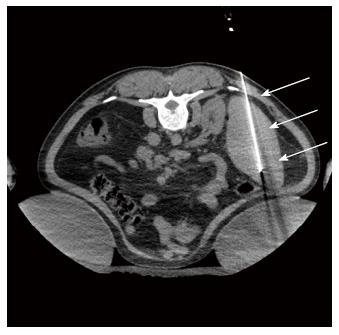
Figure 2 Axial non-enhanced computed tomography scan guiding the splenic radiofrequency ablation, with the patient in the prone position.
Note the “single” probe (arrow) in the middle/ lower third of the spleen. where the ablation zones were concentrated.
A contrast-enhanced CT scan confirmed that the ablated area was concentrated to the middle and lower thirds of the spleen and represented approximately 70% of the spleen parenchyma (initial volume of 1296.2 cc, leaving only 359.5 cc) (Figure 3).
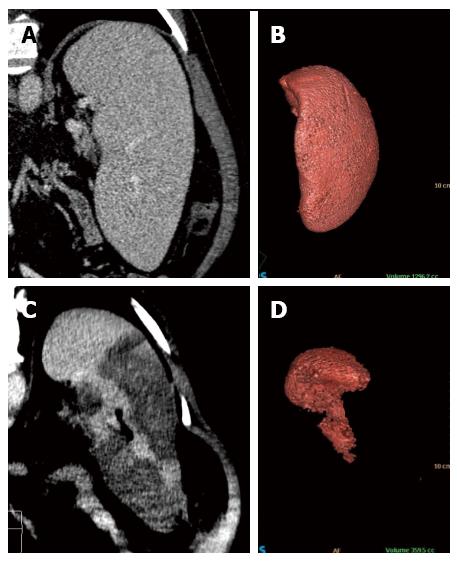
Figure 3 Coronal reformatted contrast-enhanced computed tomography and 3D volumetric reconstruction of the spleen before (A and B) and after (C and D) radiofrequency ablation.
Observe the homogeneous splenic parenchyma (A) showing a volume of 1296.2 cc (B) before treatment. After ablation, note the heterogeneity of the spleen (C), with multiple areas of coagulative necrosis mainly concentrated in the middle and lower thirds, saving the splenic hilum and its upper third. After ablation, the volumetric 3D reconstruction confirmed treatment success, leaving approximately 30% of viable parenchyma (volume of 359.5 cc) (D).
After the procedure, the patient developed mild hematuria associated with acute renal failure, which was characterized by moderate elevation of blood urea and creatinine. This complication was completely resolved after ten days of hospitalization, without the need for dialysis.
The platelet count increased to 225000/mm³ three weeks after the procedure. A post-procedure leukocytosis, which was most likely secondary to systemic inflammatory response syndrome (commonly observed in patients undergoing ablation of large volumes of tissue), also completely resolved after one week. Chemotherapy was started one month after ablation.
DISCUSSION
In the context of liver disease and portal hypertension, where an enlarged spleen often leads to thrombocytopenia, the need for chemotherapy can present special problems. Cytotoxic chemotherapeutic regimens often induce bone marrow suppression, which can also result in thrombocytopenia. When this condition occurs, many patients must stop their treatment because serious and potentially lethal side effects may occur due to severe leucopenia and thrombocytopenia[29].
Patients with thrombocytopenia who are offered a splenectomy must present good functional status, preferably with thrombocytopenia as the only factor limiting surgical treatment. By using these stringent preoperative criteria prior to splenectomy, perioperative morbidity and mortality can be minimized[29]. Splenectomy can eliminate hypersplenism, but morbidity ranges from 9.6% to 26.6%, when considering both laparoscopic and open approaches[30-33]. Perioperative complications include bleeding, atelectasis, portal vein thrombosis (range: 1.6% to 11%) and subphrenic abscess formation[15,28,34]. The major long-term risk after splenectomy is overwhelming sepsis (up to a 30-fold increase when compared to the normal population). Other complications are thrombophilia, pulmonary hypertension, and death[28,30,34].
Patients who are not good candidates for splenectomy may opt for splenic artery embolization, as this treatment can also potentially reverse hypersplenism-induced thrombocytopenia[35]. Embolization is considered to be the second line of treatment because it can lead to significant postoperative pain and splenic abscesses[36]. In a large series, Zhu et al[30] found that in partial splenic embolization, the splenic infarction rate should be limited to 50%-70% to ensure long-term efficacy in alleviating hypersplenism and thrombocytopenia, as assessed by good outcome at the 5-year follow-up. In their series, the most common complications were post-embolization syndrome, followed by transient pleural effusion and/or ascites, as well as small left-sided atelectasis[30]. Portal vein thrombosis[37], severe bacterial peritonitis[38] and splenic abscess resulting in death have also been reported[37,38].
Because of severe comorbidities, high surgical risk, significant stenosis of the celiac trunk, and the need to prevent splenic embolization (first and second lines of treatment), our patient underwent splenic ablation as a last resort to revert thrombocytopenia.
There are no standardized selection criteria for the use of splenic RFA to treat hypersplenism; therefore, the indication of this procedure is individualized and case-based. In several published studies, the indication was based on the individual authors’ experience, which included severe disease with persistent thrombocytopenia (between 15000 and 100000 platelets), persistent leucopenia, splenic volumes of less than 1500 mL, absence of esophageal varices and/or previous treatment of the varices, absence of portal vein or hepatic veins thrombosis, and prothrombin time less than 22 s[15,23,26,39].
The RFA ablation can be performed percutaneously, laparoscopically or with open surgery. The percutaneous approach is preferred because it is minimally invasive, results in a shorter recovery time, and allows for good visualization of the entire needle and the insertion site. Ultrasound or computed tomography may be used for guidance, and multiple regions of the spleen can be ablated or multiple needles can be used simultaneously, keeping in mind that the needle should be positioned at a certain distance from the splenic capsule and the vessels of the hilum. Although radiofrequency electrodes can be inserted into any portion of the spleen, the middle and lower thirds may be the best sites to avoid thermal damage to surrounding organs and reduce postoperative complications (particularly pleural effusion)[15,16,25,26,39]. Because of its superior resolution, we chose to use a percutaneous CT-guided approach, and we concentrated the ablation zones to the middle and lower thirds of the spleen, avoiding the central and upper portions, as previously described in the literature.
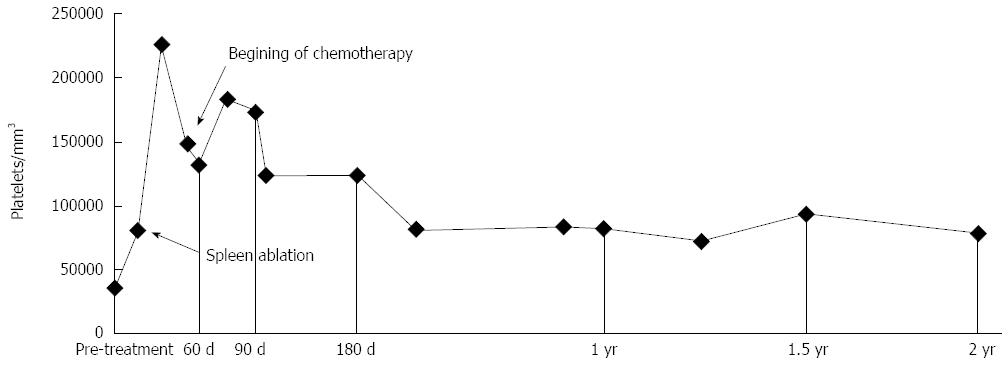
There is still no consensus regarding how much parenchyma should be ablated to ensure the resolution of thrombocytopenia while also avoiding complications. It has been shown that splenic ablation can lead to good outcomes in the short-term; however, the long-term results depend on the volume of ablated parenchyma[39]. Liang et al[39] divided patients into 3 groups based on the volume of ablation performed (less than 20%, 20%-40% and over 40%) and observed that larger ablation volumes (> 40%) yielded better clinical results, which was similar to the findings by Liu et al[25], who observed positive results after ablation of ≥ 40% of the spleen. Moreover, Feng et al[15] reported that patients with more than 50% of ablated parenchyma showed better clinical control than patients with smaller ablated volumes and that the results were even better when the ablation volume was greater than 70%. After 5 years of follow-up, these authors concluded that the ablation volume should ideally be between 50% and 70%. In the present case, resolution of thrombocytopenia in the short and medium terms was essential for the systemic treatment indicated for the patient. After ablation, the platelet count reached 225000 mm³ in 3 wk and remained between 80000 and 120000/mm³ at the 24-mo follow-up (Figure 4).
Figure 4 Graph above shows good recovery of platelet levels in a short period of time following the procedure, maintaining adequate levels during follow-up, and allowing chemotherapy to be conducted.
The procedure lasted 4 h, and only one single radiofrequency probe was used. Therefore, to achieve adequate ablation volumes with single electrodes, multiple overlapping sessions are required[40,41]; in clinical practice, repositioning electrodes is time consuming and even technically challenging. Several types of electrodes and even different techniques have been developed to overcome this limitation and achieve greater ablation zones, including clustered and multipolar electrodes[42,43], monopolar radiofrequency ablation with a multiple-electrode switching system[44,45] and even the use of microwave ablation[39,46]. Some studies have demonstrated that all of these techniques are promising and safe, sometimes allowing more energy delivery, with the advantage of being more efficient in creating a larger and confluent coagulation zone within a clinically acceptable and lower time frame than with conventional consecutive radiofrequency ablation, but with an increased cost.
The patient developed mild hematuria associated with acute renal failure after the procedure, which was characterized by moderate elevation of blood urea and creatinine and change in urine color. A literature search revealed that RFA can induce hemolysis in the experimental setting[47], which might be due to thermal injury of erythrocytes, leading to release of hemoglobin into the circulating blood[48], once blood hemolysis occurs when warmed up to 50 °C[49]. In addition, the procedure had an overall duration of 4 h, and patient positioning and prolonged immobilization are normally associated with rhabdomyolysis in surgical patients; unfortunately, no serum markers were measured in this case[50]. Nevertheless, there are no proven factors that can predict acute renal failure in cases such as this, but patients who require larger ablation volumes and longer operating times, and those with low-flow state should be closely monitored for this complication[50]. In conclusion, we believe that the most likely cause of transient acute renal failure in our case was hemolysis from prolonged and large volume radiofrequency ablation and that measures such as intravenous hydration and alkalinization of urine should be adopted to avoid it when indicated[50].
The most common complications described in the literature associated with splenic RFA are transient low-grade fever, symptomatic pleural effusion (some patients required thoracentesis) and mild abdominal and left shoulder pain[15,25,26,39]. Less common complications include mild transient hemoglobinuria and mild hematuria[25,39], skin bruises[25], portal vein thrombosis[39] and intra-abdominal hemorrhage[39]. Splenic rupture, refractory ascites, thermal injury to adjacent organs (including stomach, pancreas and colon), liver dysfunction and acute pancreatitis are other life-threatening complications that could potentially occur but have not been reported in the literature.
In summary, splenic ablation was the last-resort chosen to resolve this patient’s thrombocytopenia. Despite the occurrence of a major complication that was clinically monitored and no further observed consequences, in this case percutaneous splenic RFA was successful in managing hypersplenism thrombocytopenia in a safe, effective, and minimally invasive manner, which makes it a treatment option in patients who are not candidates for surgery or an endovascular intervention. Nevertheless, more studies are needed to determine the best technique for each case, taking into consideration the success and complication rates associated with each procedure.
COMMENTS
Case characteristics
This is a 60-year-old male patient with a diagnosis of hepatosplenic schistosomiasis and persistent thrombocytopenia, who presented with a moderately differentiated thymoma.
Clinical diagnosis
Dullness to percussion and pain on palpation of the left hypochondrium, associated with an increased spleen.
Differential diagnosis
Hypersplenism, abdominal tumor in the left hypochondrium.
Laboratory diagnosis
WBC 1.81 k/uL; HGB 14.2 gm/dL; Platelets: between 37000 and 44000/mm³; metabolic panel and liver function test were within normal limits.
Imaging diagnosis
Thoracic CT scan showed an anterior mediastinal mass measuring approximately 9.2 cm × 7.3 cm. Abdominal imaging showed signs of chronic liver disease associated with portal hypertension and an estimated spleen volume of 1296.2 cc.
Pathological diagnosis
Biopsy revealed a moderately differentiated thymoma (type B3). Hypersplenism had no pathological diagnosis.
Treatment
The patient was treated for hypersplenism with radiofrequency ablation (initial volume of 1296.2 cc, leaving only 359.5 cc) to revert thrombocytopenia.
Related reports
Chemotherapy was proposed to treat the patient´s thymoma; however, it was necessary to revert thrombocytopenia to initiate the treatment, as cytotoxic chemotherapeutic regimens often induce bone marrow suppression, and serious and potentially lethal side effects may occur due to severe leucopenia and thrombocytopenia.
Term explanation
Thermal ablation showed great progress with new technological advances, and radiofrequency ablation (RFA) is the most used among our group and has attracted much attention because it is minimally invasive and well accepted. RFA is based on the principle of coagulative necrosis of tumors at temperatures above 50 °C, generating a tissue dehydration and protein denaturation.
Experiences and lessons
This case report presents radiofrequency ablation as an alternative treatment for hypersplenism, proving to be not only a safe procedure, but also effective in controlling thrombocytopenia, which makes it a viable option especially when surgery or an endovascular intervention are contraindicated.
Peer-review
The authors have described one case of thrombocytopenia secondary to hypersplenism that needed to be reverted to initiate chemotherapy for the patient. The article highlights RFA as an alternative option for treating hypersplenism and provides some technical aspects and clinical outcome after spleen radiofrequency ablation.