Published online May 28, 2015. doi: 10.3748/wjg.v21.i20.6384
Peer-review started: December 9, 2014
First decision: January 8, 2015
Revised: February 7, 2015
Accepted: March 18, 2015
Article in press: March 19, 2015
Published online: May 28, 2015
Processing time: 172 Days and 18.3 Hours
Patients with metastasized carcinoma of the pancreas have a very poor prognosis, and long-term survival cannot be expected. This case report describes two patients with an initial diagnosis of metastatic pancreatic cancer, both with hepatic metastases and one with an additional peritoneal carcinomatosis. Initially, both patients were treated intravenously with the FOLFIRINOX chemotherapy regimen, consisting of 5-FU, folinic acid, irinotecan and oxaliplatin. Surprisingly, the FOLFIRINOX treatment resulted in complete resolution of the hepatic metastases in both patients, with no lesions detectable by computed tomography scan. Furthermore, treatment response included decreased diameter of the primary tumor in the tail of the pancreas and disappearance of the additional peritoneal carcinomatosis. Both patients were discussed by our multidisciplinary tumor board, which recommended surgical resections of the carcinoma. The R0 resection of the primary tumor was successful in both cases and, interestingly, the resected tissues showed no evidence of the hepatic metastases intraoperatively. In the first case, the patient received a postoperative 6-mo course of adjuvant chemotherapy with gemcitabine. In the second case, the patient continued to receive the FOLFIRINOX regimen for an additional 6 mo postoperatively. At 12 mo after the operation, a nonresectable retroperitoneal lymph node metastasis was detected in the first patient, whereas the second patient remained in complete remission at the time of this report (5 mo after the adjuvant therapy was discontinued). This case report is the first of its kind to describe two cases of hepatic metastatic pancreatic carcinoma that were resectable following treatment with FOLFIRINOX. Further studies are required to examine the role of FOLFIRINOX as a neoadjuvant treatment option in subgroups of patients with initially metastasized pancreatic carcinoma.
Core tip: The FOLFIRINOX regimen is a promising treatment option for metastatic pancreatic carcinoma. This case report describes two cases in which treating metastatic pancreatic carcinoma with neoadjuvant FOLFIRINOX therapy prior to R0 resection was possible. The positive outcomes for these patients provide hope that metastatic pancreatic neoplasms may be cured in certain cases.
- Citation: Schneitler S, Kröpil P, Riemer J, Antoch G, Knoefel WT, Häussinger D, Graf D. Metastasized pancreatic carcinoma with neoadjuvant FOLFIRINOX therapy and R0 resection. World J Gastroenterol 2015; 21(20): 6384-6390
- URL: https://www.wjgnet.com/1007-9327/full/v21/i20/6384.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i20.6384
It is generally accepted that the prognosis of pancreatic cancer is very poor, with 5-year survival rates of 8%[1]. Since the disease onset manifests few symptoms, diagnosis of pancreatic carcinomas is frequently made after the cancer has already metastasized. Currently, the only curative option is radical surgery for patients with non-metastatic and locally resectable lesions. However, only 10% to 20% of the patients who undergo surgical intervention are cured[2]. In the palliative setting, gemcitabine (as a monotherapy or in combination with nab-paclitaxel or erlotinib) is available as a treatment option[3-6]. In 2011, a new and effective form of chemotherapy was described for treating metastatic pancreatic cancer patients; this chemotherapy regimen, FOLFIRINOX (consisting of 5-FU/folinic acid, irinotecan and oxaliplatin), was reported to achieve an average life extension of 11.1 mo compared to the extension of 6.8 mo that was achieved using gemcitabine monotherapy[7]. Since then, several studies have reported interesting findings from neoadjuvant treatment with FOLFIRINOX in patients with borderline resectable pancreatic cancer[2,8,9]. In terms of locally advanced pancreatic cancers, neoadjuvant therapy also provides an opportunity for resection[8]. This report describes two patients with metastatic pancreatic neoplasms that became secondarily resectable following treatment with FOLFIRINOX.
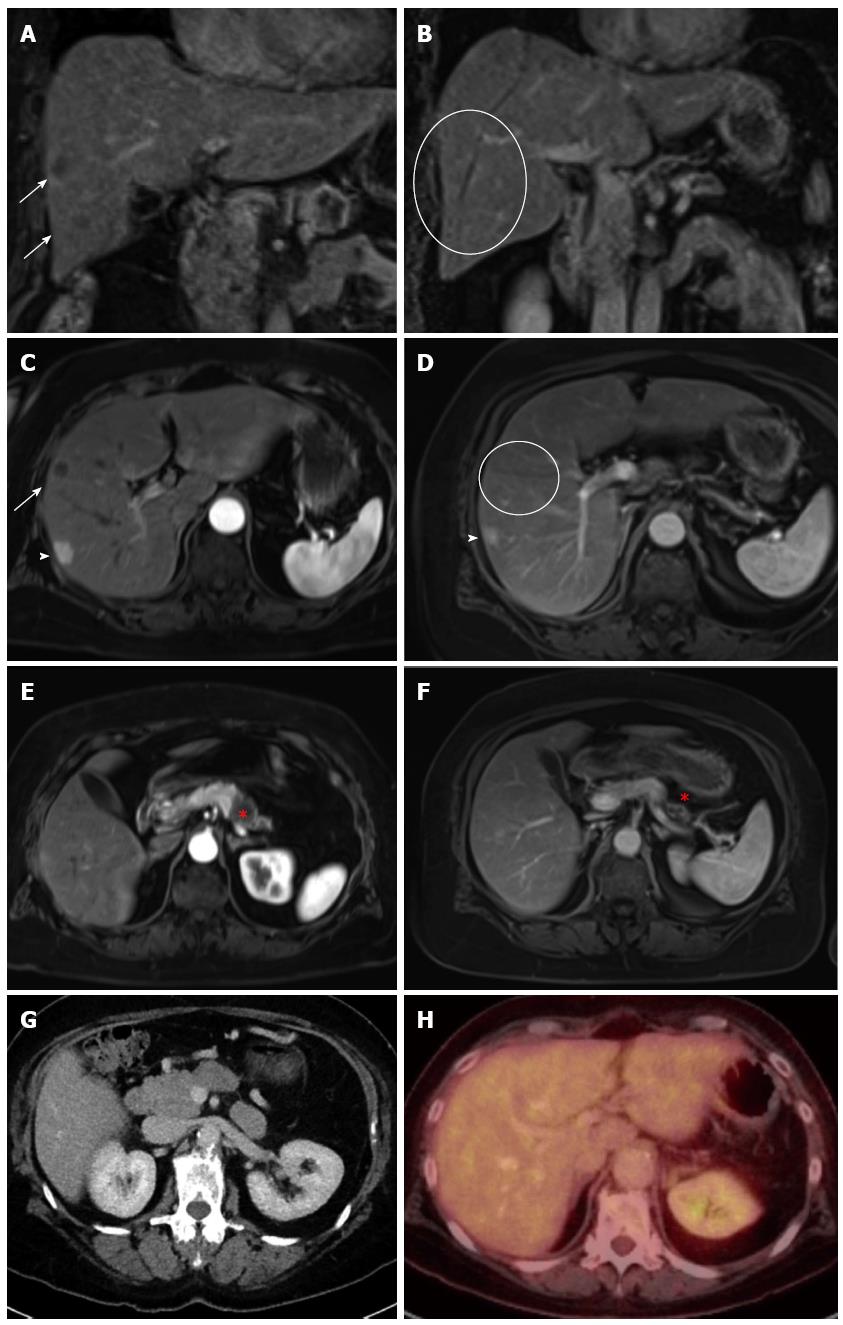
A routine check-up by ultrasound detected suspicious lesions in the liver of a 65-year-old Caucasian female, whose medical history included a case of pneumonia in 1985, as well as cervical cancer and subsequent hysterectomy in 1994, high blood pressure, hip arthrosis and the effects of chronic nicotine abuse. The patient was entirely symptom-free and had an unremarkable clinical examination. The patient had been taking medication to treat high blood pressure. Subsequent computed tomography (CT)-, magnetic resonance imaging (MRI)-scans and an endoscopic ultrasound (Figure 1A, C, E) performed in August of 2012 indicated a carcinoma in the tail of the pancreas with metastasis to the liver. Liver biopsies confirmed the suspected diagnosis and showed liver metastases of a ductal pancreatic adenocarcinoma (G2). Tumor histological findings included a positive dye reaction to antibodies against cytoceratin 7, MUC1 (indicting partial coexpression) and cytoceratin 20 (indicating coexpression in individual cells). Furthermore, there was a negative dye reaction with antibodies against CDX2, CA19-9 and TTF1. While there was no observed elevated level of the CA19-9 biomarker for pancreatic neoplasms, the carcinoembryonic antigen (CEA) tumor marker was slightly elevated (4.7 μg/L; normal: < 3.4 μg/L). The patient was treated with FOLFIRINOX (85 mg/m2 of oxaliplatin, 180 mg/m2 of irinotecan, 400 mg/m2 of folinic acid, 400 mg/m2 bolus of 5-FU, with 2400 mg/m2 > 46 h of 5-FU; 5 cycles of 100% dose) from October 2012 to January 2013.
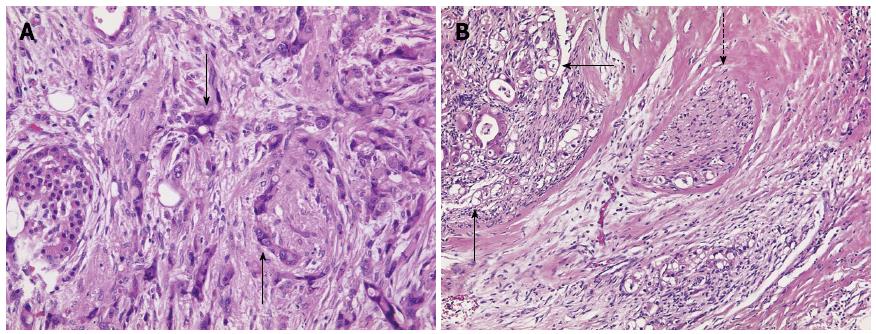
Subsequent to this treatment, the CT scan showed that the pancreatic carcinoma had decreased in size (Figure 1F) and no signs of liver metastases (Figure 1B and D). The multidisciplinary tumor board recommended resection of the tail of the pancreas as an individual therapeutic strategy. The surgery was performed in February of 2013 and included a resection of the tail of the pancreas as well as of the spleen, a radical dissection of the lymph nodes, a gall bladder resection, and a non-anatomical resection of liver segments II, VI and VII (1.4 cm × 1.2 cm × 0.9 cm, 2.7 cm × 1.8 cm × 1.3 cm, 3.8 cm × 4.4 cm × 2.3 cm). The pathological stage was ypT3 ypN0 (0/16), L0, V0, Pn1 G2 R0 M0 (Figure 2A). The resected liver tissue showed scars and fibrosis without any evidence of persistent cancer cells. Adjuvant treatment with gemcitabine was administered from April to October, 2013. The patient was cancer-free in subsequent check-ups with CT, the last of which was performed in November of 2013 (Figure 1G and H). The patient returned in April of 2014, due to a complaint of pain in her lower left side. CT scan showed metastases in a retroperitoneal lymph node and muscles of the back. The FOLFIRINOX chemotherapy regimen was resumed in May of 2014. The most recent CT scan (performed in August of 2014) showed partial remission of the retroperitoneal lymph node metastasis. No liver metastases have been detected since the initial diagnosis in January of 2013 (Table 1).
| Course | Patient 1 | Patient 2 |
| First diagnosis | 08/2012 | 01/2013 |
| Start of neoadjuvant therapy | 10/2012 | 02/2013 |
| Length of neoadjuvant therapy | 3 mo | 6 mo |
| Operation | 02/2013 | 09/2013 |
| Start of adjuvant therapy | 04/2013 | 11/2013 |
| Length of adjuvant therapy | 6 mo | 6 mo |
| Relapse | 04/2014 | - |
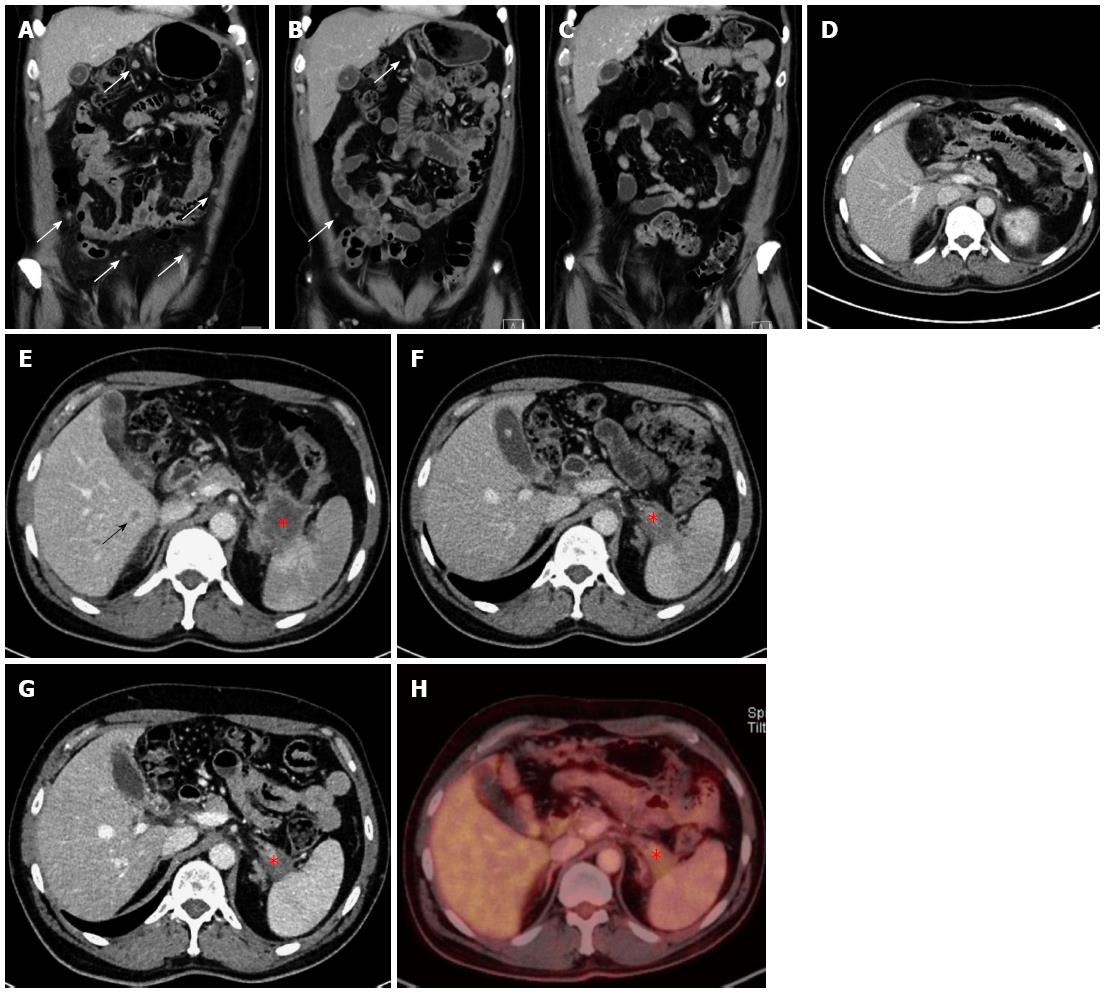
A 45-year-old Caucasian male was admitted to the hospital via the emergency room in January of 2013. The patient’s symptoms included increasing dyspnea, nausea and abdominal pain, and chronic pain in the left shoulder. The patient had been diagnosed with non-Hodgkin lymphoma in 1996, which had been resolved by treatment with rituximab and the standard cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP) regimen. Epigastric pain was the only pathological clinical finding during the clinical exam. The patient reported unintentional weight loss (2 kg) within the last 2 mo, during which time he had been taking the following standard medications: Tilidin/Naloxon (at 100 mg/8 mg; extended release tablet 1-0-1), ibuprofen (at 600 mg; 1-0-1), pantoprazol (at 40 mg; 0-0-1), metamizol as needed (at 4 × 40°) and bromazepam as needed daily (1/4 of a 3 mg tablet). The patient presented with elevated levels of C-reactive protein (CRP: 2.3 mg/dL; normal: < 0.5 mg/dL), gamma-glutamyl transferase (99 U/L; normal: < 55 U/L), β2-microglobulin (2.01 mg/L; normal: 0.8-1.8 mg/L), CEA (3.8 μg/L; normal: < 3.4 μg/L), and D-dimer (1.61 mg/dL; normal: < 0.50 mg/L). However, the CA19-9 marker level was within normal range (16.9 U/mL; normal: < 27 U/mL). A chest CT ruled out pulmonary embolism, but showed an area in the tail of the pancreas with possibility as a neoplasm, as well as positive lymph nodes and suspicious peritoneal areas. Subsequent ultrasound showed a tumor in the tail of the pancreas that was in contact with the splenic artery. An abdominal CT confirmed the presence of a 4.3 cm × 5.4 cm neoplasm in the tail of the pancreas with infiltration of the spleen (Figure 3F and 3G), as well as multiple hepatic (Figure 3E), nodal and peritoneal metastases (Figure 3A and B). An endoscopic ultrasound indicated the stage was T4, N1, Mx. Ultimately, fine needle biopsy of the lesion via oral endoscopic ultrasound led to the diagnosis of a ductal pancreatic adenocarcinoma (G2) in the tail of the pancreas. Immunohistochemistry analysis of the biopsied tissue showed a significant positive reaction to cytokeratin 7 antibodies, as well as a positive focal reaction to CA19-9 antibodies. Immunoreactivity to MIB1 antibodies showed expression in approximately 50% of the tumor tissue, and immunoreactivity to p53 antibodies showed moderate to strong expression throughout. In February of 2013, the patient was started on the FOLFIRINOX regimen (85 mg/m2 of oxaliplatin, 180 mg/m2 of irinotecan, 400 mg/m2 of folinic acid, 400 mg/m2 bolus of 5-FU, and 2400 mg/m2 > 46 h of 5-FU; 6 cycles of 100% dose). The patient suffered from complications during chemotherapy, including pneumonia in the left lung and a deep vein thrombosis with pulmonary embolism.
After 3 mo of the chemotherapy treatment, a decline in tumor growth was observed and the decision to continue treatment with FOLFIRINOX was made (6 cycles of 75%-100% dose). A subsequent CT scan performed in August of 2013 showed neither liver nor peritoneal metastases (Figure 3C), and these findings were confirmed by positron emission tomography (PET)-CT (Figure 3G and H). In September of 2013, the patient underwent surgery with a multivisceral resection of the tail and body of the pancreas, an adrenal gland resection on the left side, a resection of the spleen, a large bowel resection on the left side with transverso-descendostomy, a resection of the cranial part of the kidney on the left side, a gall bladder resection, an atypical liver resection segment V (1.7 cm × 1.5 cm × 0.7 cm), an aortic lymph nodal dissection and a celiac lymph node dissection. Postoperatively, the patient’s stage was ypT3, ypN0 (0/10), V1, L0, Pn0, R0 and Dworak TRG 3 (Figure 2B). As there were no previous cases for adjuvant treatment involving initial metastatic pancreatic carcinoma and an initially advanced metastatic stage, the decision was made in November of 2013 to continue treatment with FOLFIRINOX. The chemotherapy regimen, however, was delivered at 75% of the full dose to address the patient’s frequent presentation of leukopenia in the adjuvant phase. The FOLFIRINOX treatment was discontinued in May of 2014 (Table 1), and the last CT scan (performed in June of 2014) showed no metastatic lesions (Figure 3D).
The prognosis of carcinoma of the pancreas is very poor, especially when metastasis has occurred. One-year survival rates of patients with metastatic pancreatic carcinoma treated with gemcitabine are between 18% and 20%[3]. In recent years, new treatment options, such as FOLFIRINOX[7,10] or gemcitabine/nab-paclitaxel, have been introduced and have provided life-extending benefits for patients with metastasized pancreatic carcinomas[4]. The FOLFIRINOX chemotherapy regimen extends life by an average of 11.1 mo and prolongs progression-free survival rate to 6.4 mo longer than the gemcitabine therapy[7].
This report describes two patients with hepatic metastases who achieved complete remission following treatment with FOLFIRINOX and a subsequent R0 resection of the primary tumors in the tail of the pancreas. For the first case, the patient is still alive 26 mo after the initial diagnosis; although the patient developed a new retroperitoneal lymph node metastasis at 1 year after the surgical resection. For the second case, the patient was still in complete remission at the time of this report (22 mo after the initial diagnosis). Surgical resection may have provided a complete cure, but this conclusion is subject to the findings that will come from future clinical follow-ups. Interestingly, to date neither patient has shown recurrent hepatic metastases, though hepatic resections have not been taken.
A previously published case reported similar findings for a patient with pancreatic neoplasia and pathological findings in the lymph nodes near the superior mesenteric artery, as well as a paraaortal lesion[11]. Among the few studies on borderline resectable or nonresectable locally advanced carcinomas in response to neoadjuvant chemotherapy or radiochemotherapy that have been described in the literature[2,8,9,12], none investigated the potential resectability for cases of pancreatic carcinomas diagnosed with hepatic metastases.
Both of the cases described herein involved carcinomas in the tail of the pancreas. A study by Lorgis et al[13] examined the influence of tumor localization, as it relates to the effectiveness of chemotherapy, and found that carcinomas in the head of the pancreas are not as responsive to FOLFIRINOX treatment as carcinomas in other locations. In terms of the embryonic development of the pancreas, it is well known that development can be divergent between the head, body and tail, arguably resulting in varying malignant potential among the various kinds of cells present in each section[14]. Therefore, varying responses to chemotherapy regimens may be expected depending on the location of the carcinoma within the pancreas. Independent of this feature, Lau et al[15] analyzed data from the Surveillance, Epidemiology and End RESULTS Program (SEER) of the National Cancer Institute in the United States and found that the incidence rates of pancreatic cancer in the body and tail of the pancreas are increasing, while the incidence rate of pancreatic cancer in the head of the pancreas has remained stable over time. Such a trend may lead to larger incidences of metastasizing carcinomas in the tail of the pancreas, which may respond positively to chemotherapy in a neoadjuvant setting.
In summary, both patients described in this case report may be representative of a wider subgroup of pancreatic carcinoma patients with a better prognosis. Further studies must be undertaken to analyze the impact of tumor localization, biomarkers and the stage and progression of this disease, and to determine whether FOLFIRINOX as a neoadjuvant therapy with subsequent resection is a suitable treatment option for all patients within this subset.
A symptom-free 65-year-old patient with suspected hepatic lesions (Case 1) and a 45-year-old male patient with increasing dyspnea, stomach pain and shoulder pain (Case 2).
The clinical examination was unremarkable for Case 1. Case 2 presented with pathological epigastric pain.
There were no differential diagnoses pertaining to the unremarkable clinical examination for Case 1, who presented with no symptoms. For Case 2, the differential diagnoses were pulmonary embolism, atypical pneumonia, and lymphoma.
For Case 1, the level of CA19-9 was unremarkable, but the level of carcinoembryonic antigen (CEA) was elevated (4.7 μg/L). Case 2 showed elevated levels of C-reactive protein (CRP; 2.3 mg/dL), gamma-glutamyl transferase (99 U/L), CEA (3.8 μg/L) and D-dimer (1.61 mg/L), but the level of CA19-9 was unremarkable.
For Case 1, computed tomography (CT), endoscopic ultrasound (EUS), magnetic resonance imaging (MRI) and contrast-enhanced ultrasound were performed. The findings showed distension (2 cm) in the tail of the pancreas, enlargement of the distal pancreatic duct, three suspected metastatic lesions in segments V and VI, and three hepatic hemangiomas. For Case 2, the CT, EUS and contrast-enhanced ultrasound studies showed an invasive, growing carcinoma in the tail of the pancreas, with a size of approximately 45 mm × 34 mm and with hepatic metastases (8-11 mm in size) and peritoneal metastases.
For Case 1, liver biopsy showed moderately differentiated ductal adenocarcinoma (G2). For Case 2, the EUS-guided fine needle biopsy showed moderately differentiated ductal adenocarcinoma (G2).
Case 1 was given neoadjuvant treatment with FOLFIRINOX (85 mg/m2 of oxaliplatin, 180 mg/m2 of irinotecan, 400 mg/m2 of folinic acid, a 400 mg/m2 bolus of 5-FU and 2400 mg/m2 > 46 h of 5-FU) followed by resection surgery with subsequent adjuvant treatment with gemcitabine (1000 mg/m2). The FOLFIRINOX treatment continued after recurrence. Case 2 was given neoadjuvant treatment with FOLFIRINOX and subsequent multivisceral resection that was followed by adjuvant treatment with FOLFIRINOX.
This case report highlights the consideration of whether a subgroup of initially metastasized patients with pancreatic carcinoma may be treated effectively with chemotherapy in a neoadjuvant setting in order to achieve secondary resectability.
Pancreatic neoplasms have very poor prognosis since the disease onset is frequently asymptomatic, and diagnosis usually occurs after metastasis. FOLFIRINOX (5-FU, folinic acid, irinotecan and oxaliplatin) is a new and promising chemotherapy regimen that has been shown to increase life expectancy in patients with metastatic pancreatic neoplasms. Neoadjuvant treatments are administered with the aim of shrinking a tumor prior to administration of the primary treatment. Curative surgery involves the radical removal of a partial or entire organ in which a cancer has originated.
This case report indicates that the treatment of pancreatic carcinomas may be at a turning point following decades of poor prognoses for patients. New treatment options have resulted in new clinical outcomes that could result in improved prognoses.
The authors reported two cases of hepatic metastasized pancreatic carcinoma, both of which were resectable after neoadjuvant treatment with FOLFIRINOX. This finding emphasizes the importance of considering whether carcinomas in the tail of the pancreas differ from those with other localizations in the pancreas, especially in regards to their response to FOLFIRINOX.
P- Reviewer: Hsieh CC, Li SD, Makisalo H, Wagener G, Wang DS, Zhang ZM S- Editor: Ma YJ L- Editor: A E- Editor: Ma S
| 1. | Gesellschaft der epidemiologischen Krebsregister in Deutschland e. V., RKI, Gesundheitsberichterstattung des Bundes, Berlin: Robert Koch Institute. Available from: http://www.krebsdaten.de/Krebs/DE/Content/Krebsarten/Bauchspeicheldruesenkrebs/bauchspeicheldruesenkrebs_node.html vom 31.08.2014. |
| 2. | Hosein PJ, Macintyre J, Kawamura C, Maldonado JC, Ernani V, Loaiza-Bonilla A, Narayanan G, Ribeiro A, Portelance L, Merchan JR. A retrospective study of neoadjuvant FOLFIRINOX in unresectable or borderline-resectable locally advanced pancreatic adenocarcinoma. BMC Cancer. 2012;12:199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 174] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 3. | Sultana A, Smith CT, Cunningham D, Starling N, Neoptolemos JP, Ghaneh P. Meta-analyses of chemotherapy for locally advanced and metastatic pancreatic cancer. J Clin Oncol. 2007;25:2607-2615. [PubMed] |
| 4. | Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4035] [Cited by in RCA: 4876] [Article Influence: 406.3] [Reference Citation Analysis (0)] |
| 5. | Sun C, Ansari D, Andersson R, Wu DQ. Does gemcitabine-based combination therapy improve the prognosis of unresectable pancreatic cancer? World J Gastroenterol. 2012;18:4944-4958. [PubMed] |
| 6. | Hu J, Zhao G, Wang HX, Tang L, Xu YC, Ma Y, Zhang FC. A meta-analysis of gemcitabine containing chemotherapy for locally advanced and metastatic pancreatic adenocarcinoma. J Hematol Oncol. 2011;4:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4838] [Cited by in RCA: 5628] [Article Influence: 402.0] [Reference Citation Analysis (1)] |
| 8. | Heinemann V, Haas M, Boeck S. Neoadjuvant treatment of borderline resectable and non-resectable pancreatic cancer. Ann Oncol. 2013;24:2484-2492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 9. | Marthey L, Sa-Cunha A, Blanc JF, Gauthier M, Cueff A, Francois E, Trouilloud I, Malka D, Bachet JB, Coriat R. FOLFIRINOX for locally advanced pancreatic adenocarcinoma: results of an AGEO multicenter prospective observational cohort. Ann Surg Oncol. 2015;22:295-301. [PubMed] |
| 10. | Conroy T, Gavoille C, Samalin E, Ychou M, Ducreux M. The role of the FOLFIRINOX regimen for advanced pancreatic cancer. Curr Oncol Rep. 2013;15:182-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 11. | Sasaki T, Isayama H, Aoki T, Tanaka M, Hamada T, Nakai Y, Sakamoto Y, Hasegawa K, Morikawa T, Fukayama M. A R0 resection case of initially unresectable metastatic pancreatic cancer downstaged by FOLFIRINOX therapy. Pancreas. 2014;43:972-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Satoi S, Yamaue H, Kato K, Takahashi S, Hirono S, Takeda S, Eguchi H, Sho M, Wada K, Shinchi H. Role of adjuvant surgery for patients with initially unresectable pancreatic cancer with a long-term favorable response to non-surgical anti-cancer treatments: results of a project study for pancreatic surgery by the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci. 2013;20:590-600. [PubMed] |
| 13. | Lorgis V, Chauffert B, Gentil J, Ghiringhelli F. Influcence of localization of primary tumor on effectiveness of 5-fluorouracil/leucovorin combined with irinotecan and oxaliplatin (FOLFIRINOX) in patients with metastatic pancreatic adenocarcinoma: a retrospective study. Anticancer Res. 2012;32:4125-4130. [PubMed] |
| 14. | Ling Q, Xu X, Zheng SS, Kalthoff H. The diversity between pancreatic head and body/tail cancers: clinical parameters and in vitro models. Hepatobiliary Pancreat Dis Int. 2013;12:480-487. [PubMed] |
| 15. | Lau MK, Davila JA, Shaib YH. Incidence and survival of pancreatic head and body and tail cancers: a population-based study in the United States. Pancreas. 2010;39:458-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 135] [Article Influence: 9.0] [Reference Citation Analysis (0)] |