Published online May 28, 2015. doi: 10.3748/wjg.v21.i20.6252
Peer-review started: November 7, 2014
First decision: December 11, 2014
Revised: January 14, 2015
Accepted: January 30, 2015
Article in press: January 30, 2015
Published online: May 28, 2015
Processing time: 204 Days and 16.2 Hours
AIM: To elucidate the role of contrast-enhanced endoscopic ultrasonography (CE-EUS) in the diagnosis of branch duct intraductal papillary mucinous neoplasm (BD-IPMN).
METHODS: A total of 50 patients diagnosed with BD-IPMN by computed tomography (CT) and endoscopic ultrasonography (EUS) at our institute were included in this study. CE-EUS was performed when mural lesions were detected by EUS. The diagnostic accuracy for identifying mural nodules (MNs) was evaluated by CT, EUS, and EUS combined with CE-EUS. In the patients who underwent resection, the accuracy of measuring MN height with each imaging modality was compared. The cut-off values to diagnose malignant BD-IPMNs based on MN height for each imaging modality were determined using receiver operating characteristic curve analysis.
RESULTS: Fifteen patients were diagnosed with BD-IPMN with MNs and underwent resection. The remaining 35 patients were diagnosed with BD-IPMN without MNs and underwent follow-up monitoring. The pathological findings revealed 14 cases with MNs and one case without. The accuracy for diagnosing MNs was 92% using CT and 72% using EUS; the diagnostic accuracy increased to 98% when EUS and CE-EUS were combined. The accuracy for measuring MN height significantly improved when using CE-EUS compared with using CT or EUS (median measurement error value, CT: 3.3 mm vs CE-EUS: 0.6 mm, P < 0.05; EUS: 2.1 mm vs CE-EUS: 0.6 mm, P < 0.01). A cut-off value of 8.8 mm for MN height as measured by CE-EUS improved the accuracy of diagnosing malignant BD-IPMN to 93%.
CONCLUSION: Using CE-EUS to measure MN height provides a highly accurate method for differentiating benign from malignant BD-IPMN.
Core tip: Both the presence and the height of mural nodules (MNs) are important for differentiating benign from malignant branch duct intraductal papillary mucinous neoplasm (BD-IPMN). However, no studies have determined the ability of contrast-enhanced endoscopic ultrasonography (CE-EUS) to accurately measure MN height. In this study, we demonstrated that CE-EUS is the optimal imaging modality for measuring MN height. Using CE-EUS to measure MN height improved the accuracy of the differential diagnosis of benign vs malignant BD-IPMN, therefore enabling patients to avoid unnecessary surgery.
- Citation: Harima H, Kaino S, Shinoda S, Kawano M, Suenaga S, Sakaida I. Differential diagnosis of benign and malignant branch duct intraductal papillary mucinous neoplasm using contrast-enhanced endoscopic ultrasonography. World J Gastroenterol 2015; 21(20): 6252-6260
- URL: https://www.wjgnet.com/1007-9327/full/v21/i20/6252.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i20.6252
Intraductal papillary mucinous neoplasm (IPMN) is defined as an intraductal, grossly visible (typically ≥ 1.0 cm) epithelial neoplasm of mucin-producing cells that arises in the main pancreatic duct (MPD) or its branches. The neoplastic epithelium is usually papillary, and the degrees of mucin secretion, duct dilation (cyst formation), and dysplasia are variable[1]. IPMN can be subdivided into main duct IPMN (MD-IPMN) and branch duct IPMN (BD-IPMN) depending on the location of the primary lesion[2]. Most BD-IPMNs are less invasive and can be monitored; thus, the differential diagnosis of benign and malignant BD-IPMN must be accurate to appropriately indicate surgical resection[3-6]. In 2006, an international panel of experts published the International Consensus Guidelines for the Management of IPMN (ICG2006)[7]. These guidelines were updated in 2012 (ICG2012)[8]. According to the most recent guidelines, all BD-IPMNs diagnosed with mural nodules (MNs) by computed tomography (CT), magnetic resonance imaging (MRI) or endoscopic ultrasound (EUS) are recommended for resection. However, certain studies have indicated that both the presence of MNs and their height may be risk factors for malignancy[9-13]. If MN height is included in the factors used to determine resection, superior criteria for BD-IPMN resection might be created. Therefore, in pre-surgical examinations of BD-IPMN, the identification of MNs as well as accurate measurements of MN height is important.
Recently, many reports have noted the effectiveness of contrast-enhanced EUS (CE-EUS) in the diagnosis of pancreatic tumors[14-17]. With regard to BD-IPMN, it has been reported that CE-EUS is effective at differentiating MNs from mucinous clots[18,19]. Furthermore, CE-EUS is effective at identifying the mucosal fluid attached to MNs, thus enabling more accurate measurements of MN height than when using EUS alone. However, to the best of our knowledge, no study has determined the ability of CE-EUS to accurately measure MN height.
Therefore, in this study, the utility of CE-EUS to accurately evaluate the presence and height of MNs was determined. The purpose of this study was to elucidate the role of CE-EUS in the differential diagnosis of benign and malignant BD-IPMN.
This study was approved by the Institutional Review Board of Yamaguchi University Graduate School of Medicine. The clinical records, EUS images, radiologic data, pathology, and surgical reports in this study were all reviewed retrospectively.
A total of 50 patients diagnosed with BD-IPMN by CT and EUS at our institute between April 2009 and March 2014 were included in this study. Of these, 15 patients diagnosed with MNs underwent resection, and 35 patients diagnosed without MNs were monitored during follow-up. However, two of the 35 patients underwent resection after becoming symptomatic or presenting with an increased MPD diameter despite being diagnosed without MNs. The remaining 33 patients diagnosed without MNs were followed up without intervention.
According to ICG2012, IPMN can be classified into three types: MD-IPMN, BD-IPMN, and mixed type[8]. According to the ICG2012 criteria, most cases of IPMN are classified as mixed type. Therefore, we defined all cases of IPMN, including mixed type, as BD-IPMN if branch duct dilation was the primary symptom. The pathological results were determined based on the World Health Organization classification system published in 2010[1]. In this study, noninvasive IPMN was considered benign, and only IPMN associated with an invasive carcinoma (IC) was defined as malignant.
EUS was performed using an electric radial-type endoscope (GF-UE260-AL5; Olympus, Tokyo, Japan) and an ultrasound system (ProSound SSD α-10; Aloka, Tokyo, Japan). When mural lesions were detected by EUS, a contrast-enhanced evaluation was conducted. To perform CE-EUS, we used Sonazoid (Daiichi Sankyo, Tokyo, Japan), which is a second-generation ultrasonographic contrast agent composed of perfluorobutane microbubbles with a median diameter of 2-3 μm. After reconstitution with 2 mL of sterile water for injection, 0.5 mL of the agent was administered through a peripheral vein. Each mural lesion was observed for two minutes, during which time the presence or absence of vascularity in the mural lesions was evaluated. Mural lesions that demonstrated vascularity were diagnosed as MNs, and mural lesions without detectable vasculature were diagnosed as mucinous clots. The evaluations were conducted by four or five on-site physicians specializing in biliopancreatic diseases.
Contrast-enhanced CT imaging was performed using a 64-section multidetector CT scanner (Definition and Somatom Sensation 64; Siemens Medical Solutions, Forchheim, Germany). Solid tumors demonstrating contrast effects within the cyst were diagnosed as having MNs. The evaluations were conducted by two or three radiologists specializing in digestive organs.
(1) In all 50 patients, the ability to diagnose the presence of MNs using each imaging modality (CT, EUS alone, EUS combined with CE-EUS) was calculated. The presence or absence of MNs was ultimately determined based on pathological findings in the 17 patients who underwent a resection. The 33 patients who were followed up without intervention were deemed to have no MNs if obvious malignant findings were not detected during the follow-up periods; (2) in the 15 patients who underwent resection due to being diagnosed with BD-IPMN with MNs, the accuracy of measuring MN height with each imaging modality was compared. In this study, the MN heights measured using CT, EUS, CE-EUS or pathological specimens were expressed as HCT, HEUS, HCE-EUS or HPath, respectively. HCT, HEUS and HCE-EUS were compared with HPath, and the absolute differences were calculated (|HCT-HPath|, |HEUS-HPath| and |HCE-EUS-HPath|). These numerical values were defined as the measurement error value and were compared; and (3) in the 15 patients who underwent resection after being diagnosed with BD-IPMN with MNs, the cut-off value for MN height as measured using each imaging modality or pathological specimens was established to differentiate between benign and malignant BD-IPMN.
Spearman’s correlation coefficient was used to identify correlations between MN height as measured by each imaging modality and MN height as measured on pathological specimens. The Wilcoxon t test with the Bonferroni correction was used to compare the measurement accuracy of MN height for each imaging modality. A receiver operating characteristic (ROC) curve analysis was used to determine the optimal cut-off value for MN height measured using each imaging modality or pathological specimens to differentiate between benign and malignant BD-IPMN. JMP 9 statistical software (SAS Institute Inc., Cary, North Carolina, United States) was used for the analysis. P < 0.05 was considered statistically significant.
The mean age of the patients was 67.7 ± 9.8 years. The cohort included 29 males and 21 females. The mean cyst diameter was 27.9 ± 10.9 mm, and the mean MPD diameter was 4.6 ± 3.3 mm.
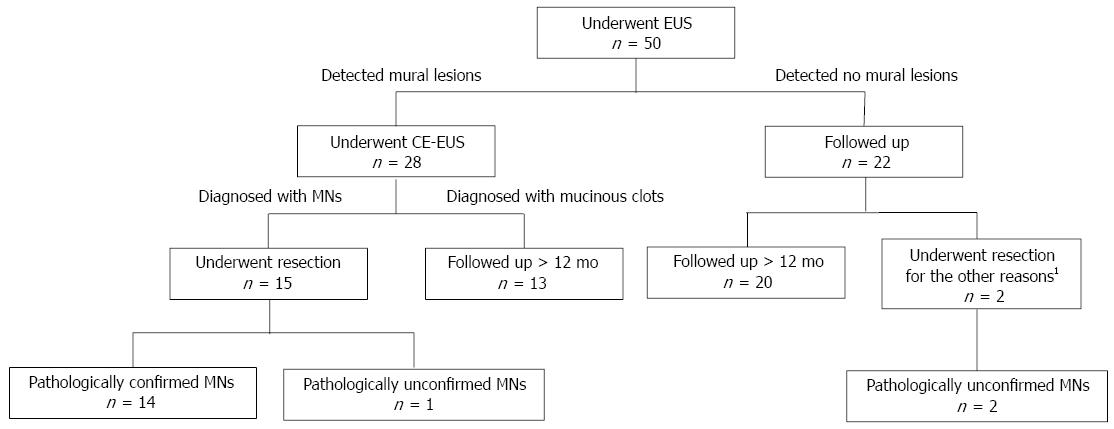
The flow chart presented in Figure 1 illustrates the clinical course of all the BD-IPMN patients. Of a total of 50 patients, mural lesions were detected by EUS in 28, and all of these patients subsequently underwent CE-EUS. Of these 28 patients, 15 were diagnosed with MNs by CE-EUS; these tumors were then surgically resected. Among these 15 patients, 14 cases were pathologically confirmed as MNs. Using CT, 10 out of the same 50 patients were determined to have MNs; these MNs were all pathologically confirmed. However, CT did not detect the remaining four cases of MNs that were diagnosed by CE-EUS.
Thirteen patients were diagnosed with mucinous clots by CE-EUS, and twenty-two patients were diagnosed as having no mural lesions by EUS. All 35 of these cases were monitored. The clinical features of these cases are presented in Table 1. MNs were not detected during the follow-up period using various imaging modalities. Of these, however, two patients underwent resection due to repeated pancreatitis or an increased MPD diameter to more than 3 mm. MNs could not be confirmed pathologically in either case. The remaining 33 cases were observed for more than 12 mo without obvious malignant findings.
| Follow-up cases (n = 35) | |
| Sex, M/F | 18/17 |
| Mean age ± SD, yr | 67.9 ± 10.2 |
| Mean follow-up period ± SD, mo | 27.4 ± 16.7 |
| Cyst size | |
| Initial examination ± SD, mm | 27.0 ± 11.8 |
| Last examination ± SD, mm | 30.1 ± 13.1 |
| Changes of the cyst size | |
| No change | 29 |
| Enlarged (≥ 10 mm) | 5 |
| Reduced (≥ 10 mm) | 1 |
| MPD diameter | |
| Initial examination ± SD, mm | 3.2 ± 1.8 |
| Last examination ± SD, mm | 3.5 ± 2.2 |
| Changes in MPD diameter | |
| No change | 26 |
| Enlarged (≥ 1 mm) | 7 |
| Reduced (≥ 1 mm) | 2 |
| Appearance of MNs during follow-up period | 0 |
| Followed up > 12 mo | 33 |
| Resected after follow-up | 2 |
| Pathological diagnosis | |
| Low-grade dysplasia | 0 |
| Intermediate-grade dysplasia | 0 |
| High-grade dysplasia | 2 |
| Invasive adenocarcinoma | 0 |
The sensitivity of CT for diagnosing MNs was 71%; the sensitivity of EUS alone for diagnosing MNs was 100%, although the specificity and positive predictive value (PPV) for diagnosing MNs were 61% and 50%, respectively. When EUS was combined with CE-EUS, the specificity and PPV for diagnosing MNs increased to 97% and 93%, respectively. The accuracy of CT, EUS alone and EUS combined with CE-EUS for diagnosing MNs was 92%, 72% and 98%, respectively (Table 2).
| Sensitivity (95%CI) | Specificity (95%CI) | PPV (95%CI) | NPV (95%CI) | Accuracy (95%CI) | |
| CT | 71% (0.42-0.92) | 100% (0.90-1.00) | 100% (0.69-1.00) | 90% (0.76-0.98) | 92% (0.80-0.98) |
| EUS alone | 100% (0.77-1.00) | 61% (0.43-0.77) | 50% (0.31-0.70) | 100% (0.85-1.00) | 72% (0.58-0.84) |
| EUS combined with CE-EUS | 100% (0.76-1.00) | 97% (0.85-1.00) | 93% (0.66-1.00) | 100% (0.90-1.00) | 98% (0.89-1.00) |
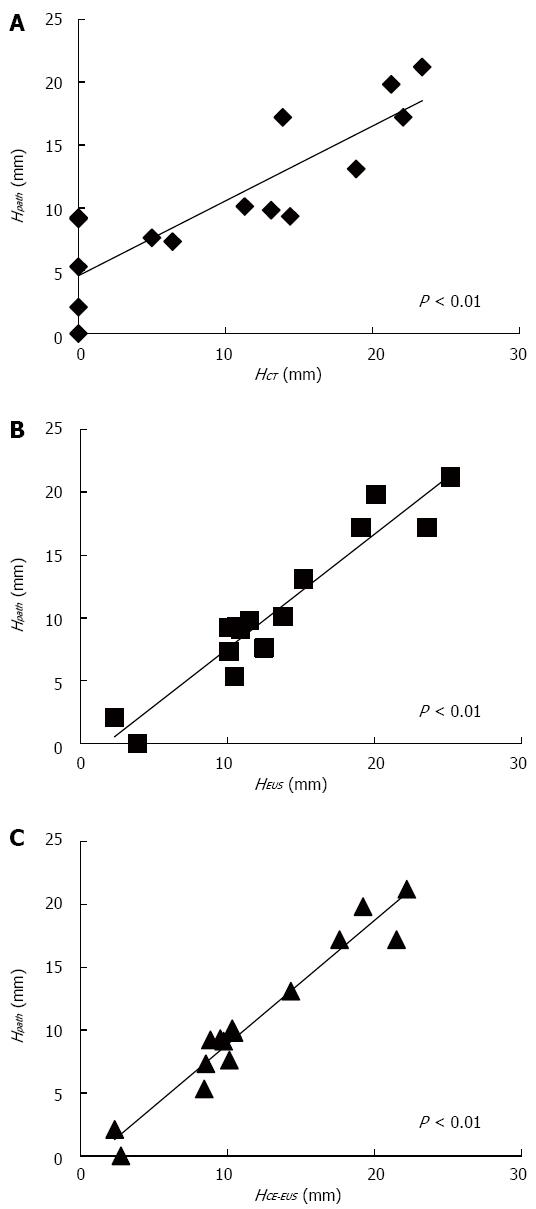
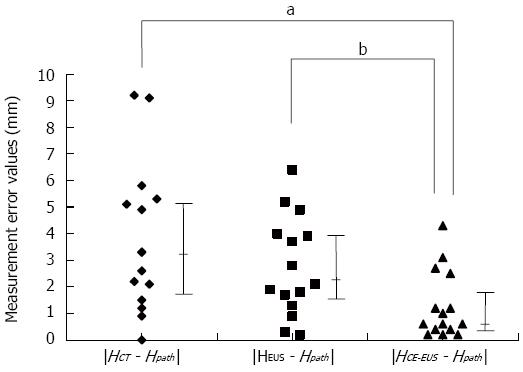
Table 3 shows the clinicopathologic features of the 15 patients who underwent resection after being diagnosed with MNs. There were significant positive correlations between MN height as measured by each imaging modality and MN height as measured on pathological specimens (Figure 2). The calculated measurement error values for each imaging modality are presented in Figure 3. The measurement error values for CE-EUS were significantly lower than those for CT or EUS (median measurement error value, CT: 3.3 mm vs CE-EUS: 0.6 mm, P < 0.05; EUS: 2.1 mm vs CE-EUS: 0.6 mm, P < 0.01).
| Case | Cyst size (mm) | MPD diameter (mm) | MNs | Pathological diagnosis | |||||||
| CT | EUS | CE-EUS | Pathology | ||||||||
| Presence | HCT (mm) | Presence | HEUS (mm) | Presence | HCE-EUS (mm) | Presence | HPath (mm) | ||||
| 1 | 36 | 12 | + | 23.4 | + | 25.2 | + | 22.2 | + | 21.2 | IC |
| 2 | 30 | 12 | + | 21.3 | + | 20.1 | + | 19.2 | + | 19.8 | IC |
| 3 | 40 | 12 | + | 13.9 | + | 19.1 | + | 17.6 | + | 17.2 | IC |
| 4 | 20 | 8 | + | 22.1 | + | 23.6 | + | 21.5 | + | 17.2 | IC |
| 5 | 50 | 2 | + | 18.9 | + | 15.2 | + | 14.3 | + | 13.1 | IC |
| 6 | 20 | 6 | + | 13.1 | + | 11.5 | + | 10.4 | + | 9.8 | IC |
| 7 | 30 | 9 | + | 14.4 | + | 10.6 | + | 9.5 | + | 9.3 | IC |
| 8 | 18 | 8 | - | 0 | + | 10.1 | + | 8.8 | + | 9.2 | IC |
| 9 | 38 | 6 | - | 0 | + | 10.9 | + | 9.7 | + | 9.1 | IC |
| 10 | 27 | 3 | + | 5.0 | + | 12.5 | + | 10.1 | + | 7.6 | IC |
| 11 | 25 | 13 | + | 11.3 | + | 13.8 | + | 10.3 | + | 10.1 | HGD |
| 12 | 30 | 6 | + | 6.4 | + | 10.1 | + | 8.5 | + | 7.3 | HGD |
| 13 | 30 | 6 | - | 0 | + | 10.5 | + | 8.4 | + | 5.3 | HGD |
| 14 | 31 | 12 | - | 0 | + | 2.3 | + | 2.3 | + | 2.1 | HGD |
| 15 | 28 | 3 | - | 0 | + | 3.9 | + | 2.7 | - | 0 | ImGD |
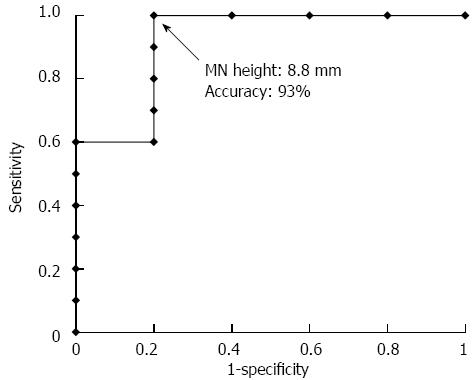
Of the 15 resected cases that were diagnosed with MNs, a pathological examination revealed that 10 cases were malignant BD-IPMN and five cases were benign BD-IPMN (Table 2). The ROC curve related to the diagnosis of malignant BD-IPMN based on MN height measured using CT, EUS, CE-EUS or pathological specimens yielded area under the curve values of 0.82, 0.87, 0.92 and 0.90, respectively (Table 4). Based on the ROC curve, 8.8 mm was determined to be the optimal threshold value for MN height measured by CE-EUS. With this cut-off value, the diagnosis of malignant BD-IPMN had a sensitivity, specificity, and accuracy of 100%, 86%, and 94%, respectively (Figure 4).
| AUC | Cutoff value (mm) | Sensitivity (95%CI) | Specificity (95%CI) | Accuracy (95%CI) | |
| CT | 0.82 | 13.1 | 70 (0.35-0.93) | 100 (0.48-1.00) | 80 (0.51-0.96) |
| EUS | 0.87 | 10.6 | 90 (0.53-1.00) | 80 (0.27-1.00) | 87 (0.58-0.99) |
| CE-EUS | 0.92 | 8.8 | 100 (0.69-1.00) | 80 (0.27-1.00) | 93 (0.66-1.00) |
| Pathological specimens | 0.90 | 7.6 | 100 (0.69-1.00) | 80 (0.27-1.00) | 93 (0.66-1.00) |
Although the frequency of IC in MD-IPMN is high (43.1%; 11%-81%), the frequency of IC in BD-IPMN is relatively low at only 17.7% (1.4%-36.7%). Deliberation is necessary before performing a resection for BD-IPMN because these lesions mostly occur in elderly patients, and the annual malignancy rate is only 2%-3%[8]. ICG2006 suggests that when encountering suspicious findings for malignant BD-IPMN, surgery is recommended in cases with (1) an MPD diameter of 6 mm or greater; (2) a cyst size of 30 mm or greater; or (3) the presence of MNs as determined by diagnostic imaging[7]. However, a recent meta-analysis demonstrated that rather than cyst size and MPD diameter, the presence of MNs is strongly indicative of malignant BD-IPMN[20]. In this study, all BD-IPMN cases in which MNs were pathologically confirmed were ultimately diagnosed with either high-grade dysplasia (HGD) or IC. Along with diagnostic imaging, the methods for diagnosing malignant BD-IPMN include pancreatic fluid cell examination and cyst fluid examination; however, both methods have disadvantages. Pancreatic fluid cell examination has the advantage of high specificity, but the sensitivity can vary widely from 11%-92%[21-23], leading to a high risk of false negatives. Although EUS-guided fine needle aspiration cytology and laboratory analysis of cyst fluid have provided excellent results in certain studies[24-27], the safety of this method remains unclear because other reports have indicated the possibility of peritoneal dissemination due to the leakage of the cyst contents[28,29]. Therefore, we believe that diagnosing malignant BD-IPMN by evaluating MNs is both accurate and safe.
CT has been reported as effective for diagnosing MNs in addition to providing the information on BD-IPMN morphology, specifically the location and the presence of any communication with the MPD[30,31]. Nakagawa et al[32] reported that using CT to detect MNs in BD-IPMN yielded a sensitivity, specificity and accuracy of 68%, 100%, and 77%, respectively. In our study, using CT to diagnose MNs in BD-IPMN yielded a sensitivity, specificity, and accuracy of 71%, 100%, and 92%, respectively, which were superior values to those reported by Nakagawa et al[32] Despite this, out of 14 pathologically confirmed BD-IPMN cases with MNs, CT failed to detect four cases (29%) of MNs during the pre-surgical examination. Furthermore, of these four cases, two were eventually diagnosed with IC based on pathological examination, thus indicating that there are limits to differentiating benign and malignant BD-IPMN using CT alone.
EUS is an invaluable modality for evaluating pancreatic diseases because of its high spatial resolution. Compared with CT and MRI, EUS is excellent at detecting small pancreatic lesions and is also useful for diagnosing IPMN[33]. Thus far, EUS has demonstrated a high level of sensitivity at diagnosing MNs in BD-IPMN in addition to producing very few false negatives[34,35]. In this study, no false negatives were obtained with EUS. The 22 BD-IPMN patients diagnosed without MNs using EUS at the initial examinations did not have detectable MNs during the entire follow-up period. However, because it is difficult to differentiate MNs from mucinous clots using EUS alone, the specificity of this methodology is low, and there is a high risk of false positives[32]. In this study, 13 of the 28 mural lesions (46%) detected by EUS alone were, in fact, mucinous clots. The accuracy of EUS alone (72%) was insufficient. Zhong et al[36] reported that ascertaining the features of MNs can improve the ability to differentiate mucinous clots from MNs; however, this only increases the accuracy to 79%, which is not ideal. Therefore, it is difficult to accurately diagnose the presence of MNs using EUS alone.
Originally, a contrast-enhanced imaging technique was not available for EUS because the transducer of the echoendoscope was too small to produce enough acoustic power to perform contrast-enhanced imaging using a first-generation ultrasound contrast agent. A recently developed second-generation contrast agent now allows for the production of harmonic signals, even at lower acoustic power, making CE-EUS available for clinical use[14]. To date, there have been relatively few reports of CE-EUS being used to diagnose pancreatic cystic tumors, and only a few reports exist on the utility of CE-EUS for diagnosing MNs in BD-IPMN[18,19]. In this study, we looked not only at cases in which MNs were diagnosed using CE-EUS and resected but also at cases in which mucinous clots were diagnosed using CE-EUS and followed up. The results indicated that 14 of the 15 cases (93%) diagnosed with MNs using CE-EUS were subsequently pathologically confirmed to have MNs. In addition, the 13 cases diagnosed with mucinous clots by CE-EUS showed no malignant findings during follow-up, and they presented no contradictions regarding the absence of MNs. The accuracy of diagnosing MNs in BD-IPMN increased from 72% to 98% when EUS was combined with CE-EUS. The accuracy of EUS combined with CE-EUS was better than that for CT or EUS alone. As a result, we believe that CE-EUS is the most appropriate method for detecting the presence of MNs in BD-IPMN.
Certain studies have indicated the possibility that MN height is a risk factor for malignant BD-IPMN[9-13]. Therefore, accurately detecting the presence of MNs and accurately measuring MN height may contribute to the differential diagnosis of benign and malignant BD-IPMN. To the best of our knowledge, no previous studies including CE-EUS have determined the optimal imaging modality for measuring MN height. In this study, the measurement error values, calculated as the absolute values of the difference between the MN height measured by specific imaging modalities and that measured on pathological specimens, were compared. The results indicated that the measurement error values for CE-EUS were significantly lower than those for CT or EUS, supporting our conclusion that CE-EUS is the optimum imaging modality for measuring MN height in BD-IPMN. CE-EUS most likely both differentiates the mucosal fluid attached to MNs and clarifies the structure of the cystic wall, making it possible to accurately measure MN height. The threshold value of 8.8 mm, which was determined from a ROC curve related to the diagnosis of malignant BD-IPMN based on MN height as measured by CE-EUS, gave an excellent accuracy of 93%. CE-EUS accurately measures MN height and has potential applicability in differentiating benign and malignant BD-IPMN.
There were several limitations to our research. Firstly, because this study was a retrospective study with a limited cohort, there is the possibility of selection bias. Secondly, the study involved patients who underwent resection as well as those who were merely monitored. As a result, it was not possible to pathologically confirm whether MNs were actually present in each case. Despite these limitations, CE-EUS demonstrated effectiveness at diagnosing the presence of MNs and in accurately measuring MN height. Recent studies have already begun to adopt MN height to inform treatment policy in BD-IPMN cases[37]. According to our results, CE-EUS is the optimum modality for measuring the height of MNs. If indications of resection in BD-IPMN cases can be determined from both the presence of MNs and their height as measured by CE-EUS, many unnecessary surgeries can be avoided.
In conclusion, CE-EUS offers the potential to accurately diagnose the presence of MNs in BD-IPMN. According to our results, in cases of a diagnosis of BD-IPMN with MNs, it is highly probable that the pathological diagnosis will be HGD or IC, in which case resection is indicated; this is consistent with ICG2012. Furthermore, if the MN height, as measured by CE-EUS, is 8.8 mm or greater, it is highly probable that the pathological diagnosis will be indicative of IC, resulting in a strong positive recommendation for resection. CE-EUS not only diagnoses the presence of MNs but also facilitates the measurement of MN height, thereby playing an important role in differentiating benign and malignant BD-IPMN. CE-EUS should therefore be considered necessary for the accurate pre-surgical evaluation of BD-IPMN.
Intraductal papillary mucinous neoplasm (IPMN) represents one type of pancreatic tumor. IPMN can be subdivided into main duct IPMN and branch duct IPMN (BD-IPMN). Most BD-IPMNs are less invasive and can be routinely monitored; thus, the differential diagnosis of benign and malignant BD-IPMN must be accurate to indicate surgical resection.
A meta-analysis demonstrated that the presence of mural nodules (MNs) is a highly suspicious finding for malignant BD-IPMN. Furthermore, certain studies have indicated that both the presence of MNs and their height may be risk factors for malignant BD-IPMN.
This is the first study to demonstrate that contrast-enhanced endoscopic ultrasonography (CE-EUS) is the optimal imaging modality for detecting the presence of MNs and measuring MN height. CE-EUS enabled the detection of MNs with an accuracy of 98%. CE-EUS measured MN height significantly better than computed tomography or endoscopic ultrasonography (EUS). A receiver operating characteristic curve analysis related to the diagnosis of malignant BD-IPMN based on MN height as measured by CE-EUS was performed, and the cut-off value was determined to be 8.8 mm, which yielded an excellent accuracy of 93%.
The results of this study suggest that using CE-EUS for the pre-surgical evaluation of BD-IPMN will improve the diagnostic accuracy of malignant BD-IPMN and will help patients avoid unnecessary surgery.
Originally, a contrast-enhanced imaging technique was not available for EUS, but a second-generation contrast agent now allows for the clinical use of CE-EUS. Recently, many reports have noted the effectiveness of CE-EUS in the diagnosis of pancreatic lesions.
The authors elucidated the role of CE-EUS in the differential diagnosis of benign and malignant BD-IPMN. And the result of research is inspiring and helpful for clinical practice to diagnose BD-IPMN.
P- Reviewer: Gao CM, Gu GL S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN
| 1. | Adsay NV, Fukushima N, Furukawa T, Hruban RH, Klimstra DH, Klöppel G, Offerhaus GJA, Pitman MB, Shimizu M, Zamboni G. Intraductal neoplasms of the pancreas. WHO classification of tumors of the digestive system. Lyon: IARC Press 2010; 304-313. |
| 2. | Kobari M, Egawa S, Shibuya K, Shimamura H, Sunamura M, Takeda K, Matsuno S, Furukawa T. Intraductal papillary mucinous tumors of the pancreas comprise 2 clinical subtypes: differences in clinical characteristics and surgical management. Arch Surg. 1999;134:1131-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 229] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 3. | Terris B, Ponsot P, Paye F, Hammel P, Sauvanet A, Molas G, Bernades P, Belghiti J, Ruszniewski P, Fléjou JF. Intraductal papillary mucinous tumors of the pancreas confined to secondary ducts show less aggressive pathologic features as compared with those involving the main pancreatic duct. Am J Surg Pathol. 2000;24:1372-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 278] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 4. | Matsumoto T, Aramaki M, Yada K, Hirano S, Himeno Y, Shibata K, Kawano K, Kitano S. Optimal management of the branch duct type intraductal papillary mucinous neoplasms of the pancreas. J Clin Gastroenterol. 2003;36:261-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 162] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 5. | Lévy P, Jouannaud V, O’Toole D, Couvelard A, Vullierme MP, Palazzo L, Aubert A, Ponsot P, Sauvanet A, Maire F. Natural history of intraductal papillary mucinous tumors of the pancreas: actuarial risk of malignancy. Clin Gastroenterol Hepatol. 2006;4:460-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 163] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 6. | Kang MJ, Jang JY, Kim SJ, Lee KB, Ryu JK, Kim YT, Yoon YB, Kim SW. Cyst growth rate predicts malignancy in patients with branch duct intraductal papillary mucinous neoplasms. Clin Gastroenterol Hepatol. 2011;9:87-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 158] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 7. | Tanaka M, Chari S, Adsay V, Fernandez-del Castillo C, Falconi M, Shimizu M, Yamaguchi K, Yamao K, Matsuno S. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1539] [Cited by in RCA: 1441] [Article Influence: 75.8] [Reference Citation Analysis (0)] |
| 8. | Tanaka M, Fernández-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, Kimura W, Levy P, Pitman MB, Schmidt CM. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1714] [Cited by in RCA: 1614] [Article Influence: 124.2] [Reference Citation Analysis (0)] |
| 9. | Kubo H, Chijiiwa Y, Akahoshi K, Hamada S, Harada N, Sumii T, Takashima M, Nawata H. Intraductal papillary-mucinous tumors of the pancreas: differential diagnosis between benign and malignant tumors by endoscopic ultrasonography. Am J Gastroenterol. 2001;96:1429-1434. [PubMed] |
| 10. | Kobayashi G, Fujita N, Noda Y, Ito K, Horaguchi J, Takasawa O, Akaishi S, Tsuchiya T, Kobari M. Mode of progression of intraductal papillary-mucinous tumor of the pancreas: analysis of patients with follow-up by EUS. J Gastroenterol. 2005;40:744-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Okabayashi T, Kobayashi M, Nishimori I, Sugimoto T, Namikawa T, Okamoto K, Okamoto N, Kosaki T, Onishi S, Araki K. Clinicopathological features and medical management of intraductal papillary mucinous neoplasms. J Gastroenterol Hepatol. 2006;21:462-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Hirono S, Tani M, Kawai M, Ina S, Nishioka R, Miyazawa M, Fujita Y, Uchiyama K, Yamaue H. Treatment strategy for intraductal papillary mucinous neoplasm of the pancreas based on malignant predictive factors. Arch Surg. 2009;144:345-39; discussion 345-39;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Shimizu Y, Yamaue H, Maguchi H, Yamao K, Hirono S, Osanai M, Hijioka S, Hosoda W, Nakamura Y, Shinohara T. Predictors of malignancy in intraductal papillary mucinous neoplasm of the pancreas: analysis of 310 pancreatic resection patients at multiple high-volume centers. Pancreas. 2013;42:883-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 14. | Kitano M, Sakamoto H, Komaki T, Kudo M. New techniques and future perspective of EUS for the differential diagnosis of pancreatic malignancies: contrast harmonic imaging. Dig Endosc. 2011;23 Suppl 1:46-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Matsubara H, Itoh A, Kawashima H, Kasugai T, Ohno E, Ishikawa T, Itoh Y, Nakamura Y, Hiramatsu T, Nakamura M. Dynamic quantitative evaluation of contrast-enhanced endoscopic ultrasonography in the diagnosis of pancreatic diseases. Pancreas. 2011;40:1073-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 16. | Săftoiu A, Dietrich CF, Vilmann P. Contrast-enhanced harmonic endoscopic ultrasound. Endoscopy. 2012;44:612-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 17. | Gong TT, Hu DM, Zhu Q. Contrast-enhanced EUS for differential diagnosis of pancreatic mass lesions: a meta-analysis. Gastrointest Endosc. 2012;76:301-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 18. | Ohno E, Hirooka Y, Itoh A, Ishigami M, Katano Y, Ohmiya N, Niwa Y, Goto H. Intraductal papillary mucinous neoplasms of the pancreas: differentiation of malignant and benign tumors by endoscopic ultrasound findings of mural nodules. Ann Surg. 2009;249:628-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 146] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 19. | Yamashita Y, Ueda K, Itonaga M, Yoshida T, Maeda H, Maekita T, Iguchi M, Tamai H, Ichinose M, Kato J. Usefulness of contrast-enhanced endoscopic sonography for discriminating mural nodules from mucous clots in intraductal papillary mucinous neoplasms: a single-center prospective study. J Ultrasound Med. 2013;32:61-68. [PubMed] |
| 20. | Kim KW, Park SH, Pyo J, Yoon SH, Byun JH, Lee MG, Krajewski KM, Ramaiya NH. Imaging features to distinguish malignant and benign branch-duct type intraductal papillary mucinous neoplasms of the pancreas: a meta-analysis. Ann Surg. 2014;259:72-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 133] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 21. | Yamaguchi K, Nakamura M, Shirahane K, Kawamoto M, Konomi H, Ohta M, Tanaka M. Pancreatic juice cytology in IPMN of the pancreas. Pancreatology. 2005;5:416-21; discussion 421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Hirono S, Tani M, Kawai M, Okada K, Miyazawa M, Shimizu A, Kitahata Y, Yamaue H. The carcinoembryonic antigen level in pancreatic juice and mural nodule size are predictors of malignancy for branch duct type intraductal papillary mucinous neoplasms of the pancreas. Ann Surg. 2012;255:517-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 113] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 23. | Sai JK, Nobukawa B, Matsumura Y, Watanabe S. Pancreatic duct lavage cytology with the cell block method for discriminating benign and malignant branch-duct type intraductal papillary mucinous neoplasms. Gastrointest Endosc. 2013;77:726-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Maire F, Couvelard A, Hammel P, Ponsot P, Palazzo L, Aubert A, Degott C, Dancour A, Felce-Dachez M, O’toole D. Intraductal papillary mucinous tumors of the pancreas: the preoperative value of cytologic and histopathologic diagnosis. Gastrointest Endosc. 2003;58:701-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 87] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 25. | Pelaez-Luna M, Chari ST, Smyrk TC, Takahashi N, Clain JE, Levy MJ, Pearson RK, Petersen BT, Topazian MD, Vege SS. Do consensus indications for resection in branch duct intraductal papillary mucinous neoplasm predict malignancy? A study of 147 patients. Am J Gastroenterol. 2007;102:1759-1764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 205] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 26. | de Jong K, Poley JW, van Hooft JE, Visser M, Bruno MJ, Fockens P. Endoscopic ultrasound-guided fine-needle aspiration of pancreatic cystic lesions provides inadequate material for cytology and laboratory analysis: initial results from a prospective study. Endoscopy. 2011;43:585-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 27. | Thornton GD, McPhail MJ, Nayagam S, Hewitt MJ, Vlavianos P, Monahan KJ. Endoscopic ultrasound guided fine needle aspiration for the diagnosis of pancreatic cystic neoplasms: a meta-analysis. Pancreatology. 2013;13:48-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 191] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 28. | Hirooka Y, Goto H, Itoh A, Hashimoto S, Niwa K, Ishikawa H, Okada N, Itoh T, Kawashima H. Case of intraductal papillary mucinous tumor in which endosonography-guided fine-needle aspiration biopsy caused dissemination. J Gastroenterol Hepatol. 2003;18:1323-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 148] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 29. | Yamao K, Yanagisawa A, Takahashi K, Kimura W, Doi R, Fukushima N, Ohike N, Shimizu M, Hatori T, Nobukawa B. Clinicopathological features and prognosis of mucinous cystic neoplasm with ovarian-type stroma: a multi-institutional study of the Japan pancreas society. Pancreas. 2011;40:67-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 181] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 30. | Kawamoto S, Lawler LP, Horton KM, Eng J, Hruban RH, Fishman EK. MDCT of intraductal papillary mucinous neoplasm of the pancreas: evaluation of features predictive of invasive carcinoma. AJR Am J Roentgenol. 2006;186:687-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 105] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 31. | Yamada Y, Mori H, Matsumoto S, Hijiya N, Hongo N, Moriyama M. Invasive carcinomas originating from intraductal papillary mucinous neoplasms of the pancreas: conspicuity and primary sites of the solid masses on triple-phase dynamic CT imaging. Abdom Imaging. 2010;35:181-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Nakagawa A, Yamaguchi T, Ohtsuka M, Ishihara T, Sudo K, Nakamura K, Hara T, Denda T, Miyazaki M. Usefulness of multidetector computed tomography for detecting protruding lesions in intraductal papillary mucinous neoplasm of the pancreas in comparison with single-detector computed tomography and endoscopic ultrasonography. Pancreas. 2009;38:131-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 33. | Kamata K, Kitano M, Kudo M, Sakamoto H, Kadosaka K, Miyata T, Imai H, Maekawa K, Chikugo T, Kumano M. Value of EUS in early detection of pancreatic ductal adenocarcinomas in patients with intraductal papillary mucinous neoplasms. Endoscopy. 2014;46:22-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 34. | Sugiyama M, Atomi Y, Saito M. Intraductal papillary tumors of the pancreas: evaluation with endoscopic ultrasonography. Gastrointest Endosc. 1998;48:164-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 63] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Baba T, Yamaguchi T, Ishihara T, Kobayashi A, Oshima T, Sakaue N, Kato K, Ebara M, Saisho H. Distinguishing benign from malignant intraductal papillary mucinous tumors of the pancreas by imaging techniques. Pancreas. 2004;29:212-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 36. | Zhong N, Zhang L, Takahashi N, Shalmiyev V, Canto MI, Clain JE, Deutsch JC, DeWitt J, Eloubeidi MA, Gleeson FC. Histologic and imaging features of mural nodules in mucinous pancreatic cysts. Clin Gastroenterol Hepatol. 2012;10:192-18, 192-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 37. | Kobayashi G, Fujita N, Maguchi H, Tanno S, Mizuno N, Hanada K, Hatori T, Sadakari Y, Yamaguchi T, Tobita K. Natural history of branch duct intraductal papillary mucinous neoplasm with mural nodules: a Japan Pancreas Society multicenter study. Pancreas. 2014;43:532-538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |