Published online May 28, 2015. doi: 10.3748/wjg.v21.i20.6157
Peer-review started: July 26, 2014
First decision: September 27, 2014
Revised: November 10, 2014
Accepted: December 20, 2014
Article in press: December 22, 2014
Published online: May 28, 2015
Processing time: 309 Days and 2.8 Hours
AIM: To evaluate the efficacy of the improved thrombospondin mimetic peptide ABT-898 in a murine model of ulcerative colitis.
METHODS: The dextran sodium sulfate (DSS) was used for the induction of colitis in both TSP-1 deficient (TSP-1-/-) and wild type (WT) mice during 7 d. While mice were receiving the DSS dissolved in the drinking water, the ABT-898 peptide was dissolved in sterile 5% glucose solution and delivered using mini pumps subcutaneously implanted. Plasma samples were analyzed for interleukin (IL)-6 by ELISA assay and colonic tissues were harvested, fixed and processed for histological evaluation. Immunohistochemistry using antibodies for the detection of CD31 and MECA in endothelial cells was performed. Inflammation was graded in colonic sections and the number of microvessels in each lesion was assessed. Activation of signal transducer and activator of transcription 3 (STAT3) in colonic samples was quantified by immunohistochemistry and Western blotting using antibodies against total STAT3 and phosphorylated STAT3 (pSTAT3) (Ser727).
RESULTS: Treatment with ABT-898 considerably diminished the inflammatory response in WT and TSP-1-/- mice (P < 0.0001 in both groups vs control). Identification of blood vessels highlighted by CD31/MECA immunohistochemistry, showed significantly reduced vessel counts in colitic lesions of WT and TSP-1-/- mice treated with ABT898 (TSP-1-/- controls/TSP-1-/- treated, P = 0.0002; WT controls/WT treated, P = 0.0005). Consistently, IL-6 was significantly diminished in plasma samples of TSP-1-/- and WT treated with the peptide when compared to the control mice (P = 0.0002 and P = 0.0148, respectively). pSTAT3 positive cells were quantified in WT and TSP-1-/- treated with ABT-898. A significant decrease in positive cells for pSTAT3 was observed in treated mice (TSP-1-/- controls/TSP-1-/- treated, P = 0.0089; WT/WT treated, P = 0.0110). These results were confirmed by Western blotting analyses showing lower levels of pSTAT3 in colitic lesions from mice treated with the peptide ABT-898.
CONCLUSION: These findings indicate that the new peptide ABT-898 ameliorates inflammation and angiogenesis and might be a therapeutic alternative in IBD and inflammatory diseases.
Core tip: Inflammatory bowel disease is still incurable and a major burden in the patient’s life and health care system. The discovery of new and safe therapeutic alternatives is urgently needed. This study tested the efficacy of a new thrombospondin- derived peptide, ABT-898 in a murine model of colitis. Our results indicate that this peptide was able to ameliorate inflammation and angiogenesis. In addition, mice treated with ABT-898 showed significant decrease of plasmatic Interleukin-6 and lesser activation of signal transducer and activator of transcription 3 in colitic lesions. These findings suggest that ABT-898 may indeed be an alternative treatment for inflammatory bowel disease.
- Citation: Gutierrez LS, Ling J, Nye D, Papathomas K, Dickinson C. Thrombospondin peptide ABT-898 inhibits inflammation and angiogenesis in a colitis model. World J Gastroenterol 2015; 21(20): 6157-6166
- URL: https://www.wjgnet.com/1007-9327/full/v21/i20/6157.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i20.6157
Thrombospondin 1 (TSP-1) is a well-known anti-angiogenic protein that induces apoptosis in endothelial cells and inhibits their proliferation[1]. This protein regulates inflammation by multiple mechanisms[2] and its expression has been detected in inflammatory diseases such as rheumatoid arthritis and dermatitis[3]. TSP-1 is expressed in kidney diseases including glomerulonephritis[4], suggesting a close association between TSP-1, inflammation and early fibrosis.
TSP-1[5] as well as factors regulating angiogenesis such as vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) are upregulated in inflamed colonic tissues. Angiopoetin-1 and VEGF could activate the innate immune system of the vessel wall, stimulating the production of pro-angiogenic inflammatory cytokines[6]. In addition, these factors have been found altered in the serum of patients with inflammatory bowel disease (IBD)[7]. Mice with a targeted deficiency of TSP-1 (TSP-1-/-) show high levels of plasmatic VEGF and bFGF during chronically induced colitis[8].
TSP-1 also modulates vascular leakage and remodeling in acute hypersensitivity[9]. TSP-1-/- mice exhibit a delayed resolution of the inflammation as well as an enhanced vascular remodeling[9]. These mice also display leukocytic infiltrates in the lung, suffer from leukocytosis and augmented colitis[10,11].
As a major activator of transforming growth factor beta 1 (TGFβ1), TSP-1 may play an important role in inflammatory processes[12]. Actually, TGFβ1-deficient mice show enhanced inflammatory phenotype and augmented tumor burden in experimental models of colitis and colorectal carcinogenesis, a phenotype also observed in TSP-1-/- mice[13,14].
The anti-inflammatory and anti-angiogenic properties of TSP-1 are mediated by its interaction with the transmembrane receptors CD36 and CD47[15,16]. TSP-1 induces endothelial cell death upon its binding to CD36. This process upregulates the Fas-Fas ligand system and initiates the apoptotic cascade of caspases[15]. By inducing apoptosis, TSP-1 might also regulate the secretion of cytokines and growth factors implicated in the immune response. TSP-1 also modulates the functions of nitric oxide (NO), a critical molecule involved in a variety of physiological events such as vasodilation and chemotaxis[17].
Peptides corresponding to specific domains of TSP-1, have shown antiangiogenic and anti-inflammatory properties in pre-clinical studies[16,17] and in combined therapies in clinical trials as well[18]. One of these peptides ABT-510 is a nonapeptide peptide simulating the sequence GVITRIR, enclosed within the second type 1 repeat of thrombospondin 1 (TSR). TSP-1 mimetic nonapeptide has the sequence: acetyl-sarcosine-glycine-valine-D-alloisoleucine-threonine-norvaline-isoleucine-arginine-proline-ethylamide (NAcSarGly-Val-D-Ile-T-N-Ile-Arg-ProNHEt). This domain directly interacts with CD36[16] and induces cell death predominantly in endothelial and smooth muscle cells. As a result, this domain has major anti-angiogenic functions. Most recently, a modified and improved TSP mimetic peptide is available, A-428898 (ABT-898; Abbott Laboratories). This peptide is an octapeptide with a sequence that provides more stability and longer half-life (NAcGly-Val-D-Ile-Ser-Gln-Ile-Arg-ProNHEt). Data herein show that mice treated with ABT-898 display reduced inflammation and angiogenesis in colons, lower levels of interleukin (IL)-6 in plasma and decreased signal transducer and activator of transcription 3 (STAT3) activation in colitic lesions. TSP-1 mimetic peptides may ameliorate inflammation and serve as alternative treatment for inflammatory diseases.
All animal procedures were performed with the approval of the Wilkes University Institutional Animal Care and Use Committee, in accordance with the Declaration of Helsinki and the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the NIH. DSS (MW: 36000-40000, MP Biomedical, Aurora, OH) was dissolved in the drinking water at a dilution of 2.5% (wt/v) and administered for 7 d to induce acute colitis in WT and TSP-1-/- mice (Jackson Laboratories, Bar Harbor, Maine).
WT (n = 16) and TSP-1-/- (n = 13) mice were anesthetized and osmotic mini-pumps (Alzet, Cupertino, CA) were subcutaneously implanted. These mini-pumps contained the peptide ABT-898 dissolved in sterile 5% glucose solution (Abbott Laboratories, Chicago, IL). Pumps delivered this solution at controlled rates (0.5 μL/h). The dose for ABT-898 was 60 mg/kg per day. Pumps containing only sterile 5% glucose were also implanted in WT (n = 15) and TSP-1-/- mice (n = 13) as controls.
Intestines were removed, opened longitudinally and rinsed with ice-cold phosphate buffer solution (PBS). For morphological studies, tissues were fixed with Histochoice (Electron Microscopy Sciences, Hartfield, PA), processed and cut in serial sections (5 μm). Sections were stained with hematoxylin and eosin for histopathological analysis. Immunohistochemistry (IHC) sections were incubated overnight a purified rat anti-mouse CD31 (BD Pharmingen, San Diego, CA), MECA 32 and STAT3 and phospho-STAT3 (Ser727) (all from BioLegend, San Diego, CA). Sections were immersed in biotinylated goat anti-rat IgG Impress (Vector Laboratories,) diluted in PBS for 30 min. Color was developed using a 3, 3'-diaminobenzidine substrate kit (Vector Laboratories).
Inflammation grading and evaluations of MECA 34/CD31 were performed in colonic sections from mice drinking DSS for 7 d. Sections were screened at low magnification (× 40) to detect areas with colitis. After areas of inflammation were identified, computer digitized images were taken at × 100 or × 400 magnifications using a color digital camera (Olympus Corporation, Tokyo, Japan). Pictures were stored in a memory card and recoded as frame numbers. Frames were blindly analyzed by multiple observers in a monitor in a blindly fashion. A minimum of 5 microphotographs was taken from each colonic section.
Colonic inflammation was graded in hematoxylin-eosin stained sections as follows: 0, no inflammation; 1, modest numbers of infiltrating leukocytes in the lamina propria; 2, infiltration of leukocytes leading to separation of crypts and mild mucosal hyperplasia; 3, massive infiltration of inflammatory cells accompanied by complete disruption of the mucosal architecture and loss of epithelium. The number of pSTAT3 positive cells was recorded for each frame. The number of vessels/field was measured as mean vascular density (MVD). MVD was assessed by counting the vessels in each colitic lesions showing positive stain for MECA/CD31. The number of pSTAT3 positive cells (brown staining) was recorded for each frame/field as well.
Plasma samples were collected from WT and TSP1-/- mice under acute DSS induced colitis after 7 d treated or not with ABT-898. A mouse cytokine Multi-Analyte ELISArray Kit (SABiosciences Frederick, MD) was used to measure the cytokine production of IL1A, IL1B, IL2, IL4, IL5, IL6, IL8, IL10, IL12, IL13, IL17A and Granulocyte-Macrophage Colony Stimulating Factor (GM-CSF) in mice with or without ABT-898 treatment. The arrays were performed according to the manufacturer’s instructions. The absorbance levels of the cytokines were measured on a plate reader (Beckman Coulter DTX 880) at 450 nm. The IL-6 concentrations in the plasma were measured with R&D ELISA (Minn, United States) kit. Briefly, 100 μL of plasma were applied to the immunoplate precoated with anti-human or anti-mouse monoclonal antibody. Secondary detection antibody of each assay was then added to the immobilized IL-6 in the sample. The conjugation of anti-IL-6 with their antigens was visualized using Avidin-HRP substrate. The absorbance levels of proteins were measured on a plate reader at 450 nm. Sampling was performed in triplicates. All the results were representative of multiple and separated experiments. Sampling was performed in triplicates including standards.
Intestinal tissues were collected and briefly rinsed with cold PBS and stored at -80 °C until use. Protein was extracted with buffer A (25 mmol/L Tris-HCl pH 7.6, 150 mmol/L NaCl, 0.5% NP-40, 0.5% Sodium deoxycholate, 0.05% SDS and 5 mmol/L β-mercaptoethanol) supplemented with protease inhibitors cocktail (BP-477, Boston Bioproducts, Worchester, MA) and phosphatase inhibitors cocktail (BP-480, Boston Bioproducts) prior to use. Tissue was homogenized in buffer A followed by brief sonication (10 s each for 3 times) on ice.
The extract was then centrifuged at 12000 rpm for 10 min at 4 °C and the supernatant taken as the total protein lysate for protein analysis. Protein concentration was measured using the Bradford assay (Coomassie Plus, Pierce Rockford, IL). Equal amounts of protein were separated by SDS-PAGE and transferred onto the nitrocellulose membrane by a semi-dry transfer procedure (Bio-Rad, Hercules, CA). The quantitative Western blot (WB) was carried out according to the Odyssey protocol (LI-COR, Inc, Lincoln, NE). Briefly, the membrane was blocked for 1 h at room temperature in the blocking buffer. Then, it was hybridized with the primary antibody against total STAT3 or phospho-STAT3 (Ser727) (p-STAT3) at 1:1000 dilution in TBST (50 mmol/L Tris/HCl, pH 7.5, 150 mmol/L NaCl, 0.1% Tween-20) containing 1% bovine serum albumin overnight at 4 °C. The membrane was then washed with TBST for 3-5 times, followed by hybridization with infrared IRDye®-labeled secondary antibody at a dilution of 1:5000 in TBST/1% BSA for 1 h. The membranes were washed with TBST (× 5) and PBS (× 2) for image acquisition using an Odyssey infrared scanner (Li-cor, United States). Data were analyzed using the Odyssey software 3.0 to quantify of pSTAT3 in three independent Western blotting analyses.
Data was analyzed for significance by a one-way ANOVA (analysis of variance). Calculations were performed using StatView system for Macintosh (Abacus Concepts, Berkeley, CA). P < 0.05 was considered significant. Where appropriate, values are expressed as the mean ± SE.
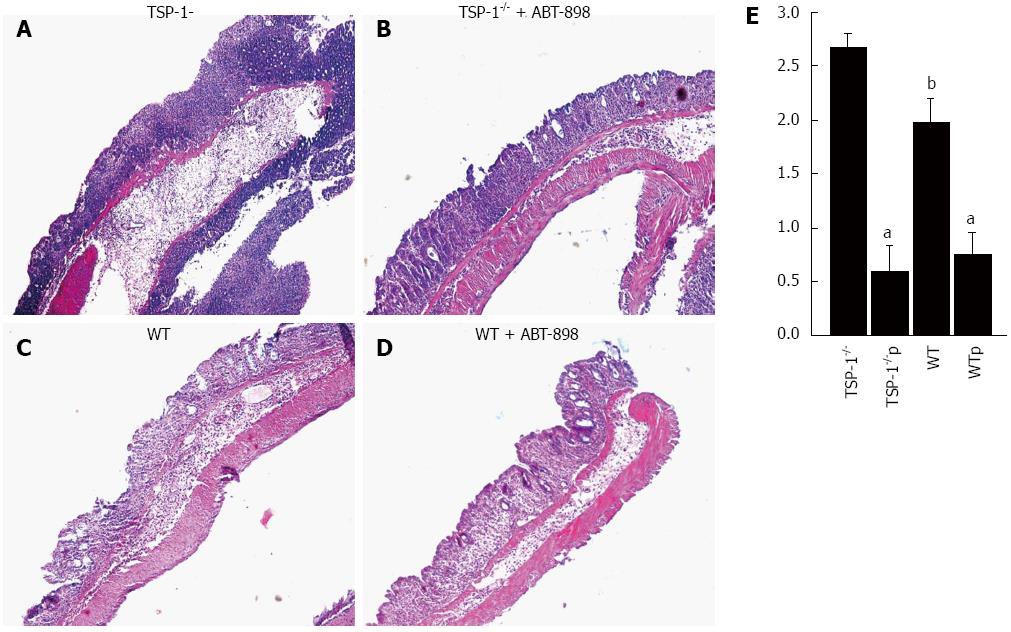
Inflammation was graded by evaluating hematoxylin-eosin stained sections in a blindly matter (Figure 1A-D). TSP-1 deficient mice showed enhanced inflammation grade at DSS doses of 2.5% (Figure 1A). The leukocytic infiltrate was usually involving all the layers of the colonic wall with multiple erosions. WT mice treated with DSS only, also showed erosions and heavy inflammation (Figure 1B). However, the inflammatory infiltrate was mostly confined to the mucosa of the colon. Treatment with ABT-898 considerably diminished the inflammatory response in WT and TSP-1-/- mice (Figure 1C and D, respectively; P < 0.0001 vs control). The peptide ABT-898 significantly reduced the leukocytic infiltration in both WT and TSP-1-/- colitic lesions when compared with their controls (Figure 1E; P < 0.0001 vs control). TSP-1 deficient mice receiving glucose showed a higher grade of inflammation when compared to WT mice controls (Figure 1A and C). These results were statistically significant (Figure 1E; P = 0.0157).
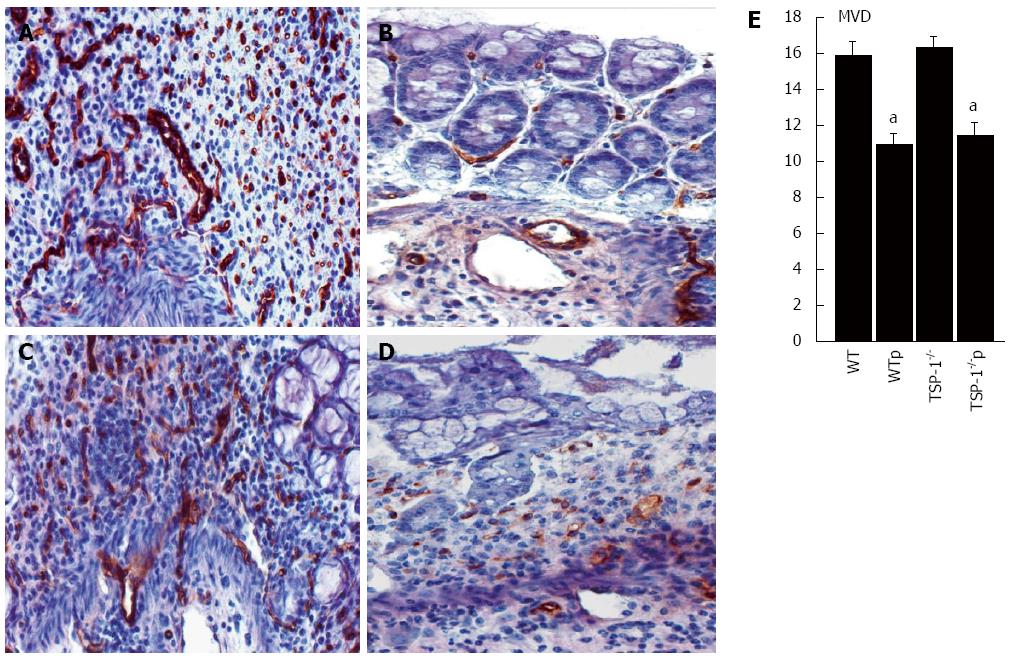
TSP-1 is an angiogenic regulator that induces apoptosis in endothelial and smooth muscle vascular cells. Colonic tissues of both genotypes TSP-1-/- and WT showed both a high density of microvessels (MVD) in the DSS- induced lesions (Figure 2A and C, respectively). Conversely, CD31/MECA positive blood vessels were significantly reduced in colons from WT and TSP-1-/- mice treated with ABT898, TSP-1-/- controls/TSP-1-/- treated (P = 0.0002; Figure 2A, 2B and 2E, respectively). WT controls and WT treated (P = 0.0005; Figure 2C-E, respectively). No significant changes were detected in MVC between WT and TSP-1-/-mice, indicating that endogenous TSP-1 does not inhibit angiogenesis. Changes in angiogenesis in this model may be related to the concentration of DSS used and the duration of the treatment. TSP-1 deficient mice have shown increased MVC and secretion of pro-angiogenic factors only when using higher concentrations of DSS or when it was delivered during multiple cycles[19,20].
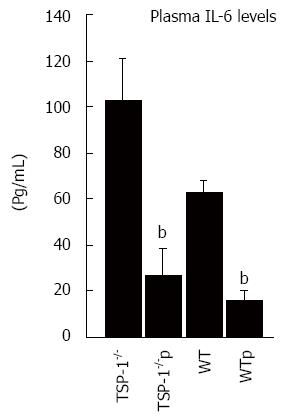
A cytokine ELISA array was used to screen 12 cytokines in plasma of mice treated and untreated with ABT-898. Levels of IL-6 were particularly higher in control mice and very much reduced after the treatment with ABT-898. In order to validate these results plasma levels of IL-6 from treated and untreated mice were measured by using specific sandwich-based ELISA for each cytokine. Consistently, IL-6 was significantly diminished in plasma of TSP-1-/- treated with the peptide (P = 0.0002). WT samples under the same treatment showed similar results (P = 0148). TSP-1 deficient mice untreated displayed higher levels of IL-6 compared to WT mice treated with saline solutions as well (P = 0.0114; Figure 3).
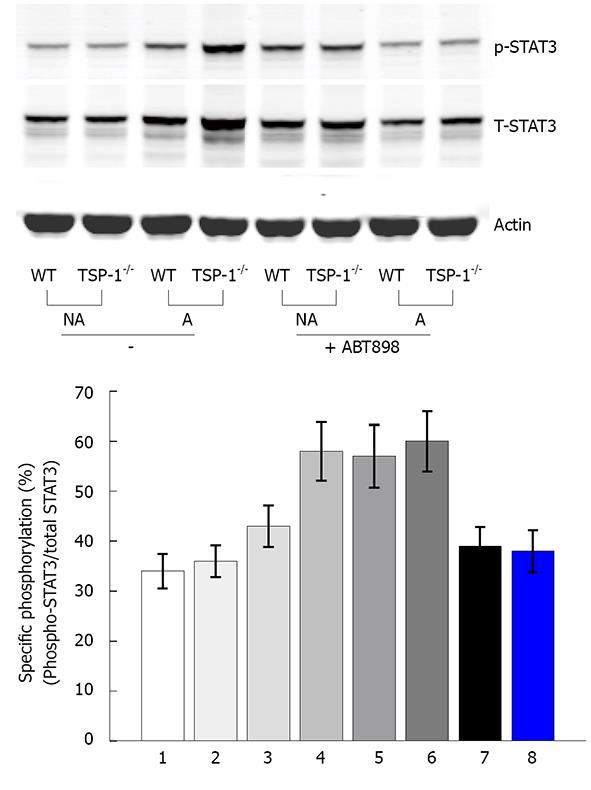
Activation of STAT3 is one of the main signaling mechanisms in response to IL-6 action[20]. The activation of STAT3 is mediated through the phosphorylation at Tyr705 or Ser727 site. This step is required for dimerization, nuclear translocation and DNA binding. Western blot with pSTAT3 (Ser727) antibody showed that pSTAT3 was increased 2-fold in the inflamed TSP-1-/- colon tissues treated with DSS, as compared to the WT. When the colon tissues were not affected by DSS treatment, the levels of pSTAT3 were about the same (Figure 4A). However, both WT and TSP-1-/- showed lower levels of p-STAT3 when treated with the peptide ABT-898 and DSS. pSTAT3 levels were quantified after normalization with total STAT3 in three independent western blotting experiments. These analyses showed that the specific pSTAT3 is increased under induced colitis in TSP1-/- and decreased by ABT-898 (Figure 4B).
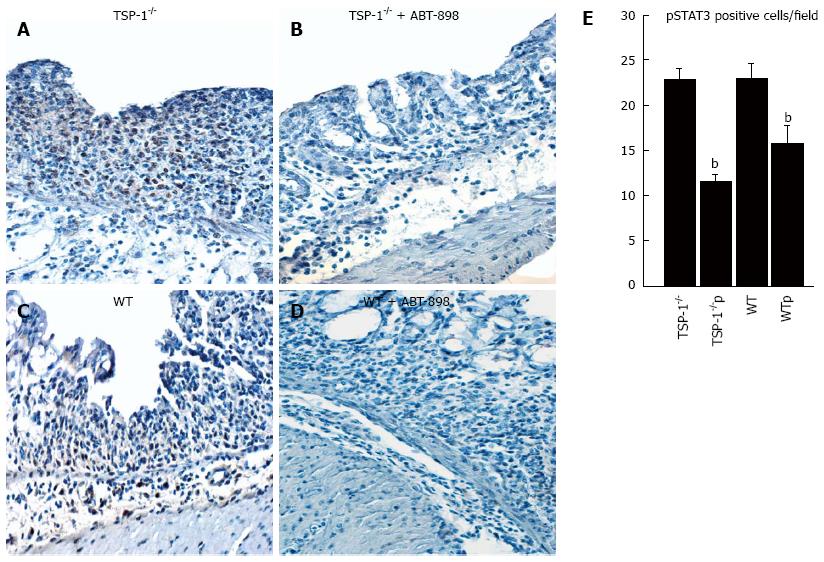
IHC with p-STAT3 (Ser727) revealed that active STAT3 was distributed mainly in the nuclei of epithelial cells. Nuclear staining was also observed in the luminal epithelium and crypts of both groups. Nuclei of the endothelial cells in some venules and arterioles were strongly positive. Cytoplasmic staining was observed in cells undergoing mitosis. p-STAT3 was predominantly expressed in the inflamed epithelium and leukocytic infiltrate of TSP-1-/- and WT intestines controls (Figure 5A and C, respectively). When positive cells were quantified in WT and TSP-1-/- treated with ABT-898 mice (Figure 5B and D, respectively) a significant decrease in positive cells for pSTAT3 was observed (Figure 5F, TSP-1-/-/TSP-1-/- p, P = 0.0089; WT/WTp, P = 0110).
Previous results have shown that TSP-1 significantly diminishes inflammation and angiogenesis in the DSS model of colitis[11]. Secretion of pro-angiogenic factors such as VEGF and basic fibroblast growth factor were significantly higher in TSP-1-/- mice under multiple cycles of DSS[8]. In this study, a second generation TSP-1 derived peptide; ABT-898 significantly ameliorates inflammation and angiogenesis in the DSS mouse model. Data shown herein indicate that ABT-898 significantly reduces the plasmatic levels of IL-6 in these colitic tissues[21].
IL-6 levels have been found elevated in several models of colitis[22,23]. Baseline levels of the anti-inflammatory cytokines IL-10 and TGFβ1 were significantly elevated in IL-6 deficient mice compared with WT mice[23]. IL-6 regulates the secretion of TSP-1 by monocytes[24] and modulates the localization of TSP-1 in the vascular compartment[25]. Several reports have underlined the importance of IL-6 in angiogenesis and inflammation. This cytokine is inhibited when VEGF is silenced using RNAi technology[26]. TSP-1-/- vascular cells could modulate the innate immune system directly and indirectly through production of cytokines such as IL-6. It has been documented that VEGF induces the production of IL-6 in endothelial cells but not in leukocytes[27]. Vascular cells may be a natural source of such production since vascular changes and angiogenesis are critical components of any inflammatory process.
IL-6 interacts with TGFβ1[28,29] and plays an important role in the development of IBD. Inhibition of IL-6 signaling decreases IL-6 secretion in an inflammatory colon cancer model[28] and high levels of IL-6 have been found in patients suffering of IBD[30]. The functions of IL-6 are regulated by a series of events, starting with the binding to its receptor (IL-6R). Two subunits of the IL-6R have been identified: an 80 kDa ligand-binding subunit, known as IL-6 receptor alpha (IL-6Rα), and a 130 kDa signal-transducing subunit, gp130 (IL-6Rβ). The gp130 is associated with the IL-6/IL-6Rα, initiating the phosphorylation of the C-terminal domain of gp130 by JAK1/2 and the recruitment of STAT3 and its subsequent phosphorylation. STAT3 is considered a potent anti-apoptotic factor, directly involved in IBD and cancer[31].
STAT3 is recruited to the receptor subunit gp130 and activated through phosphorylation by JAK1/2. The phosphorylation then renders the dimerization of STAT3 and its translocation into the nucleus to regulate the transcription of responsive genes, including cytokines and growth factors[19,20]. IL-6 and activated STAT3 have been reported as crucial for intestinal carcinogenesis in a model of colitis-associated cancer[32].
The activity of IL-6 through the STAT3 pathway was evaluated in this study and the status of pSTAT3 was determined. The phosphorylated form of STAT3 is expressed in both WT and TSP-1-/- colons, and almost abolishes in mice treated with the antiangiogenic peptide ABT-898. This peptide inhibits angiogenesis and chemotaxis to the colitic areas, as was evidenced by the diminished inflammation and MVD in both genotypes after the treatment with ABT-898. Treatment of mice under colitis with this peptide decreases MVD in both genotypes even at lower doses of DSS as we show herein. TSP-1 induces apoptosis in endothelial cells through its interaction with CD36. CD36 and IL-6 regulate oxidative stress and both are associated with metabolic and inflammatory diseases[33]. In addition, soluble CD36 has been associated with high levels of IL-6 in men with impaired glucose tolerance[34]. Our results here indicate that ABT-898 is able to inhibit the activation of STAT3 in colitic tissues. These data also confirm the importance of regulating the secretion of IL-6 for the resolution of the inflammatory response. IL-6 thus represents an alternative mechanism by which TSP-1 and its derived peptides could be a protective factor against chronic inflammation and carcinogenesis.
Recent studies have shown that the activation of the axis IL-6/STAT3 enhances the secretion of TSP-1[35,36]. STAT-3 as TSP-1 both exhibit paradoxical roles in inflammation and cancer. In addition, the functions of TSP-1 are dose and tissue depending and may vary due the presence of specific receptors with which TSP-1 may interact[37]. While the expression of TSP-1 in the normal colon is not significant, in DSS colitic lesions is highly upregulated[5,11]. TSP-1 is intensely expressed during the acute phase of inflammation in the several models[38]. The releasing of TSP-1 upon injury suggests a protective role in intestinal homeostasis. In addition, lacking of endogenous TSP-1 enhances angiogenesis and inflammation in colitis[11].
Data herein indicate that TSP-1 could diminish the level of pSTAT-3. These results could be solely a consequence of the severe inflammation observed in TSP-1 deficient mice. However, results from our gene microarray data might provide some insights. S100A9 is an important marker for inflammation that can activate STAT3[39,40]. TSP-1 derived peptides upregulated S100A9 at the transcriptional level in the same model of colitis[41]. However, the TSP-1 peptide containing the activating domain of TGFβ1 showed the lowest expression levels of S100A9 among the treated groups. These results suggest that TSP-1 could reduce pSTAT3 in the colon by TGFβ1 related mechanisms.
Our data demonstrate that ABT-898 is effective in controlling colonic inflammation and angiogenesis. Treatment of cancers with ABT-898 has shown to be quite effective in pre-clinical studies as well[42,43]. In addition the treatment with this peptide significantly reduces the secretion of IL-6 and inhibits the activation of the STAT3 system. As clinical trials are testing the efficacy of IL-6 antibodies in cancers, the possibility of using peptides such as ABT-898 warrants further investigation.
We thank Abbott Laboratories for providing the peptide ABT-898. We also acknowledge Dr. Wilbur Hayes and Dr. Zenaida Lopez-Dee for their comments to this manuscript.
Inflammatory bowel disease (IBD) consists of two major forms of chronic intestinal inflammation, ulcerative colitis and Crohn’s disease. Its etiology remains unknown and no cure is yet available. Strong evidence suggests that IBD is the result of an immune deregulation of the intestinal mucosa. Vascular changes and enhanced angiogenesis (formation of new blood vessels from pre-existent ones) indicate that an abnormal angiogenic response may be also implicated, enhancing the recruitment of inflammatory cells and contributing to the mucosal damage. Therapeutic approaches targeting angiogenesis may be safe alternatives for treating inflammatory diseases such as IBD.
Thrombospondin 1 (TSP-1) is natural antiangiogenic that has a role in inflammation and cancer. This protein has multifunctional domains that interact with a variety of growth factors and extracellular proteins. During the last decade, peptides derived from specific domains of TSP-1 have been developed. One of these peptides ABT-510 mimicked the anti-angiogenic properties of TSP-1 and it was evaluated in pre-clinical experiments and proved safe in clinical trials. However, this peptide seemed to be effective only when combined with cytotoxic therapies. This study evaluates the efficacy of a second-generation peptide ABT-898, designed to be more effective by providing a longer half-life and better solubility.
This study is using a model of colitis to test the therapeutic effects of a new and improved TSP-derived peptide. ABT-510 was effective not only inhibiting angiogenesis but also ameliorating the inflammation and mucosal damage. These results link an antiangiogenic peptide with the signal transducer and activator of transcription 3 (STAT3), a key factor in inflammation and cancer. These data suggest an alternative mechanism by which TSP-1 might decrease inflammation warranting further investigation.
The study suggests that the TSP-1 peptide ABT-510 might be effective as a therapeutic agent for inflammatory bowel disease and other inflammatory conditions.
Thrombospondin 1 is a disulfide-linked homotrimeric protein. This protein is an adhesive glycoprotein secreted by a variety of cells but it is storage in the extracellular matrix. TSP-1 has the following major domains: an amino-terminal heparin-binding domain, a procollagen domain, a properdin-like type I repeats, and a globular carboxy-terminal domain. The protein also contains type II repeats with epidermal growth factor-like homology and type III repeats that contain an RGD sequence. ABT-510 (Abbott Laboratories) was formulated based on the sequence GVITRIR within the type I repeats: Ac-Sar-GV-DalloIle-T-Nva-IRP-ethylamide. ABT-898: Ac-GV-DalloIle-SQIRP-ethylamide.
This is an interesting study evaluating the efficacy of second generation TSP-1 derived peptide in a colitis model. These data suggest that ABT-898 could be an effective therapeutic tool for inhibiting inflammation and angiogenesis in inflammatory bowel disease.
P- Reviewer: Roberts DD S- Editor: Yu J L- Editor: A E- Editor: Zhang DN
| 1. | Jiménez B, Volpert OV, Crawford SE, Febbraio M, Silverstein RL, Bouck N. Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nat Med. 2000;6:41-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 742] [Cited by in RCA: 759] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 2. | Gutierrez LS. The role of thrombospondin 1 on intestinal inflammation and carcinogenesis. Biomark Insights. 2008;3:171-178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Rico MC, Castaneda JL, Manns JM, Uknis AB, Sainz IM, Safadi FF, Popoff SN, Dela Cadena RA. Amelioration of inflammation, angiogenesis and CTGF expression in an arthritis model by a TSP1-derived peptide treatment. J Cell Physiol. 2007;211:504-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Hugo C, Daniel C. Thrombospondin in renal disease. Nephron Exp Nephrol. 2009;111:e61-e66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Chidlow JH, Langston W, Greer JJ, Ostanin D, Abdelbaqi M, Houghton J, Senthilkumar A, Shukla D, Mazar AP, Grisham MB. Differential angiogenic regulation of experimental colitis. Am J Pathol. 2006;169:2014-2030. [PubMed] |
| 6. | Aplin AC, Gelati M, Fogel E, Carnevale E, Nicosia RF. Angiopoietin-1 and vascular endothelial growth factor induce expression of inflammatory cytokines before angiogenesis. Physiol Genomics. 2006;27:20-28. [PubMed] |
| 7. | Pousa ID, Maté J, Gisbert JP. Angiogenesis in inflammatory bowel disease. Eur J Clin Invest. 2008;38:73-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Zak S, Treven J, Nash N, Gutierrez LS. Lack of thrombospondin-1 increases angiogenesis in a model of chronic inflammatory bowel disease. Int J Colorectal Dis. 2008;23:297-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Velasco P, Huegel R, Brasch J, Schröder JM, Weichenthal M, Stockfleth E, Schwarz T, Lawler J, Detmar M, Lange-Asschenfeldt B. The angiogenesis inhibitor thrombospondin-1 inhibits acute cutaneous hypersensitivity reactions. J Invest Dermatol. 2009;129:2022-2030. [PubMed] |
| 10. | Lawler J, Sunday M, Thibert V, Duquette M, George EL, Rayburn H, Hynes RO. Thrombospondin-1 is required for normal murine pulmonary homeostasis and its absence causes pneumonia. J Clin Invest. 1998;101:982-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 356] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 11. | Punekar S, Zak S, Kalter VG, Dobransky L, Punekar I, Lawler JW, Gutierrez LS. Thrombospondin 1 and its mimetic peptide ABT-510 decrease angiogenesis and inflammation in a murine model of inflammatory bowel disease. Pathobiology. 2008;75:9-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SM, Lawler J, Hynes RO, Boivin GP, Bouck N. Thrombospondin-1 is a major activator of TGF-beta1 in vivo. Cell. 1998;93:1159-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 902] [Cited by in RCA: 902] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 13. | Gutierrez LS, Suckow M, Lawler J, Ploplis VA, Castellino FJ. Thrombospondin 1--a regulator of adenoma growth and carcinoma progression in the APC(Min/+) mouse model. Carcinogenesis. 2003;24:199-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 89] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Adrian K, Strouch MJ, Zeng Q, Barron MR, Cheon EC, Honasoge A, Xu Y, Phukan S, Sadim M, Bentrem DJ. Tgfbr1 haploinsufficiency inhibits the development of murine mutant Kras-induced pancreatic precancer. Cancer Res. 2009;69:9169-9174. [PubMed] |
| 15. | Janin A, Deschaumes C, Daneshpouy M, Estaquier J, Micic-Polianski J, Rajagopalan-Levasseur P, Akarid K, Mounier N, Gluckman E, Socié G. CD95 engagement induces disseminated endothelial cell apoptosis in vivo: immunopathologic implications. Blood. 2002;99:2940-2947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 90] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Greenaway J, Henkin J, Lawler J, Moorehead R, Petrik J. ABT-510 induces tumor cell apoptosis and inhibits ovarian tumor growth in an orthotopic, syngeneic model of epithelial ovarian cancer. Mol Cancer Ther. 2009;8:64-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 195] [Reference Citation Analysis (0)] |
| 17. | Hasina R, Martin LE, Kasza K, Jones CL, Jalil A, Lingen MW. ABT-510 is an effective chemopreventive agent in the mouse 4-nitroquinoline 1-oxide model of oral carcinogenesis. Cancer Prev Res (Phila). 2009;2:385-393. [PubMed] |
| 18. | Yang Q, Tian Y, Liu S, Zeine R, Chlenski A, Salwen HR, Henkin J, Cohn SL. Thrombospondin-1 peptide ABT-510 combined with valproic acid is an effective antiangiogenesis strategy in neuroblastoma. Cancer Res. 2007;67:1716-1724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 758] [Reference Citation Analysis (0)] |
| 19. | Atreya R, Mudter J, Finotto S, Müllberg J, Jostock T, Wirtz S, Schütz M, Bartsch B, Holtmann M, Becker C. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in crohn disease and experimental colitis in vivo. Nat Med. 2000;6:583-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 945] [Cited by in RCA: 1026] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 20. | Carey R, Jurickova I, Ballard E, Bonkowski E, Han X, Xu H, Denson LA. Activation of an IL-6: STAT3-dependent transcriptome in pediatric-onset inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:446-457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 121] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 21. | Ball EM, Gibson DS, Bell AL, Rooney MR. Plasma IL-6 levels correlate with clinical and ultrasound measures of arthritis in patients with systemic lupus erythematosus. Lupus. 2014;23:46-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Alex P, Zachos NC, Nguyen T, Gonzales L, Chen TE, Conklin LS, Centola M, Li X. Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis. Inflamm Bowel Dis. 2009;15:341-352. [PubMed] |
| 23. | Gay J, Kokkotou E, O’Brien M, Pothoulakis C, Karalis KP. Interleukin-6 genetic ablation protects from trinitrobenzene sulfonic acid-induced colitis in mice. Putative effect of antiinflammatory cytokines. Neuroimmunomodulation. 2006;13:114-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Yamauchi Y, Kuroki M, Imakiire T, Abe H, Uchida H, Beppu R, Yamashita Y, Kuroki M, Shirakusa T. Thrombospondin-1 differentially regulates release of IL-6 and IL-10 by human monocytic cell line U937. Biochem Biophys Res Commun. 2002;290:1551-1557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Loganadane LD, Berge N, Legrand C, Fauvel-Lafève F. Endothelial cell proliferation regulated by cytokines modulates thrombospondin-1 secretion into the subendothelium. Cytokine. 1997;9:740-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Forooghian F, Das B. Anti-angiogenic effects of ribonucleic acid interference targeting vascular endothelial growth factor and hypoxia-inducible factor-1alpha. Am J Ophthalmol. 2007;144:761-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Hao Q, Wang L, Tang H. Vascular endothelial growth factor induces protein kinase D-dependent production of proinflammatory cytokines in endothelial cells. Am J Physiol Cell Physiol. 2009;296:C821-C827. [PubMed] |
| 28. | Becker C, Fantini MC, Schramm C, Lehr HA, Wirtz S, Nikolaev A, Burg J, Strand S, Kiesslich R, Huber S. TGF-beta suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity. 2004;21:491-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 530] [Cited by in RCA: 589] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 29. | Rose-John S, Mitsuyama K, Matsumoto S, Thaiss WM, Scheller J. Interleukin-6 trans-signaling and colonic cancer associated with inflammatory bowel disease. Curr Pharm Des. 2009;15:2095-2103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 30. | León AJ, Gómez E, Garrote JA, Bernardo D, Barrera A, Marcos JL, Fernández-Salazar L, Velayos B, Blanco-Quirós A, Arranz E. High levels of proinflammatory cytokines, but not markers of tissue injury, in unaffected intestinal areas from patients with IBD. Mediators Inflamm. 2009;2009:580450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 31. | Mitchell TJ, John S. Signal transducer and activator of transcription (STAT) signalling and T-cell lymphomas. Immunology. 2005;114:301-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 129] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 32. | Bromberg J, Wang TC. Inflammation and cancer: IL-6 and STAT3 complete the link. Cancer Cell. 2009;15:79-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 444] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 33. | Okamura DM, Pennathur S, Pasichnyk K, López-Guisa JM, Collins S, Febbraio M, Heinecke J, Eddy AA. CD36 regulates oxidative stress and inflammation in hypercholesterolemic CKD. J Am Soc Nephrol. 2009;20:495-505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 119] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 34. | Handberg A, Lopez-Bermejo A, Bassols J, Vendrell J, Ricart W, Fernandez-Real JM. Circulating soluble CD36 is associated with glucose metabolism and interleukin-6 in glucose-intolerant men. Diab Vasc Dis Res. 2009;6:15-20. [PubMed] [DOI] [Full Text] |
| 35. | Sarközi R, Hauser C, Noppert SJ, Kronbichler A, Pirklbauer M, Haller VM, Grillari J, Grillari-Voglauer R, Mayer G, Schramek H. Oncostatin M is a novel inhibitor of TGF-β1-induced matricellular protein expression. Am J Physiol Renal Physiol. 2011;301:F1014-F1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 36. | Tyzack GE, Sitnikov S, Barson D, Adams-Carr KL, Lau NK, Kwok JC, Zhao C, Franklin RJ, Karadottir RT, Fawcett JW. Astrocyte response to motor neuron injury promotes structural synaptic plasticity via STAT3-regulated TSP-1 expression. Nat Commun. 2014;5:4294. [PubMed] |
| 37. | Lopez-Dee Z, Pidcock K, Gutierrez LS. Thrombospondin-1: multiple paths to inflammation. Mediators Inflamm. 2011;2011:296069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 201] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 38. | Koch AE, Szekanecz Z, Friedman J, Haines GK, Langman CB, Bouck NP. Effects of thrombospondin-1 on disease course and angiogenesis in rat adjuvant-induced arthritis. Clin Immunol Immunopathol. 1998;86:199-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 39. | Schiopu A, Cotoi OS. S100A8 and S100A9: DAMPs at the crossroads between innate immunity, traditional risk factors, and cardiovascular disease. Mediators Inflamm. 2013;2013:828354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 203] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 40. | Lee MJ, Lee JK, Choi JW, Lee CS, Sim JH, Cho CH, Lee KH, Cho IH, Chung MH, Kim HR. Interleukin-6 induces S100A9 expression in colonic epithelial cells through STAT3 activation in experimental ulcerative colitis. PLoS One. 2012;7:e38801. [PubMed] |
| 41. | Lopez-Dee ZP, Chittur SV, Patel B, Stanton R, Wakeley M, Lippert B, Menaker A, Eiche B, Terry R, Gutierrez LS. Thrombospondin-1 type 1 repeats in a model of inflammatory bowel disease: transcript profile and therapeutic effects. PLoS One. 2012;7:e34590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 42. | Sahora AI, Rusk AW, Henkin J, McKeegan EM, Shi Y, Khanna C. Prospective study of thrombospondin-1 mimetic peptides, ABT-510 and ABT-898, in dogs with soft tissue sarcoma. J Vet Intern Med. 2012;26:1169-1176. [PubMed] |
| 43. | Campbell N, Greenaway J, Henkin J, Petrik J. ABT-898 induces tumor regression and prolongs survival in a mouse model of epithelial ovarian cancer. Mol Cancer Ther. 2011;10:1876-1885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |