Published online May 28, 2015. doi: 10.3748/wjg.v21.i20.6374
Peer-review started: October 17, 2014
First decision: October 29, 2014
Revised: November 26, 2014
Accepted: December 19, 2014
Article in press: January 5, 2015
Published online: May 28, 2015
Processing time: 232 Days and 4.5 Hours
AIM: To investigate the diagnostic ability of single-shot echo-planar imaging (EPI) diffusion-weighted imaging (DWI) to differentiate between malignant and benign pancreatic lesions.
METHODS: A computerized search was performed on PubMed, MEDLINE and EMBASE up to August 2014. Nine studies (10 sets of data) with a total of 304 malignant pancreatic lesions and 188 benign pancreatic lesions were included. The characteristics of each study included the study name, year of publication, magnetic resonance modalities used, patient population, strength of field, pulse time, repetition time, echo time (TE), maximum b factor, mean age, mean body weight, fat suppression, number of benign and malignant lesions, and true positive, true negative, false positive and false negative results. All analyses were performed using Meta-DiSc and Stata 11.0.
RESULTS: The pooled sensitivity and specificity of single-shot EPI DWI were 0.83 (95%CI: 0.79-0.87) and 0.77 (95%CI: 0.70-0.83), respectively. The positive likelihood ratio and negative likelihood ratio were 5.09 (95%CI: 2.19-11.84) and 0.23 (95%CI: 0.15-0.36), respectively. The P value for the χ2 heterogeneity for all pooled estimates was < 0.05. From the fitted summary receiver operating characteristic curve, the area under the curve and Q* index were 0.89 and 0.82, respectively. Publication bias was not present (t = 0.58, P = 0.58). Meta-regression analysis indicated that fat suppression, mean age, TE, and maximum b factor were not sources of heterogeneity (all P > 0.05).
CONCLUSION: Single-shot EPI DWI is useful to differentiate between malignant and benign pancreatic lesions. Lesion size ≥ 2 cm is the limit for the diagnosis of early lesions.
Core tip: We performed a meta-analysis to investigate the diagnostic capability of single-shot echo-planar imaging (EPI) diffusion-weighted imaging (DWI) to differentiate between malignant and benign pancreatic lesions. Single-shot EPI DWI was useful to differentiate between malignant and benign pancreatic lesions. Lesion size ≥ 2 cm was the limit for the diagnosis of early lesions.
- Citation: Hong BZ, Li XF, Lin JQ. Differential diagnosis of pancreatic cancer by single-shot echo-planar imaging diffusion-weighted imaging. World J Gastroenterol 2015; 21(20): 6374-6380
- URL: https://www.wjgnet.com/1007-9327/full/v21/i20/6374.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i20.6374
Pancreatic ductal adenocarcinoma accounts for 85%-90% of all solid pancreatic tumors and is the fourth leading cause of cancer-related death[1]. In 2014, it is estimated that there will be 46420 new cases of pancreatic cancer and an estimated 39590 people will die from this disease (http://seer.cancer.gov/). The only chance for a cure in pancreatic adenocarcinoma is surgery. Most of the time, by the time the tumor presents, it is already invasive. The 5-year survival rate of patients with pancreatic adenocarcinoma is dismal, being < 10%. At initial diagnosis, fewer than 10% of patients can undergo surgical resection, which is the only potential curative treatment[2]. Hence, early detection and characterization, followed by appropriate treatment, are currently the most effective strategies to reduce pancreatic cancer mortality[1,3-5].
Diffusion-weighted (DW) imaging is a magnetic resonance imaging technique that provides unique information related to the diffusion of water molecules in the tissue, and allows estimation of cellularity and tissue structure[6]. Recent reports have shown that the apparent diffusion coefficient (ADC) can be used to detect and characterize malignant and benign pancreatic lesions. Pancreatic cancer tissue has a significantly lower ADC value than that of normal pancreatic tissue, mass-forming focal pancreatitis, and autoimmune pancreatitis[7,8]. DWI of the upper abdomen is a technical challenge because of artifacts secondary to heart and bowel motion, and field inhomogeneity related to parenchyma-gas interfaces.
With EPI, the information in the k-space can be acquired in a single shot. The advantage of using single-shot EPI as a readout sequence is that only one excitation is necessary, and hence the DW images become less sensitive to subject motion[9]. The implementation of ultrafast imaging of single-shot EPI has made DWI of the upper abdomen a feasible option, and is useful to differentiate malignant from benign liver lesions[10-13]. The diagnostic ability of single-shot EPI DWI for the pancreas has not yet been defined. In the present study, we performed a meta-analysis to evaluate the diagnostic ability of single-shot EPI DWI to differentiate between malignant and benign pancreatic lesions.
We performed a search of PubMed, MEDLINE and EMBASE up to August 2014. The following search terms were used: “pancreatic and diffusion-weighted imaging”, “pancreatic and diffusion weighted imaging”, “pancreatic and diffusion”, “pancreas and diffusion-weighted imaging”, “pancreas and diffusion weighted imaging”, and “pancreas and diffusion”. The search was limited to English language studies only.
Studies were included in this analysis if: (1) single-shot EPI DWI data were obtained using either a 1.5 or 3.0 T MR scanner; (2) applied field strength was 1.5 or 3 T to represent common technical standards used for clinical pancreatic imaging; (3) the diagnostic criteria of the malignant or benign pancreatic lesions were clearly stated; and (4) data were available to fill out cross-tabs to assess true-positive (TP), true-negative (TN), false-positive (FP) and false-negative (FN) cases.
The characteristics of each study, including study name, year of publication, MR modalities used, patient population, strength of field, pulse time, repetition time (TR), echo time (TE), maximum b factor, mean age, mean body weight, fat suppression, number of benign and malignant lesions, and TP, TN, FP and FN results, are shown in Tables 1 and 2.
| No. | Ref. | Year of publication | Patient population | MRI unit | Field (T) | TR (ms) | TE (ms) | Max b factor (s/mm2) | Mean age (yr) | FS | Mean size (cm) |
| 1 | Muhi et al[21] | 2011 | Japanese | GE | 1.5 | 8000-10000 | 73 | 1000 | 66.1 | Yes | 2.6 |
| 2 | Lemke et al[20] | 2009 | Germany | Siemens | 1.5 | 1300 | 60 | 800 | 65.1 | Yes | 2.8 |
| 3 | Sandrasegaran et al[22] | 2011 | American | Siemens | 1.5 | 1500 | 71 | 800 | 68.0 | Yes | 2.0 |
| 4 | Sandrasegaran et al[23] | 2013 | American | Siemens | 1.5 | 1500 | 71 | 800 | 66.2 | Yes | 3.6 |
| 5 | Kartalis et al[17] | 2009 | Sweden | Siemens | 1.5 | 4600 | 77 | 500 | NA | Yes | NA |
| 6 | Lee et al[19] | 2008 | Korean | Siemens | 1.5 | 2100 | 72 | 1000 | 57.4 | NA | 3.6 |
| 7 | Ichikawa et al[2] | 2007 | Japanese | GE | 1.5 | 8000-10000 | 73 | 1000 | 62.0 | Yes | 2.8 |
| 8 | Huang et al[16] | 2011 | Chinese | GE | 3.0 | 5700 | 55 | 1000 | 58.9 | Yes | 3.4 |
| 9 | Klauss et al[21] | 2011 | Germany | Siemens | 1.5 | 1300 | 60 | 800 | 62.8 | Yes | 3.5 |
| No. | Ref. | Benign lesions | Malignant lesions | TP | FP | FN | TN |
| 1 | Muhi et al[21] | 10 | 54 | 52 | 0 | 2 | 10 |
| 2 | Lemke et al[20] | 14 | 23 | 17 | 2 | 6 | 12 |
| 3 | Sandrasegaran et al[22] | 45 | 25 | 20 | 26 | 5 | 19 |
| 4 | Sandrasegaran et al[23] | 23 | 13 | 9 | 4 | 4 | 19 |
| 5 | Kartalis et al[17] | 24 | 12 | 11 | 2 | 1 | 22 |
| 6a | Lee et al[19] | 13 | 47 | 34 | 3 | 13 | 10 |
| 6b | Lee et al[19] | 13 | 47 | 41 | 4 | 6 | 9 |
| 7 | Ichikawa et al[2] | 23 | 26 | 25 | 0.3 | 1 | 22.7 |
| 8 | Huang et al[16] | 14 | 37 | 31.7 | 1.9 | 5.3 | 12.1 |
| 9 | Klauss et al[18] | 9 | 20 | 13 | 0 | 7 | 9 |
The statistical methods of this study were reviewed by xiaoyuan from the Second Affiliated Hospital of Fujian Medical University. All analyses were performed using Meta-DiSc and Stata 11.0 (StataCorp, College Station, TX, United States). The DerSimonian-Laird random-effects model was used to pool together the final sensitivity, specificity, positive likelihood ratio (PLR) and negative likelihood ratio (NLR). Publication bias was evaluated. We also performed a meta-regression analysis by adding covariates to the summary receiver operating characteristic (SROC) curve using the Moses-Shapiro-Littenberg method[14,15]. For all tests, P < 0.05 was considered to indicate statistical significance.
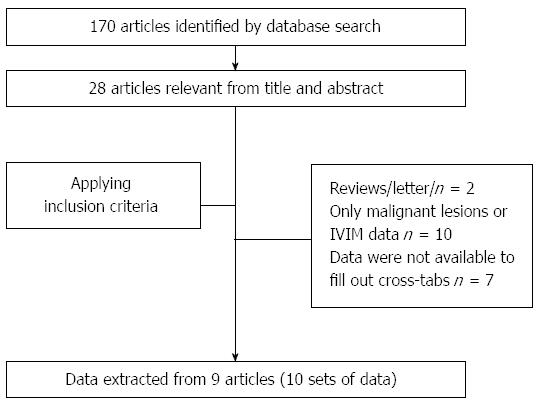
The initial database search of PubMed and EMBASE identified 170 relevant articles that were published up to August 2014. The initial screening by one reviewer reduced this to 28 articles. After applying the inclusion criteria, nine articles[2,16-23] were selected for data extraction (10 sets of data) (Figure 1).
The meta-analysis included 304 malignant and 188 benign pancreatic lesions from nine studies (10 sets of data) (Table 1).
Eight studies used a 1.5 T MRI scanner (Nos. 1-7 and 9) and the other (No. 8) used a 3 T scanner. Seven studies (Nos. 2-6, 8 and 9) used a DWI sequence with TR in the range of 1300-5700 ms, and two studies (Nos. 1 and 7) used a DWI sequence with TR of 8000-10000 ms. Typical acquisition parameters included TE of ≥ 55 ms (Nos. 1-9 range: 55-73 ms). Typical acquisition parameters included maximum b factor of 800 or 1000 ms (No. 1-4 and No. 6-9). One study (No. 6) did not provide information on the fat suppression technique used. One study (No. 5) did not provide information on the mean size of malignant tumors. The mean age of patients with malignant pancreatic lesions was 63.3 years (No. 1-4 and No. 6-9). The results of all analyses are reported in Tables 1 and 2.
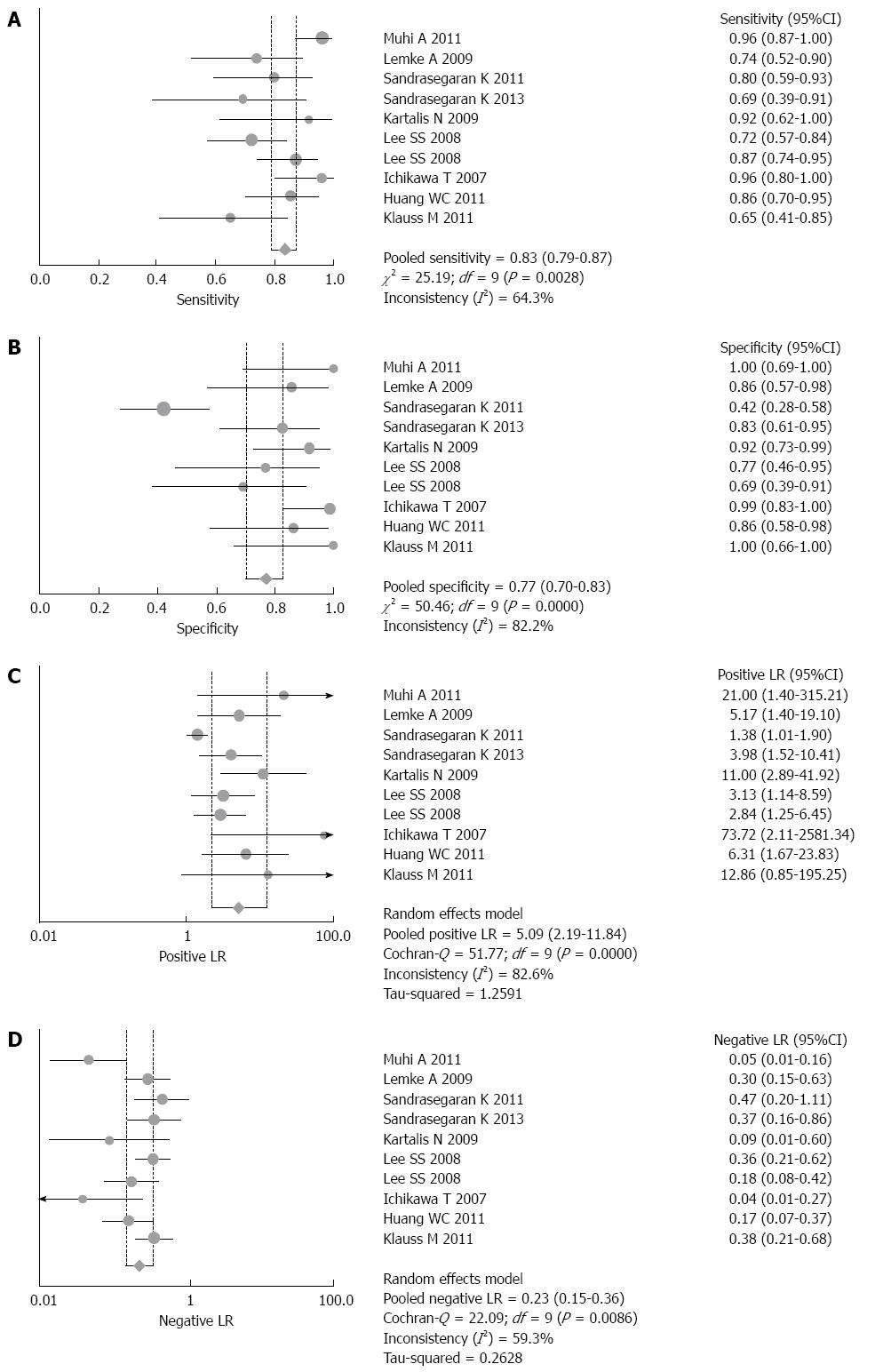
Figure 2 shows the forest plots of sensitivity (Figure 2A), specificity (Figure 2B), PLR (Figure 2C) and NLR (Figure 2D), of DWI for differential diagnoses between malignant and benign pancreatic lesions.
The pooled sensitivity and specificity of single-shot EPI DWI were 0.83 (95%CI: 0.79-0.87) and 0.77 (95%CI: 0.70-0.83), respectively. PLR and NLR were 5.09 (95%CI: 2.19-11.84) and 0.23 (95%CI: 0.15-0.36), respectively. The P value for the χ2 heterogeneity for all pooled estimates was < 0.05.
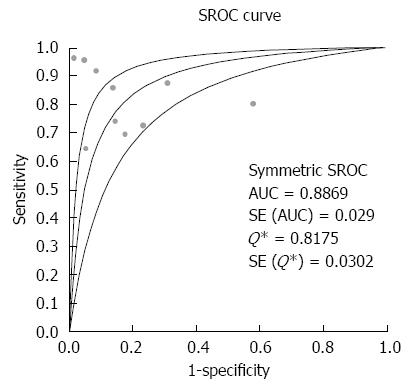
The overall accuracy was further explored by drawing SROC curves and finding the area under the curve (AUC) and Q* index (Figure 3), which were 0.89 and 0.82, respectively, indicating good diagnostic accuracy.
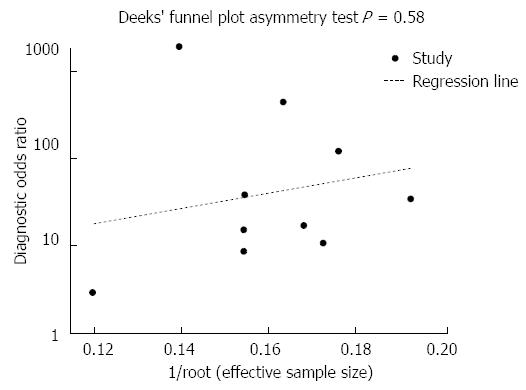
Publication bias was not observed (Figure 4; t = 0.58, P = 0.58).
The meta-regression analysis indicated that fat suppression, mean age, TE, and maximum b factor were not sources of heterogeneity (all P > 0.05).
Research concerning pancreatic DWI is rapidly expanding, and a growing amount of data are being published[5,16,24-28]. Fast imaging is important to avoid motion artifacts. The advantage of using single-shot EPI as a readout sequence is that only one excitation is necessary, and hence the DW images become less sensitive to subject motion[9].
Based on calculations of the relevant data available in the currently published articles, our systematic review and meta-analysis demonstrated that pancreatic single-shot EPI DWI was useful to differentiate between malignant and benign pancreatic lesions. The pooled sensitivity and specificity were 83% and 77%, respectively. PLR and NLR were 5.09 and 0.23, respectively. From the fitted SROC, AUC was 0.89 and Q*, the point where sensitivity equals specificity, was 0.82. All these data indicated that the overall diagnostic performance of single-shot EPI to differentiate malignant from benign pancreatic lesions was high.
There was significant heterogeneity among the studies in our analysis; therefore, it is critical to investigate the source of heterogeneity to determine the potential impact factors. Publication bias is a common source of heterogeneity in meta-analyses. However, in the present analysis, the funnel plot suggested that there may not have been publication bias. Meta-regression analysis was performed to explore other sources of heterogeneity for pancreatic DWI.
There are many factors that affect the signal intensity on DWI and affect measured ADC values[29-32]. Meta-regression analysis indicated that fat suppression, mean age, TE, and maximum b factor were not sources of heterogeneity. The best acquisition strategies for DWI data in the focal pancreatic disease are still a matter of debate. There was considerable variation in the results, which indicated that more-detailed, high-quality prospective studies on pancreatic DWI should be carried out to establish the presence of heterogeneity.
Some limitations of the present study should be mentioned. First, as described above, there was notable heterogeneity among the studies. Evaluated covariates were the sources of the heterogeneity, which requires further study. Standardization of the acquisition protocol for pancreatic DWI across the multicenter studies is recommended. Lesion size ≥ 2 cm was the limit for the diagnosis of early lesions. Optimization of the DWI protocol includes appropriate b-value selection, sufficient signal-to-noise ratio, adequate fat suppression, and artifact reduction via shimming and parallel imaging[6]. The application of those techniques may be necessary to enhance the results of the present study.
In conclusion, single-shot EPI DWI was useful to differentiate between malignant and benign pancreatic lesions. The pooled sensitivity and specificity were 83% and 77%, respectively. PLR and NLR were 5.09 and 0.23, respectively. From the fitted SROC, AUC was 0.89 and Q* was 0.82. Lesion size ≥ 2 cm was the limit for the diagnosis of early lesions. More-detailed, high-quality prospective studies on pancreatic DWI should be carried out to establish the presence of heterogeneity.
Diffusion-weighted imaging (DWI) provides tissue contrast based on the diffusion properties of water molecules in tissue, without using any contrast agents. The advantage of using single-shot echo-planar imaging (EPI) as a readout sequence is that only one excitation is necessary, and hence the DW images become less sensitive to subject motion.
There is no current consensus on the diagnostic ability of single-shot EPI DWI. We conducted a systematic review to investigate the diagnostic capability of single-shot EPI DWI to differentiate between malignant and benign pancreatic focal lesions.
Lesion size ≥ 2 cm was the limit for the diagnosis of early lesions. More detailed, high quality prospective studies on pancreatic DWI should be carried out in the presence of heterogeneity.
Single-shot EPI DWI was useful to differentiate between malignant and benign pancreatic focal lesions.
DWI provides tissue contrast based on the diffusion properties of water molecules in tissue. DWI has a potential role in the differentiation and evaluation of pancreatic masses on the basis of the high contrast between the lesion and normal tissue.
The paper discusses the prognostic value of single-shot EPI DWI in differentiation of benign vs malignant pancreatic masses. The meta-analysis is comprehensive and carefully done.
P- Reviewer: Giraldi G, Wang CX, Wang XH S- Editor: Qi Y L- Editor: Stewart G E- Editor: Wang CH
| 1. | Balachandran A, Bhosale PR, Charnsangavej C, Tamm EP. Imaging of pancreatic neoplasms. Surg Oncol Clin N Am. 2014;23:751-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Ichikawa T, Erturk SM, Motosugi U, Sou H, Iino H, Araki T, Fujii H. High-b value diffusion-weighted MRI for detecting pancreatic adenocarcinoma: preliminary results. AJR Am J Roentgenol. 2007;188:409-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 194] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 3. | Tamm EP, Bhosale PR, Vikram R, de Almeida Marcal LP, Balachandran A. Imaging of pancreatic ductal adenocarcinoma: State of the art. World J Radiol. 2013;5:98-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Taniguchi T, Kobayashi H, Nishikawa K, Iida E, Michigami Y, Morimoto E, Yamashita R, Miyagi K, Okamoto M. Diffusion-weighted magnetic resonance imaging in autoimmune pancreatitis. Jpn J Radiol. 2009;27:138-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Wang Y, Chen ZE, Nikolaidis P, McCarthy RJ, Merrick L, Sternick LA, Horowitz JM, Yaghmai V, Miller FH. Diffusion-weighted magnetic resonance imaging of pancreatic adenocarcinomas: association with histopathology and tumor grade. J Magn Reson Imaging. 2011;33:136-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 6. | Chen X, Li WL, Zhang YL, Wu Q, Guo YM, Bai ZL. Meta-analysis of quantitative diffusion-weighted MR imaging in the differential diagnosis of breast lesions. BMC Cancer. 2010;10:693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 168] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 7. | Fattahi R, Balci NC, Perman WH, Hsueh EC, Alkaade S, Havlioglu N, Burton FR. Pancreatic diffusion-weighted imaging (DWI): comparison between mass-forming focal pancreatitis (FP), pancreatic cancer (PC), and normal pancreas. J Magn Reson Imaging. 2009;29:350-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 150] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 8. | Kamisawa T, Takuma K, Anjiki H, Egawa N, Hata T, Kurata M, Honda G, Tsuruta K, Suzuki M, Kamata N. Differentiation of autoimmune pancreatitis from pancreatic cancer by diffusion-weighted MRI. Am J Gastroenterol. 2010;105:1870-1875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 9. | van Pul C, Roos FG, Derksen OS, Buijs J, Vlaardingerbroek MT, Kopinga K, Wijn PF. A comparison study of multishot vs. single-shot DWI-EPI in the neonatal brain: reduced effects of ghosting compared to adults. Magn Reson Imaging. 2004;22:1169-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Wu LM, Hu JN, Hua J, Liu MJ, Chen J, Xu JR. Diagnostic value of diffusion-weighted magnetic resonance imaging compared with fluorodeoxyglucose positron emission tomography/computed tomography for pancreatic malignancy: a meta-analysis using a hierarchical regression model. J Gastroenterol Hepatol. 2012;27:1027-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Li Y, Chen Z, Wang J. Differential diagnosis between malignant and benign hepatic tumors using apparent diffusion coefficient on 1.5-T MR imaging: a meta analysis. Eur J Radiol. 2012;81:484-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Wu LM, Hu J, Gu HY, Hua J, Xu JR. Can diffusion-weighted magnetic resonance imaging (DW-MRI) alone be used as a reliable sequence for the preoperative detection and characterisation of hepatic metastases? A meta-analysis. Eur J Cancer. 2013;49:572-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Xia D, Jing J, Shen H, Wu J. Value of diffusion-weighted magnetic resonance images for discrimination of focal benign and malignant hepatic lesions: a meta-analysis. J Magn Reson Imaging. 2010;32:130-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Baltzer PA, Dietzel M, Kaiser WA. MR-spectroscopy at 1.5 tesla and 3 tesla. Useful? A systematic review and meta-analysis. Eur J Radiol. 2012;81 Suppl 1:S6-S9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Cen D, Xu L. Differential diagnosis between malignant and benign breast lesions using single-voxel proton MRS: a meta-analysis. J Cancer Res Clin Oncol. 2014;140:993-1001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Huang WC, Sheng J, Chen SY, Lu JP. Differentiation between pancreatic carcinoma and mass-forming chronic pancreatitis: usefulness of high b value diffusion-weighted imaging. J Dig Dis. 2011;12:401-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Kartalis N, Lindholm TL, Aspelin P, Permert J, Albiin N. Diffusion-weighted magnetic resonance imaging of pancreas tumours. Eur Radiol. 2009;19:1981-1990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 131] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 18. | Klauss M, Lemke A, Grünberg K, Simon D, Re TJ, Wente MN, Laun FB, Kauczor HU, Delorme S, Grenacher L. Intravoxel incoherent motion MRI for the differentiation between mass forming chronic pancreatitis and pancreatic carcinoma. Invest Radiol. 2011;46:57-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 19. | Lee SS, Byun JH, Park BJ, Park SH, Kim N, Park B, Kim JK, Lee MG. Quantitative analysis of diffusion-weighted magnetic resonance imaging of the pancreas: usefulness in characterizing solid pancreatic masses. J Magn Reson Imaging. 2008;28:928-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 161] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 20. | Lemke A, Laun FB, Klauss M, Re TJ, Simon D, Delorme S, Schad LR, Stieltjes B. Differentiation of pancreas carcinoma from healthy pancreatic tissue using multiple b-values: comparison of apparent diffusion coefficient and intravoxel incoherent motion derived parameters. Invest Radiol. 2009;44:769-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 204] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 21. | Muhi A, Ichikawa T, Motosugi U, Sou H, Sano K, Tsukamoto T, Fatima Z, Araki T. Mass-forming autoimmune pancreatitis and pancreatic carcinoma: differential diagnosis on the basis of computed tomography and magnetic resonance cholangiopancreatography, and diffusion-weighted imaging findings. J Magn Reson Imaging. 2012;35:827-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 22. | Sandrasegaran K, Akisik FM, Patel AA, Rydberg M, Cramer HM, Agaram NP, Schmidt CM. Diffusion-weighted imaging in characterization of cystic pancreatic lesions. Clin Radiol. 2011;66:808-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | Sandrasegaran K, Nutakki K, Tahir B, Dhanabal A, Tann M, Cote GA. Use of diffusion-weighted MRI to differentiate chronic pancreatitis from pancreatic cancer. AJR Am J Roentgenol. 2013;201:1002-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Niwa T, Ueno M, Ohkawa S, Yoshida T, Doiuchi T, Ito K, Inoue T. Advanced pancreatic cancer: the use of the apparent diffusion coefficient to predict response to chemotherapy. Br J Radiol. 2009;82:28-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Puskás T, Henits I. [Diffusion-weighted MR imaging; the significance of ADC and perfusion values in the differential diagnosis of pancreatic adenocarcinoma and mass forming pancreatitis]. Orv Hetil. 2012;153:1191-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 26. | Rosenkrantz AB, Matza BW, Sabach A, Hajdu CH, Hindman N. Pancreatic cancer: lack of association between apparent diffusion coefficient values and adverse pathological features. Clin Radiol. 2013;68:e191-e197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Takeuchi M, Matsuzaki K, Kubo H, Nishitani H. High-b-value diffusion-weighted magnetic resonance imaging of pancreatic cancer and mass-forming chronic pancreatitis: preliminary results. Acta Radiol. 2008;49:383-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | Yao XZ, Yun H, Zeng MS, Wang H, Sun F, Rao SX, Ji Y. Evaluation of ADC measurements among solid pancreatic masses by respiratory-triggered diffusion-weighted MR imaging with inversion-recovery fat-suppression technique at 3.0T. Magn Reson Imaging. 2013;31:524-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 29. | Boraschi P, Donati F, Gigoni R, Salemi S, Bartolozzi C, Falaschi F. Diffusion-weighted MRI in the characterization of cystic pancreatic lesions: usefulness of ADC values. Magn Reson Imaging. 2010;28:1447-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Herrmann J, Schoennagel BP, Roesch M, Busch JD, Derlin T, Doh LK, Petersen KU, Graessner J, Adam G, Habermann CR. Diffusion-weighted imaging of the healthy pancreas: ADC values are age and gender dependent. J Magn Reson Imaging. 2013;37:886-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Soyer P, Kanematsu M, Taouli B, Koh DM, Manfredi R, Vilgrain V, Hoeffel C, Guiu B. ADC normalization: a promising research track for diffusion-weighted MR imaging of the abdomen. Diagn Interv Imaging. 2013;94:571-573. [PubMed] |
| 32. | Yoshikawa T, Kawamitsu H, Mitchell DG, Ohno Y, Ku Y, Seo Y, Fujii M, Sugimura K. ADC measurement of abdominal organs and lesions using parallel imaging technique. AJR Am J Roentgenol. 2006;187:1521-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 240] [Article Influence: 12.6] [Reference Citation Analysis (0)] |