Published online May 28, 2015. doi: 10.3748/wjg.v21.i20.6352
Peer-review started: August 28, 2014
First decision: September 15, 2014
Revised: October 14, 2014
Accepted: December 1, 2014
Article in press: December 1, 2014
Published online: May 28, 2015
Processing time: 276 Days and 19.2 Hours
AIM: To conduct a meta-analysis examining the effectiveness and safety of vedolizumab for the treatment of ulcerative colitis (UC).
METHODS: A search was conducted of MEDLINE, Cochrane, EMBASE, and Google Scholar on July 31, 2013. Inclusion criteria were: (1) Randomized controlled trial (RCT); (2) Patients treated for UC; and (3) Intervention was vedolizumab. The following information/data were extracted from studies that met the inclusion criteria: the name of the first author, year of publication, study design, patient demographic information, response rate, remission rate, and adverse events. The primary outcome was clinical response rate, and the secondary outcomes were clinical remission rate and serious adverse events. Odds ratio (OR) with 95%CI were calculated for each outcome.
RESULTS: Of 224 studies initially identified, three RCTs examining the use of vedolizumab meeting the inclusion criteria were included in the meta-analysis. All studies examined the use of vedolizumab at dosages ranging from 0.5 to 10 mg/kg body weight (one study used a standard dose of 300 mg). The follow-up periods were approximately 6 wk. The total number of patients in the intervention groups was 901, and in the control groups was 221. The mean age of the patients was approximately 41 years, and approximately half were males. The follow-up periods ranged from 43 d to 6 wk. The clinical response and remission rates were significantly higher for patients who received vedolizumab as compared to control patients (clinical response: OR = 2.69; 95%CI: 1.94-3.74, P < 0.001 and remission rate: OR = 2.72; 95%CI: 1.76-4.19, P < 0.001). Serious adverse events were not higher in patients that received vedolizumab.
CONCLUSION: This analysis supports the use of vedolizumab for the treatment of UC.
Core tip: Studies have suggested that vedolizumab may be effective in reducing intestinal inflammation in patients with ulcerative colitis (UC). This meta-analysis including three randomized controlled trials showed that treatment with vedolizumab results in significantly higher clinical response and remission rates than placebo in patients with UC. Importantly, serious adverse events were not more common in vedolizumab-treated patients than control patients. This analysis supports the use of vedolizumab for the treatment of UC.
- Citation: Jin Y, Lin Y, Lin LJ, Zheng CQ. Meta-analysis of the effectiveness and safety of vedolizumab for ulcerative colitis. World J Gastroenterol 2015; 21(20): 6352-6360
- URL: https://www.wjgnet.com/1007-9327/full/v21/i20/6352.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i20.6352
Inflammatory bowel disease (IBD), an inflammatory condition of the colon and small intestine, is an autoimmune disease in which the body’s own immune system attacks elements of the digestive system[1,2]. The main forms of IBD are Crohn’s disease (CD) and ulcerative colitis (UC), and the primary difference between the two is the location and nature of the inflammatory changes[1,2]. CD may affect any part of the gastrointestinal tract from mouth to anus, and can result in a wide variety of symptoms including abdominal pain, diarrhea, vomiting, and weight loss[1,2]. UC is more common than CD with a reported incidence of nine to 20 cases per 100000 person years, and a prevalence of approximately 150 to 300 cases per 100000 persons[1]. The incidence of both CD and UC has been increasing in China in the past two decades[3].
In UC, characteristic ulcers or open sores are restricted to the colonic mucosa, and the main symptom of active disease is typically the gradual onset of constant diarrhea mixed with blood[1]. UC can occur at any age, but onset is typically between 15 and 30 years of age[1]. Diagnostic studies include tests of blood and stool, colonoscopy or sigmoidoscopy, and imaging studies. Medical treatments for UC include anti-inflammatory agents such as 5-aminosalycilate compounds, systemic corticosteroids, topical corticosteroids, and immunomodulators, but all have certain side effects and are not effective in all cases[1,2]. Despite the success of anti-tumor necrosis factor therapies in the treatment of UC, a considerable proportion of patients are refractory to treatment[4,5]. In severe cases refractory to medical treatment colectomy is necessary.
Integrins are transmembrane receptors that mediate the attachment between a cell and its surroundings, such as other cells or the extracellular matrix[6,7]. Integrins interact with other proteins such as cadherins, immunoglobulin superfamily cell adhesion molecules, selectins, and syndecans to mediate cell-cell and cell-matrix interaction and communication[6,7]. Alpha4beta7 (α4β7) integrin is found on circulating T lymphocytes, and is involved in the recruitment of leukocytes to the gastrointestinal tract[8]. Integrin antagonists are a new class of agents that inhibit leukocyte adhesion and aim to selectively inhibit the inflammatory pathway[4]. Vedolizumab (MNL-02) is a recombinant humanized IgG1 monoclonal antibody that inhibits adhesion and migration of leukocytes into the gastrointestinal tract by binding the α4β7 integrin[9]. Early animal[10] and human studies[11] suggested that MLN-02 may be effective in reducing intestinal inflammation in patients with UC.
The purpose of this meta-analysis is to examine the efficacy and safety vedolizumab for the treatment of UC.
The procedures performed in this meta-analysis are in accordance with recent guidelines for the reporting of meta-analyses (PRISMA guidelines). Meta-analysis does not involve human subjects and does not require Institutional Review Board review.
We conducted a systematic search of electronic databases and the bibliographies of all eligible studies to identify all relevant studies. A search was conducted of MEDLINE, Cochrane, EMBASE, and Google Scholar on July 31, 2013 using combinations of the search terms vedolizumab/MLN0002/MLN-02, inflammatory bowel disease, and ulcerative colitis.
Studies were selected for inclusion in this analysis based on the following criteria: (1) Randomized controlled trial (RCT); (2) Patients treated for UC (if the study enrolled patients with IBD, only those with UC were included); and (3) The intervention was vedolizumab. Non-English publications were excluded.
Studies were identified using the search strategy by two independent reviewers. When there was uncertainty regarding eligibility, a third reviewer was consulted. References of identified studies were hand searched for other relevant studies. The following information/data were extracted from studies that met the inclusion criteria: the name of the first author, year of publication, study design, patient demographic information, response rate, remission rate, and adverse events (AEs).
The methodological quality of each study was assessed using the risk-of-bias assessment tool outlined in the Cochrane Handbook for Systematic Reviews of Interventions (version 5.1.0)[12]. Two reviewers subjectively reviewed all studies and assigned a value of “low risk,”“high risk,” or “unclear” to the following: (1) random sequence generation; (2) allocation concealment; (3) blinding (patients, personnel, and assessor); (4) adequate assessment of each outcome; (5) selective outcome reporting avoided; and (6) if the analysis included an intention-to-treat analysis.
The primary outcome was clinical response rate, and the secondary outcomes were clinical remission rate and serious adverse events. For each outcome, the odds ratio (OR) with 95%CI was calculated. Heterogeneity among the studies was assessed by the Cochran’s Q test and the I2 statistic. For Cochran’s Q, a value of P < 0.10 was considered to indicate statistically significant heterogeneity. If either the Q statistics (P < 0.1) or I2 statistic (> 50%) indicated the existence of significant heterogeneity between studies, a random-effects model of analysis (DerSimonian-Laird method) was used. Otherwise, a fixed-effect model of analysis (Mantel-Haenszel method) was used. Pooled ORs for the three outcomes were calculated; a two-sided P value < 0.05 was considered to indicate statistical significance. Sensitivity analysis was performed for the three outcomes based on the leave-one-out approach. As more than five studies are required to detect funnel plot asymmetry[13], publication bias was not assessed if less than five studies were identified with data for a particular outcome measure. All statistical analyses were performed using the statistical software Comprehensive Meta-Analysis, version 2.0 (Biostat, Englewood, NJ, United States).
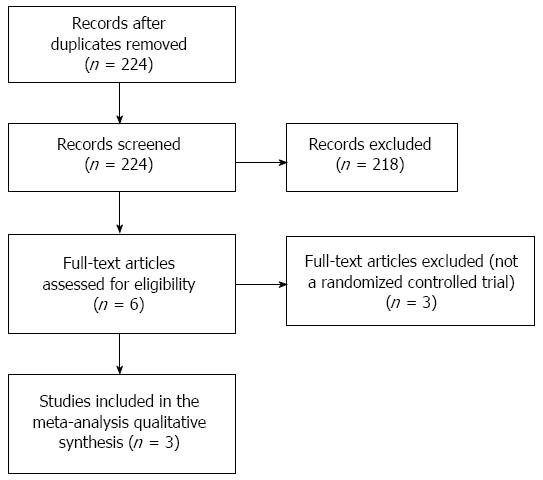
A flow diagram of study selection is shown in Figure 1. A total of 224 potentially relevant studies were identified in the literature search, and after screening 218 studies were excluded. Thus, 6 full-text articles were reviewed of which three was excluded because they were not RCT design. Finally, a total of three RCTs were included in the meta-analysis[14-16].
The characteristics of the three studies included in the meta-analysis are summarized in Table 1. All studies examined the use of vedolizumab at dosages ranging from 0.5 to 10 mg/kg body weight (one study used a standard dose of 300 mg). The total number of patients in the intervention groups was 901, and in the control groups was 221. The mean age of the patients was approximately 41 years, and approximately half were males. The follow-up periods were approximately 6 wk.
| Ref. | Study type | Follow-up periods | Group | Drug | Drug dosage | Number of cases | Age, yr | Male |
| Feaganet al[14], 2005 | RCT | 6 wk | Intervention 1 | MLN-02 | 0.5 mg/kg | 58 | 41.6 ± 14.7 | 56.9% |
| Intervention 2 | 2 mg/kg | 60 | 43.8 ± 14.6 | 50.0% | ||||
| Control | Placebo | NA | 63 | 38.9 ± 13.4 | 55.6% | |||
| Feagan et al[15], 2013 | Randomized allocation | 6 wk | Intervention | Vedolizumab | 300 mg | 746 | 40.1 ± 13.2 | 58.0% |
| Control | Placebo | NA | 149 | 41.1 ± 1.25 | 61.7% | |||
| Parikh et al[16], 2012 | RCT | 43 d | Intervention 1 | Vedolizumab | 2 mg/kg | 12 | 39 (30-49)1 | 33.3% |
| Intervention 2 | 6 mg/kg | 14 | 47 (19-61)1 | 50.0% | ||||
| Intervention 3 | 10 mg/kg | 11 | 41 (26-69)1 | 45.5% | ||||
| Control | Placebo | NA | 9 | 33 (21-51)1 | 33.3% |
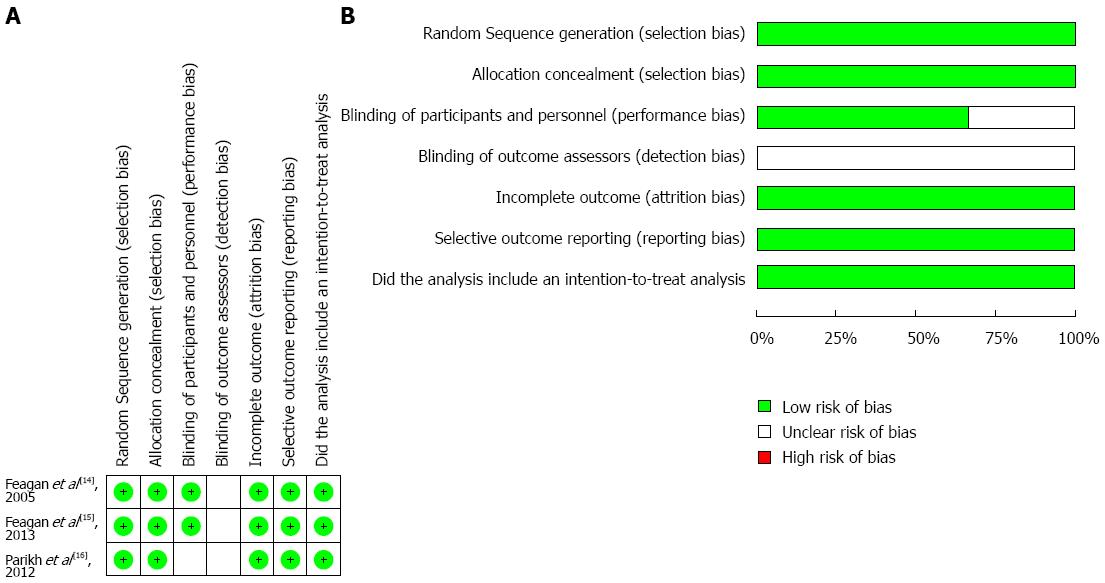
The “risk of bias” summary is presented in Figure 2A, and an overall assessment of risk of bias is presented in Figure 2B. The random sequence and allocation concealment were appropriate in all three studies. The patients and personnel were blinded in two studies; however, none of the studies provided information on the blinding of outcome assessors. All studies were at a low risk of attrition bias and reporting bias. In addition, intention-to-treat analysis was used in all three studies.
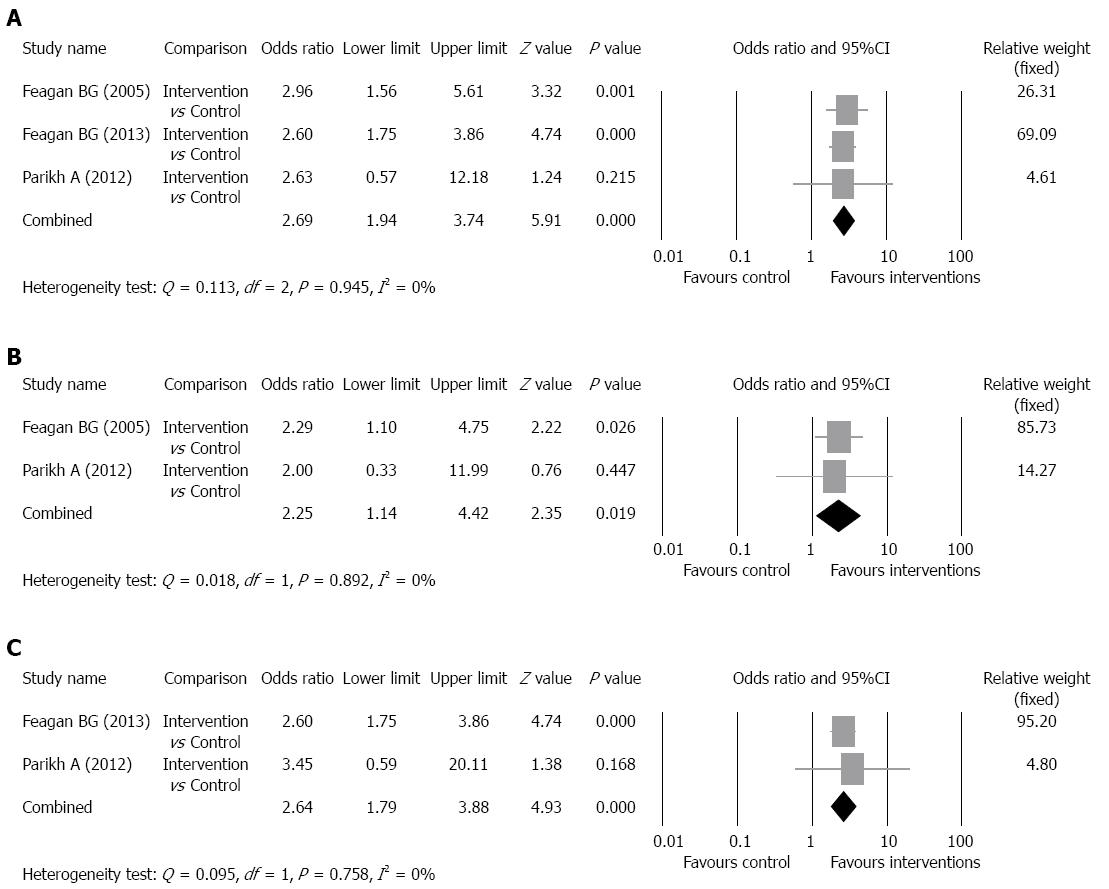
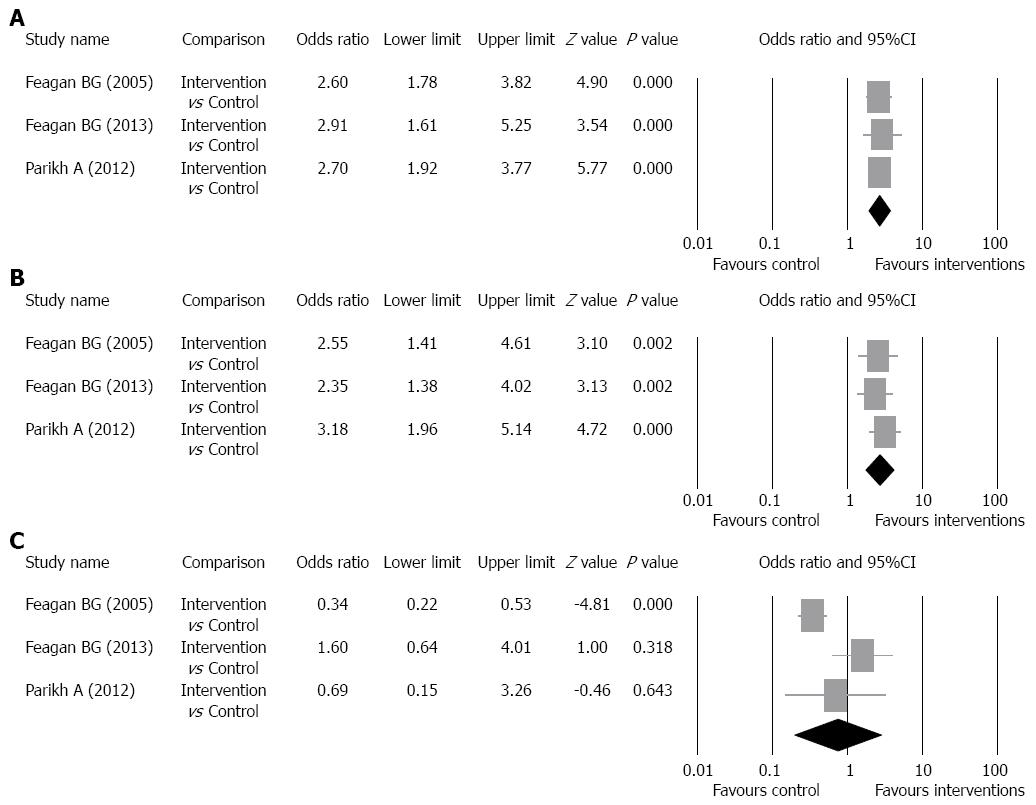
Clinical response rate: The clinical response rates of the intervention groups ranged from 47.1% to 59.3% and of the control groups ranged from 25.5% to 33.3% (Table 2). There was no evidence of significant heterogeneity when data from the studies were pooled (Q = 0.113, df = 2, P = 0.945, I2 = 0%); therefore, a fixed-effect model was used for analysis of the clinical response rate (pooled of all intervention groups) (Figure 3A). The overall analysis revealed the clinical response rate was significantly higher for patients who received vedolizumab as compared to control patients (OR = 2.69, 95%CI: 1.94-3.74, P < 0.001).
| Ref. | Group | Drug | Definition of clinical response | Clinical response rate | Definition of clinical remission | Clinical remission rate | |
| Feagan et al[14] | Intervention 1 | MLN-02 | An improvement of 3 points or more on the ulcerative colitis clinical score (modification of the Mayo Clinic Scoring system) | 66% | 59.3%1 | Ulcerative colitis clinical score of 0 or 1 and a modified Baron score of 0 or 1 with no evidence of rectal bleeding | 32.2%1 |
| Intervention 2 | 53% | ||||||
| Control | Placebo | 33% | 14.0% | ||||
| Feagan et al[15] | Intervention | Vedolizumab | A reduction in the Mayo Clinic score of at least 3 points and a decrease of at least 30% from baseline, with an accompanying decrease in the rectal bleeding subscore of at least 1 point or an absolute rectal bleeding subscore of 0 or 1 | 47.1% | Mayo Clinic score of 2 or lower and no subscore higher than 1, and mucosal healing, defined as an endoscopic subscore of 0 or 1 | 16.9% | |
| Control | Placebo | 25.5% | 5.4% | ||||
| Parikh et al[16] | Intervention 1 | Vedolizumab | A decrease from baseline in the partial Mayo score (PMS) of ≥ 2 points and ≥ 25%, with an accompanying decrease in the subscore for rectal bleeding of ≥ 1 point or an absolute subscore for rectal bleeding of 0 or 1. | 50% | 56.8%1 | PMS of ≤ 2 with no individual subscore > 1 | 58%1 |
| Intervention 2 | 63.3% | ||||||
| Intervention 3 | 53.3% | ||||||
| Control | Placebo | 33.3% | 50% | ||||
Subgroup analysis for the pooled clinical response rate was performed according to the dosage of intervention drug. The studies of Feagan et al[14] and Parikh et al[16] were included in the analysis of clinical response rate of patients who received 2 mg of drug per kilogram of body weight and the studies of Feagan et al[15] and Parikh et al[16] were included in the analysis of clinical response rate of patients who received 6 mg of drug per kilogram of body weight. Patients who received 300 mg of vedolizumab in the study of Feagan et al[15] were included in the analysis of clinical response rate of patients who received 6 mg of drug per kilogram of body weight because 300 mg is approximately 6 mg of drug per kilogram of body weight. For the 2 mg per kilogram group, the clinical response rates of the intervention groups ranged from 50% to 53%,and of the control groups ranged from 33% to 33.3%. For the 6 mg per kilogram group, the clinical response rates of the intervention groups ranged from 47.1% to 63.3%, and of the control groups ranged from 25.5% to 33.3%. There was no evidence of significant heterogeneity when data from the 2 mg per kilogram studies were pooled (Q = 0.018, df = 1, P = 0.892, I2 = 0%); therefore, a fixed-effect model was used for analysis of clinical response rate (Figure 3B). The overall analysis revealed the clinical response rate (2 mg) was significantly higher for patients who received vedolizumab as compared to control patients (P = 0.019). In addition, there was no evidence of significant heterogeneity when data from the 6 mg per kilogram studies were pooled (Q = 0.095, df = 1, P = 0.758, I2 = 0%); therefore, a fixed-effect model was used for analysis of clinical response rate (Figure 3C). The overall analysis revealed the clinical response rate (6 mg) was significantly higher for patients who received vedolizumab as compared to control patients (P < 0.001).
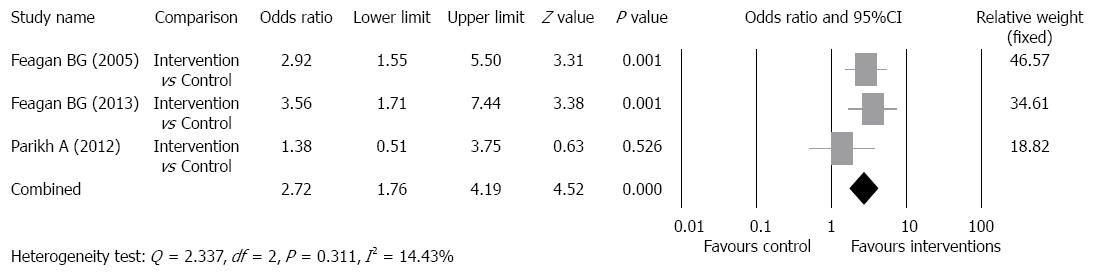
Clinical remission rate: The clinical remission rates of the intervention groups ranged from 16.9% to 58%, and of the control groups ranged from 5.4% to 50% (Table 2). There was no evidence of significant heterogeneity when data from the studies were pooled (Q = 2.337, df = 2, P = 0.311, I2 = 14.43%); therefore, a fixed-effect model was used for analysis of clinical remission rate (Figure 4). The overall analysis revealed the clinical remission rate was significantly higher for patients who received vedolizumab as compared to control patients (OR = 2.72, 95%CI: 1.76-4.19, P < 0.001).
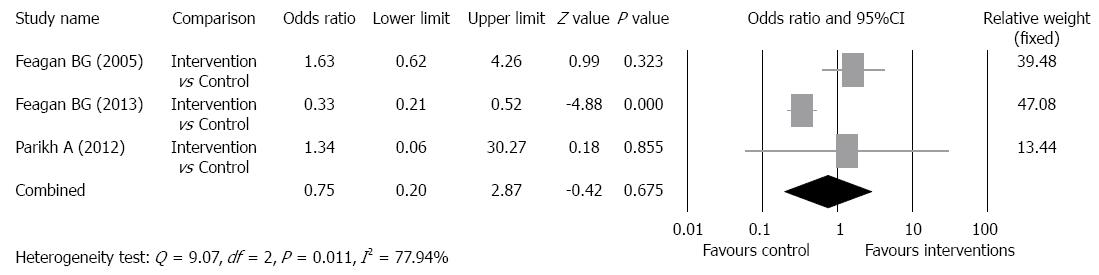
Adverse event rate: A summary of general AEs and serious adverse events (SAEs) is shown in Table 3. The most common general AEs in all studies, and in both intervention and control groups was aggravation of UC, and the incidence in the intervention groups ranged from 0% to 50%, and in the control groups from 38% to 44%. Other common general AEs included nausea, headaches, fatigue, nasopharyngitis, and abdominal pain. A statistical analysis was only performed for SAEs. In the intervention groups, the frequency of SAEs ranged from 0% to 20%, and in the control groups ranged from 0% to 25%. There was evidence of significant heterogeneity when data from the studies were pooled (Q = 9.07, df = 2, P = 0.011, I2 = 77.94%); therefore, a random-effects model was used for analysis of SAEs (Figure 5). The overall analysis revealed the SAE rate was not significantly different for patients who received vedolizumab as compared with control patients (P = 0.675).
| Ref. | Group | General adverse events1 | |||||||||
| Ulcerative colitis aggravated | Nausea/vomiting | Headache | Frequent bowel movements | Fatigue | Upper respiratory tract infection | Abdominal pain/tenderness | Arthralgia | Dizziness | Rash | ||
| Feagan et al[14] | Intervention 1 | 29 (50) | 21 (36) | 12 (21) | 10 (17) | 8 (14) | 8 (14) | 10 (17) | 4 (7) | 6 (10) | 6 (10) |
| Intervention 2 | 22 (37) | 13 (22) | 11 (18) | 5 (8) | 5 (8) | 8 (13) | 6 (10) | 7 (12) | 4 (7) | 4 (7) | |
| Control | 24 (38) | 15 (24) | 13 (21) | 10 (16) | 7 (11) | 5 (8) | 16 (25) | 5 (8) | 1 (2) | 4 (6) | |
| Feagan et al[15] | Intervention | 97 (13) | 38 (5) | 80 (11) | NA | 33 (4) | 132 (18) | 50 (7) | 56 (8) | NA | NA |
| Control | 58 (39) | 19 (13) | 28 (19) | NA | 10 (7) | 47 (32) | 10 (7) | 25 (17) | NA | NA | |
| Parikh et al[16] | Intervention 1 | 2 (17) | NA | 2 (17) | NA | NA | 4 (33) | NA | NA | 1 (8) | NA |
| Intervention 2 | 1 (7) | NA | 3 (21) | NA | NA | 3 (21) | NA | NA | 0 (0) | NA | |
| Intervention 3 | 0 (0) | NA | 2 (18) | NA | NA | 1 (9) | NA | NA | 0 (0) | NA | |
| Control | 4 (44) | NA | 1 (11) | NA | NA | 4 (44) | NA | NA | 1 (11) | NA | |
| Feagan et al[14] | Intervention 1 | 6 (10) | NA | NA | NA | NA | NA | NA | 6 (10) | 18 (15)2 | |
| Intervention 2 | 3 (5) | NA | NA | NA | NA | NA | NA | 12 (20) | |||
| Control | 8 (13) | NA | NA | NA | NA | NA | NA | 6 (10) | |||
| Feagan et al[15] | Intervention | NA | 35 (5) | 13 (2) | NA | NA | NA | NA | 77 (10) | ||
| Control | NA | 16 (11) | 36 (24) | NA | NA | NA | NA | 37 (25) | |||
| Parikh et al[16] | Intervention 1 | NA | NA | 0 (0) | 0 (0) | 0 (0) | 1 (8) | 0 (0) | 1 (8) | 2 (5)2 | |
| Intervention 2 | NA | NA | 2 (14) | 2 (14) | 2 (14) | 0 (0) | 0 (0) | 0 (0) | |||
| Intervention 3 | NA | NA | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (9) | 1 (9) | |||
| Control | NA | NA | 0 (0) | 0 (0) | 0 (0) | 1 (11) | 1 (11) | 0 (0) | |||
Figure 6 shows the results of the meta-analysis with one study removed-in-turn for the clinical response rate (pooled of all intervention groups); clinical remission rate; and SAE rate. For the clinical response rate (pooled of all intervention groups) and clinical remission rate, the direction and magnitude of the pooled estimate varied consistently, indicating good reliability. For the SAE rate, the pooled estimate was different when one study was left-out-in-turn, indicating poor reliability.
The results of this meta-analysis including three RCTs showed that the clinical response and remission rates were significantly higher for patients with UC treated with vedolizumab as compared to control patients, and SAEs were not more common in vedolizumab-treated patients than control patients.
UC is a relapsing disease that is difficult to treat, and a large percentage of patients are refractory to traditional medical management[1,2]. When medical treatment fails, the only recourse is colectomy. Since the discovery of integrins, a large amount of research has been devoted to elucidation of their functions, and they have been found to be viable therapeutic targets against thrombosis and inflammatory disease[6,7]. Because uncontrolled inflammation is the hallmark characteristic of UC, inflammatory mediators such as integrins have been investigated as therapeutic targets and shown promising results for the treatment of UC[4].
Integrin agonists block the lymphocyte-homing mechanism of T lymphocytes. In patients with IBD, there is recruitment of large numbers of T cells to the intestinal mucosa, and α4β7 integrin, which is found on circulating T lymphocytes and is involved in their recruitment to the gastrointestinal tract[4,7]. The α4β7 integrin is activated on the lymphocyte surface membrane, and binds with its glycosaminoglycan ligand [mucosal addressin-cell adhesion molecule-1 (MAdCAM-1)] located on the surface membrane of endothelial cells[9,17]. This binding results in lymphocytes migrating into the lamina propria and tissue, and subsequent inflammation[9,17]. Study has shown that there are significantly higher levels of α4β7 integrin and MAdCAM-1 in the colons of IBD patients than patients with irritable bowel syndrome[18]. It has also been shown that there are lower numbers of t-lymphocytes with α4β7 integrin in the peripheral blood of patients with inflammation of the colon[19].
Initial studies which examined natalizumab, a humanized monoclonal antibody that inhibits α4 integrin, showed that it was effective in inducing remission in patients with CD[20,21]. However, studies suggested that its use in patients with CD receiving multidrug therapy was associated with the development of progressive multifocal leukoencephalopathy (PML)[22]. Unlike natalizumab, which inhibits both α4β1 and α4β7 integrin and thus affects multiple organs, vedolizumab is gut-specific[23,24], and no cases of PML have been reported with its use[25].
The initial phase II trial of vedolizumab[14] showed that the drug was more effective than placebo for the induction of clinical and endoscopic remission in patients with active UC. Subsequent phase III trials confirmed that vedolizumab was effective for the induction and maintenance of remission in patients with UC[15]. Parikh et al[16], also included in this meta-analysis, showed that a dose of vedolizumab up to 10 mg/kg body weight was well tolerated, and that more patients treated with vedolizumab achieved a clinical response as compared with those treated with placebo. Importantly, no significant safety issues, including significant infections, have been noted in any studies using vedolizumab to treat UC[14-16,25].
The aforementioned studies were relatively short-term. A longer-term phase III study is currently being conducted in which patients who were involved in prior trials will have the option to enter a study in which they will receive vedolizumab every 4 wk for up to 100 wk[26].
While the three studies included in the meta-analysis did not all report the same general AEs, aggravation of UC as the most common AE was consistent between the studies as were other common AEs including nausea, headaches, fatigue, nasopharyngitis, and abdominal pain. These findings are consistent with those of other studies that have examined the use of vedolizumab and natalizumab for the treatment of UC[11,20,21].
The primary limitation of this meta-analysis is the small number of RCTs available for inclusion. Also, the length of follow-up in the studies was not long enough to evaluate the effectiveness of clinical remission, and endoscopic outcomes were not evaluated in all studies. Thus, care should be used when interpreting the results of this meta-analysis.
In conclusion, the results of this meta-analysis showed that treatment with vedolizumab results in significantly higher clinical response and remission rates than placebo in patients with UC. Importantly, SAEs were not more common in vedolizumab-treated patients than control patients. This analysis supports the use of vedolizumab for the treatment of UC.
Inflammatory bowel disease (IBD) is an autoimmune disease in which the body’s own immune system attacks elements of the digestive system. Ulcerative colitis (UC) is one form of IBD in which characteristic ulcers or open sores are restricted to the colonic mucosa. The main symptom of active disease is typically the gradual onset of constant diarrhea mixed with blood. To date there is no satisfactory treatment for UC, and in refractory cases colectomy is necessary.
In patients with IBD, there is recruitment of large numbers of T cells to the intestinal mucosa, and alpha4beta7 (α4β7) integrin, which is found on circulating T lymphocytes, is involved in their recruitment to the gastrointestinal tract. Integrin antagonists are new class of agents that inhibit leukocyte adhesion and aim to selectively inhibit the inflammatory pathway by blocking the lymphocyte-homing mechanism of T lymphocytes.
Vedolizumab is a recombinant humanized IgG1 monoclonal antibody that inhibits adhesion and migration of leukocytes into the gastrointestinal tract by binding the integrin associated with their recruitment. Early animal and human studies have suggested that vedolizumab may be effective in reducing intestinal inflammation in patients with UC.
The initial phase II trial of vedolizumab showed that the drug was more effective than placebo for the induction of clinical and endoscopic remission in patients with active UC. Subsequent phase III trials confirmed that vedolizumab was effective for the induction and maintenance of remission in patients with UC. While the aforementioned studies were relatively short, a longer-term phase III study is currently being conducted.
Integrins are transmembrane receptors that mediate the attachment between a cell and its surroundings, and they interact with other proteins such as cadherins, immunoglobulin superfamily cell adhesion molecules, selectins, and syndecans to mediate cell-cell and cell-matrix interaction and communication. α4β7 integrin is found on circulating T lymphocytes, and is involved in the recruitment of leukocytes to the gastrointestinal tract. Integrin antagonists, which inhibit leukocyte adhesion, have shown promise in the treatment of UC.
The results of this meta-analysis showed that treatment with vedolizumab resulted in significantly higher clinical response and remission rates than placebo in patients with UC. Further, the authors found out that serious adverse events were not more common in vedolizumab-treated patients than control patients. The data, tables and figures are clear and easy to understand.
P- Reviewer: Kato J, M’Koma AE, Suzuki H S- Editor: Gou SX L- Editor: O’Neill M E- Editor: Zhang DN
| 1. | Ordás I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet. 2012;380:1606-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1151] [Cited by in RCA: 1539] [Article Influence: 118.4] [Reference Citation Analysis (5)] |
| 2. | Assadsangabi A, Lobo AJ. Diagnosing and managing inflammatory bowel disease. Practitioner. 2013;257:13-8, 2. [PubMed] |
| 3. | Ye L, Cao Q, Cheng J. Review of inflammatory bowel disease in China. ScientificWorldJournal. 2013;2013:296470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Danese S. New therapies for inflammatory bowel disease: from the bench to the bedside. Gut. 2012;61:918-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 230] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 5. | Dignass A, Lindsay JO, Sturm A, Windsor A, Colombel JF, Allez M, D’Haens G, D’Hoore A, Mantzaris G, Novacek G. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 2: current management. J Crohns Colitis. 2012;6:991-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 728] [Cited by in RCA: 702] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 6. | Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673-687. [PubMed] |
| 7. | Yonekawa K, Harlan JM. Promises and limitations of targeting adhesion molecules for therapy. Adhesion molecules: Function and inhibition. Basel (Switzerland): Birkh user Verlag AG 2007; 289-330. |
| 8. | Erle DJ, Briskin MJ, Butcher EC, Garcia-Pardo A, Lazarovits AI, Tidswell M. Expression and function of the MAdCAM-1 receptor, integrin alpha 4 beta 7, on human leukocytes. J Immunol. 1994;153:517-528. [PubMed] |
| 9. | Tilg H, Kaser A. Vedolizumab, a humanized mAb against the α4β7 integrin for the potential treatment of ulcerative colitis and Crohn’s disease. Curr Opin Investig Drugs. 2010;11:1295-1304. [PubMed] |
| 10. | Hesterberg PE, Winsor-Hines D, Briskin MJ, Soler-Ferran D, Merrill C, Mackay CR, Newman W, Ringler DJ. Rapid resolution of chronic colitis in the cotton-top tamarin with an antibody to a gut-homing integrin alpha 4 beta 7. Gastroenterology. 1996;111:1373-1380. [PubMed] |
| 11. | Feagan BG, McDonald J, Greenberg G, Wild G, Pare P, Fedorak RN, Landau SB, Brettrnan LR. An ascending dose trial of a humanized a4b7 antibody in ulcerative colitis (UC). Gastroenterology. 2000;118:A874. [DOI] [Full Text] |
| 12. | Cochrane Handbook for Systematic. Reviews of Interventions. Version 5.1.0. (updated March, 2011). The Cochrane Collaboration. Available from: http://www.mrc-bsu.cam.ac.uk/cochrane/handbook/. |
| 13. | Sutton AJ, Duval SJ, Tweedie RL, Abrams KR, Jones DR. Empirical assessment of effect of publication bias on meta-analyses. BMJ. 2000;320:1574-1577. [PubMed] |
| 14. | Feagan BG, Greenberg GR, Wild G, Fedorak RN, Paré P, McDonald JW, Dubé R, Cohen A, Steinhart AH, Landau S. Treatment of ulcerative colitis with a humanized antibody to the alpha4beta7 integrin. N Engl J Med. 2005;352:2499-2507. [PubMed] |
| 15. | Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel JF, Sandborn WJ, Van Assche G, Axler J, Kim HJ, Danese S. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369:699-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1576] [Cited by in RCA: 1864] [Article Influence: 155.3] [Reference Citation Analysis (1)] |
| 16. | Parikh A, Leach T, Wyant T, Scholz C, Sankoh S, Mould DR, Ponich T, Fox I, Feagan BG. Vedolizumab for the treatment of active ulcerative colitis: a randomized controlled phase 2 dose-ranging study. Inflamm Bowel Dis. 2012;18:1470-1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 176] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 17. | Arihiro S, Ohtani H, Suzuki M, Murata M, Ejima C, Oki M, Kinouchi Y, Fukushima K, Sasaki I, Nakamura S. Differential expression of mucosal addressin cell adhesion molecule-1 (MAdCAM-1) in ulcerative colitis and Crohn’s disease. Pathol Int. 2002;52:367-374. [PubMed] |
| 18. | Souza HS, Elia CC, Spencer J, MacDonald TT. Expression of lymphocyte-endothelial receptor-ligand pairs, alpha4beta7/MAdCAM-1 and OX40/OX40 ligand in the colon and jejunum of patients with inflammatory bowel disease. Gut. 1999;45:856-863. [PubMed] |
| 19. | Meenan J, Spaans J, Grool TA, Pals ST, Tytgat GN, van Deventer SJ. Altered expression of alpha 4 beta 7, a gut homing integrin, by circulating and mucosal T cells in colonic mucosal inflammation. Gut. 1997;40:241-246. [PubMed] |
| 20. | Sandborn WJ, Colombel JF, Enns R, Feagan BG, Hanauer SB, Lawrance IC, Panaccione R, Sanders M, Schreiber S, Targan S. Natalizumab induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2005;353:1912-1925. [PubMed] |
| 21. | Targan SR, Feagan BG, Fedorak RN, Lashner BA, Panaccione R, Present DH, Spehlmann ME, Rutgeerts PJ, Tulassay Z, Volfova M. Natalizumab for the treatment of active Crohn’s disease: results of the ENCORE Trial. Gastroenterology. 2007;132:1672-1683. [PubMed] |
| 22. | Yousry TA, Major EO, Ryschkewitsch C, Fahle G, Fischer S, Hou J, Curfman B, Miszkiel K, Mueller-Lenke N, Sanchez E. Evaluation of patients treated with natalizumab for progressive multifocal leukoencephalopathy. N Engl J Med. 2006;354:924-933. [PubMed] |
| 23. | Fedyk ER, Wyant T, Yang LL, Csizmadia V, Burke K, Yang H, Kadambi VJ. Exclusive antagonism of the α4 β7 integrin by vedolizumab confirms the gut-selectivity of this pathway in primates. Inflamm Bowel Dis. 2012;18:2107-2119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 24. | Haanstra KG, Hofman SO, Lopes Estêvão DM, Blezer EL, Bauer J, Yang LL, Wyant T, Csizmadia V, ‘t Hart BA, Fedyk ER. Antagonizing the α4β1 integrin, but not α4β7, inhibits leukocytic infiltration of the central nervous system in rhesus monkey experimental autoimmune encephalomyelitis. J Immunol. 2013;190:1961-1973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 25. | Gledhill T, Bodger K. New and emerging treatments for ulcerative colitis: a focus on vedolizumab. Biologics. 2013;7:123-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |