Published online Jan 14, 2015. doi: 10.3748/wjg.v21.i2.484
Peer-review started: April 21, 2014
First decision: June 18, 2014
Revised: July 3, 2014
Accepted: July 30, 2014
Article in press: July 30, 2014
Published online: January 14, 2015
Processing time: 272 Days and 19.7 Hours
AIM: To observe the protective effect of glucagon-like peptide-2 (GLP-2) on the intestinal barrier of rats with obstructive jaundice and determine the possible mechanisms of action involved in the protective effect.
METHODS: Thirty-six Sprague-Dawley rats were randomly divided into a sham operation group, an obstructive jaundice group, and a GLP-2 group; each group consisted of 12 rats. The GLP-2 group was treated with GLP-2 after the day of surgery, whereas the other two groups were treated with the same concentration of normal saline. Alanine aminotransferase (ALT), total bilirubin, and endotoxin levels were recorded at 1, 3, 7, 10 and 14 d. Furthermore, on the 14th day, body weight, the wet weight of the small intestine, pathological changes of the small intestine and the immunoglobulin A (IgA) expressed by plasma cells located in the small intestinal lamina propria were recorded for each group.
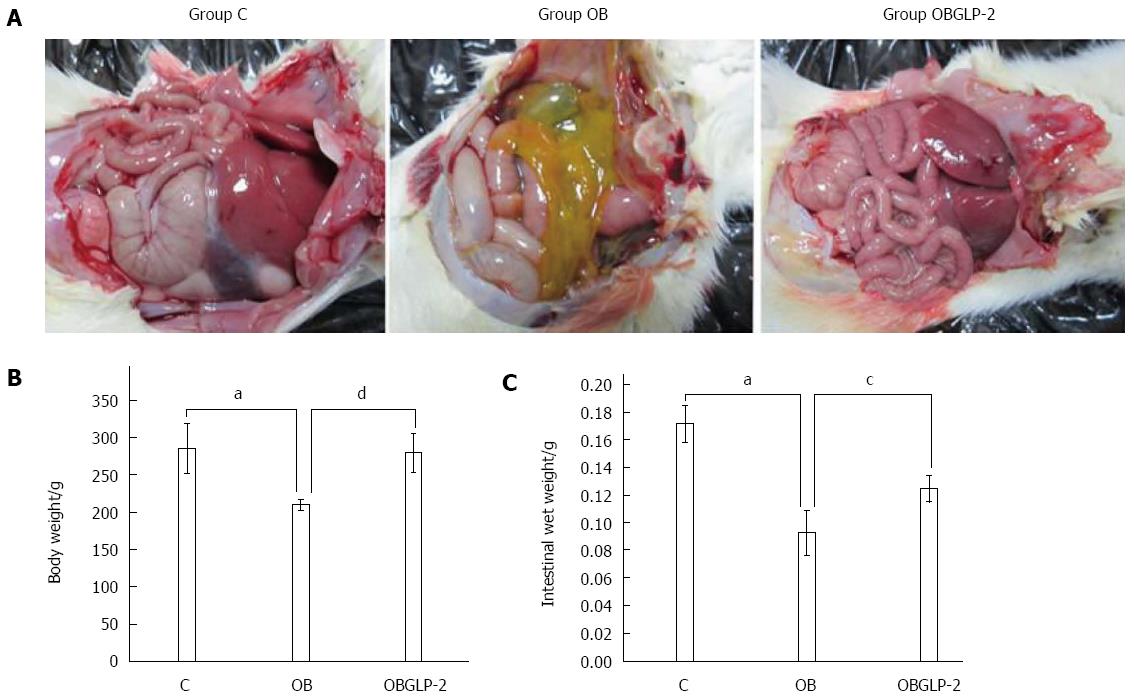
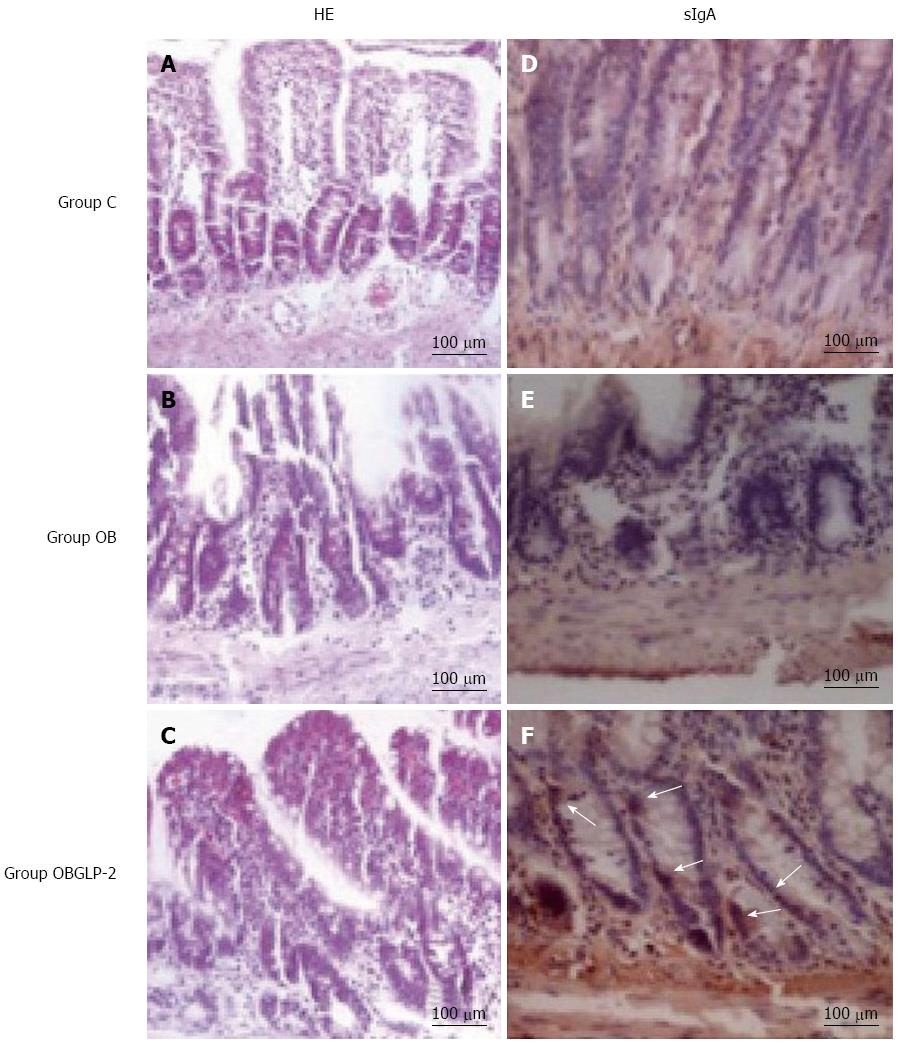
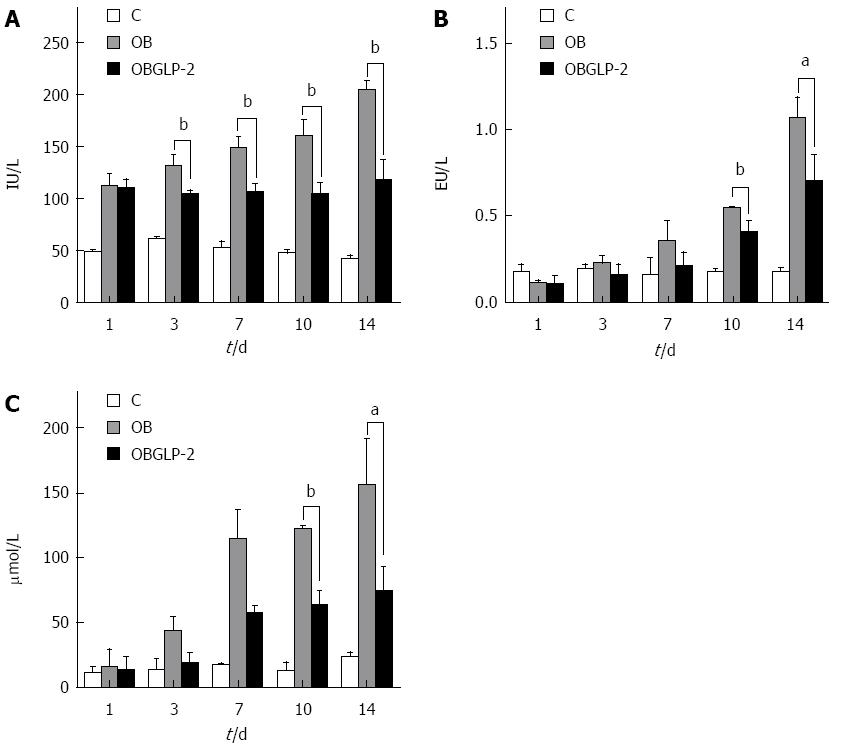
RESULTS: In the rat model, jaundice was obvious, and the rats’ activity decreased 4-6 d post bile duct ligation. Compared with the sham operation group, the obstructive jaundice group displayed increased yellow staining of abdominal visceral serosa, decreased small intestine wet weight, thinning of the intestinal muscle layer and villi, villous atrophy, uneven height, fusion, partial villous epithelial cell shedding, substantial inflammatory cell infiltration and significantly reduced IgA expression. However, no significant gross changes were noted between the GLP-2 and sham groups. With time, the levels of ALT, endotoxin and bilirubin in the GLP-2 group were significantly increased compared with the sham group (P < 0.01). The increasing levels of the aforementioned markers were more significant in the obstructive jaundice group than in the GLP-2 group (P < 0.01).
CONCLUSION: GLP-2 reduces intestinal mucosal injuries in obstructive jaundice rats, which might be attributed to increased intestinal IgA and reduced bilirubin and endotoxin.
Core tip: It has recently been demonstrated that glucagon-like peptide-2 (GLP-2) has a highly tissue-specific trophic effect on the small intestine. However, whether GLP-2 also functions as an adapter for rats with obstructive jaundice is unknown. Studies on this topic are rare, and our research clearly illustrates that exogenous GLP-2 reduces intestinal mucosal injuries in an obstructive jaundice rat model. The next step of our study is to continue focusing on the details of this research as further studies are needed.
- Citation: Chen J, Dong JT, Li XJ, Gu Y, Cheng ZJ, Cai YK. Glucagon-like peptide-2 protects impaired intestinal mucosal barriers in obstructive jaundice rats. World J Gastroenterol 2015; 21(2): 484-490
- URL: https://www.wjgnet.com/1007-9327/full/v21/i2/484.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i2.484
Glucagon-like peptide-2 (GLP-2) is a 33-amino acid peptide encoded by the carboxy terminal of GLP-1 in proglucagon. It was first reported and introduced as a specific adapter of the intestinal mucosa by Drucker et al[1] in 1996. GLP-2 is co-secreted with GLP-1, oxyntomodulin and glicentin from enteroendocrine L cells, which are primarily located in the ileum and proximal colon. It has recently been demonstrated that GLP-2 has a highly tissue-specific trophic effect on the small intestine and augments the adaptive response to intestinal resection in the rat. Additionally, GLP-2 inhibits gastric acid secretion, enhances intestinal sugar transport and slows gastric emptying[2-5]. Halaçlar et al[6] reported that bacterial translocation in samples of the liver, spleen, mesenteric lymph nodes and portal and systemic blood obtained from the GLP-2 treated group was reduced compared with samples obtained from the colitis group. In addition, the Chinese scholars Li et al[7] discovered that the rate of bacterial translocation and the level of endotoxin in rats with gut ischemia-reperfusion injury were significantly increased compared with those treated by GLP-2. Above all, the most important property of GLP-2 in the gastrointestinal (GI) tract is its enterotrophic effect[1,8-10]. Therefore, its potential therapeutic role in patients with intestinal insufficiency secondary to extensive disease or resection of the small bowel is of interest[4,9].
Intestinal barrier function is damaged in patients with obstructive jaundice, potentially leading to bacteria translocation, endotoxemia and increased mortality within the peri-operative period. An increasing number of scientists and doctors have attempted to improve intestinal function in patients or rats with obstructive jaundice. Based on the aforementioned comments, we designed a study to observe whether GLP-2 acts on the damaged intestinal mucosa of rats with obstructive jaundice.
Male Sprague-Dawley rats, weighing 200-250 g, were housed under controlled temperature, humidity and 12-h dark/light cycles; the rats were housed in stainless-steel cages and provided free access to water and rat chow before and after the operation. The rats were randomized into three groups (n = 12 in each group). Group 1 (Control; C) underwent sham operation, whereas Group 2 [obstructive jaundice (OB)] underwent common bile duct ligation. Both of the groups were given simultaneous treatment with the same amount of normal saline after the surgeries. Group 3 [obstructive jaundice with GLP-2 (OBGLP-2)] underwent common bile duct ligation and simultaneous treatment with GLP-2 [0.2 μg/(g•d)]. Either normal saline or GLP-2 was administered by peritoneal injection daily.
Using sterile techniques, a midline incision was created. The common bile duct was identified, double ligated with 5-0 silk and divided between the two ligatures. In sham-operated animals, the common bile duct was freed from the surrounding soft tissue without ligation and transection. The operation was performed using 100 g/L chloral hydrate (3 mL/kg) for intraperitoneal anesthesia.
Serum samples were obtained and analyzed on postoperative days 1, 3, 7, 10 and 14. All serum samples were measured using a Hitachi 7600 modular chemistry analyzer (Hitachi High-Technologies Corporation, Tokyo, Japan) to determine the levels of alanine aminotransferase (ALT) and total bilirubin (Tbil) using kits from Jiancheng Bioengineering Institute. The endotoxin levels in plasma were measured according to the manufacturer’s instructions with the kits (Horseshoe Crab Reagent, Xiamen, China).
After 2 wk, the animals were anesthetized, and repeat laparotomy was performed. Segments of the small bowel, approximately 3 cm long, were harvested from the terminal ileum, embedded in Optimal Cutting Temperature (OCT) compound (Sakura Finetechnical, Tokyo, Japan) and immediately snap-frozen in liquid nitrogen for immunohistochemistry. Then, the samples were fixed in 400 g/L of paraformaldehyde solution. Sections of 5 μm were cut and stained with hematoxylin and eosin (HE). The pathological changes and injuries to the intestinal mucosa were evaluated under a light microscope by an independent observer who was blinded to the experimental protocol. Additionally, secretory immunoglobulin A (sIgA) expression in the tissue samples was assessed by immunohistochemical analysis.
Experimental data were analyzed with the SPSS 13.0 statistical program (statistical product and service solutions, © 1999 to 2003; SPSS Institute Inc., Armonk, NY, United States). The results are expressed as mean ± SD. The differences among groups were evaluated by the homogeneity test of variance analysis. The means of independent samples were analyzed and compared with the independent sample t-test. Differences were considered significant at P < 0.05.
Two to three days after bile duct ligation, the ears and tails started to exhibit jaundice, and the urine turned yellow. After 4-6 d, the jaundice was obvious, the stools were pale, and the activity of the rats decreased. Four rats (1 in group C, 1 in group OBGLP-2 and 2 in group OB) died of complications, including abdominal infection, malnutrition, liver function failure and water and electrolyte disorders. The remaining rats survived until they were sacrificed at the end of the experiments.
Unlike the sham group, the organ serosa of the jaundiced rats exhibited yellow discoloration, and the root mesenteric lymph nodes displayed beadlike enlargement. However, on the 14th day, the GLP-2 group exhibited no parietal peritoneum changes (Figure 1A). The mean body weight and wet intestinal weight were reduced in the jaundiced group compared with the sham group (Figure 1B and C); however, GLP-2 reduced the reduction in both body weight and wet intestinal weight, indicating that GLP-2 protects the intestinal mucosa from damage.
The sham group exhibited normal villous formation in the intestine tissue with equal height (Figure 2A). However, the jaundiced group displayed intestinal muscularis layer thinning with villous thinning, atrophy, uneven height, villous fusion, partial villous epithelium shedding and considerable lymphocyte infiltration (Figure 2B). The GLP-2 group displayed villi that were more equal in height compared with the jaundiced group as well as slight villous edema with no epithelial shedding (Figure 2C). In the sham group, the intestinal crypts exhibited minimal sIgA-stained cells (Figure 2D). The jaundiced group had shallow and disorganized intestinal crypts with almost no IgA-stained cells (Figure 2E). The GLP-2 group exhibited deeper and more organized intestinal tissue crypts than the jaundiced group with localized positive sIgA staining (Figure 2F).
The jaundiced and GLP-2 groups exhibited obvious ALT elevations compared with the sham group. However on postoperative days 3, 7, 10, and 14, the GLP-2 group had reduced ALT levels compared with the jaundiced group (Figure 3A, P < 0.05). The jaundiced and GLP-2 groups showed obvious elevations in endotoxin levels compared with the sham group, and the level continued to rise as time passed. However, on days 10 and 14, the endotoxin level in the GLP-2 group was significantly reduced compared with the jaundiced group on the same days (Figure 3B, P < 0.05). The jaundiced and GLP-2 groups exhibited an obvious increase in the serum bilirubin compared with the sham group. However, on postoperative days 10 and 14, the GLP-2 group exhibited a significantly lower level of serum bilirubin compared with the jaundiced group (Figure 3C, P < 0.05).
Obstructive jaundice can cause a host of complex and severe pathological and physiological changes to various organs. Intestinal mucosal barrier dysfunction and intestinal immune function decline might result in endotoxemia and intestinal bacterial translocation, which are important contributors to disease progression or death in patients[11-13]. Under normal circumstances, the large number of bacteria in the intestinal tract cannot enter the body tissue or blood circulation due to the mechanical, biological, chemical and immunological barriers of the intestine. When the intestinal mechanical barrier is damaged, endotoxins enter the blood circulation, causing endotoxemia. Then, the intestinal bacteria can migrate into the intestinal lymph nodes, blood, liver or spleen, causing bacterial translocation[14-20].
In this study, we observed that rats with obstructive jaundice exhibit circular muscularis thinning and decreased mucosal thickness and villous height. Obstructive jaundice is associated with intestine mucosal structural changes and a decline in protein synthesis in the liver. These effects are coupled with a lack of proteins for gastrointestinal epithelium regeneration and renewal, leading to intestinal mechanical barrier damage and bacterial translocation. GLP-2 is an intestinal epithelial specific growth factor, and its main role is to stimulate intestinal crypt cell proliferation and inhibit cell apoptosis, promoting the growth of the intestinal mucosa and regeneration after injury[18]. GLP-2 also inhibits gastric acid secretion and gastric motility, increases the intestinal blood supply, improves intestinal barrier function and promotes the intestinal absorption of nutrients[19,20]. However, studies regarding the use of rhGLP-2 in obstructive jaundice to improve the intestinal barrier function and immune function are rare. As seen from the 14-d results of this experiment, rats receiving subcutaneous exogenous GLP-2 exhibited less structural damage to the intestinal mucosa and tall intestinal villi that were neat and relatively intact compared with the jaundiced group. This indicates that exogenously administered GLP-2 aids in the protection and improvement of the intestinal mechanical barrier in rats with obstructive jaundice.
Additionally, when obstructive jaundice occurs, the impaired local intestinal immune function is also one of the factors responsible for bacterial translocation. The local intestinal mucosal immune system primarily consists of intestinal lymphocytes, lymphoid tissue, plasma cells and immunoglobulins[21,22]. Among the immunoglobulins, sIgA plays an important role. sIgA neutralizes viruses, toxins and the biological activity of antigen enzymes and prevents bacterial adhesion on the surface of intestinal epithelial cells. sIgA displays a synergistic bactericidal effect with the complement system and lysozymes. Therefore, sIgA is an important factor facilitating the protection of the intestinal barrier function that prevents bacterial translocation[23]. In this study, ileal biopsy immunohistochemistry indicated that rats with obstructive jaundice display significantly decreased expression of ileal sIgA compared with the control group. However, the GLP-2 group exhibited significantly increased IgA expression compared with the obstructive jaundice group. These results indicate that by repairing damage to the integrity of intestinal epithelial cells and increasing sIgA synthesis in ileal epithelial cells, GLP-2 can protect rats with obstructive jaundice from intestinal barrier dysfunction and immune system damage. As an intestinal epithelium-specific growth factor, GLP-2 has a strong effect on the recovery of intestinal epithelial injury, and it is stronger than any other non-specific intestinal epithelial growth factor[24-27]. These advantages of GLP-2 suggest that it might have useful clinical applications.
With respect to its mechanism of action on the intestinal mucosa, a large number of studies have demonstrated that GLP-2 exerts its actions via a G protein-coupled receptor (GLP-2R). The human GLP-2R gene is located on chromosome 17p13.3[28,29]. GLP-2R is a member of the G protein-coupled receptor superfamily and has 7 transmembrane domains. GLP-2R and glucagon as well as GLP-1 and the glucose-dependent insulinotropic polypeptide receptor are highly homologous. GLP-2R is widely distributed in intestinal epithelial cells, gastric epithelial cells, enteric neurons, intestinal endocrine cells[29], intestinal submucosal myofibroblasts, islet A cells, the brain and lungs[30,31]. New research findings demonstrate that GLP-2 promotes the growth of normal small bowel and the recovery of pathologic intestinal mucosa through multiple pathways. On one hand, GLP-2 promotes proliferation of the intestinal mucosa by binding to GLP-2R, which is distributed in intestinal epithelial cells. On the other hand, GLP-2 protects the intestinal function indirectly by binding to the GLP-2R, which is distributed in other regions. The effects of GLP-2 involve many cell signal transduction pathways, mainly the cAMP/PKA, PI3K/Akt and Wnt/β-catenin pathways, of which the cAMP/PKA pathway is the main one. These pathways coordinate and regulate intestinal epithelial cells, promoting steady development and intestinal adaptation. However, the mechanism(s) by which these pathways coordinate and integrate key control points and feedback inhibition are not entirely clear, and further studies are required[32-35].
Above all, GLP-2 has widely been accepted as an adapter of the intestinal mucosa. In this study, we also observed its protective function in an obstructive jaundice rat model. This effect might be attributed to increased intestinal IgA and reduced bilirubin and endotoxin. In December 2012, the United States Food and Drug Administration approved the use of Teduglutide, a GLP-2 analog, to treat adults with short bowel syndrome who need additional nutrition from intravenous feeding (parenteral nutrition). Will the drug’s application expand if more experiments clarify the protection of the intestinal mucosa in rats or humans with obstructive jaundice?
The authors would like to thank Dr. Ying Zhou and Lei Yu from Zhongshan Hospital of Fudan University for their contributions to this research as well as Dr. Ru-Chuan Shen from the University of Queensland Australia for critical review of and language revisions to the manuscript.
Obstructive jaundice can cause a host of complex and severe pathological injuries to the intestinal mucosal barrier and result in intestinal immune function decline, endotoxemia and intestinal bacterial translocation, which are important factors in disease progression or death in patients. Many recent studies have demonstrated that glucagon-like peptide-2 (GLP-2) can help protect and improve intestinal mechanical barrier function in rats. However, few direct studies on whether GLP-2 has a protective role in obstructive jaundice animal models are available.
Many researchers have discussed the mechanism by which GLP-2 acts on the intestinal mucosa and signal pathways. Many scientists are attempting to determine whether GLP-2 is related to neoplastic development. In addition, the new GLP-2 analog Teduglutide was approved by the United States Food and Drug Administration to treat adults with short bowel syndrome. Unfortunately, to date, few studies have discussed the relationship between GLP-2 and injuries caused by obstructive jaundice.
Recent reports have highlighted the function of GLP-2 as an adapter of intestinal mucosa. Almost no direct studies evaluating whether GLP-2 has a protective function on obstructive jaundice rats were identified from a PubMed search. This study is the first to report that GLP-2 efficiently prevents intestinal mucosa injury after bile duct ligation operations in a rat model. These effects might be attributed to an increase in intestinal IgA, reduced bilirubin and endotoxin and improved liver function.
Due to its specific protective function in the intestinal mucosa, GLP-2 can be widely used in patients with intestinal barrier injury despite the cause.
GLP-2 is a pleiotropic hormone that affects multiple facets of intestinal physiology, including growth, barrier function, digestion, absorption, motility and blood flow. The mechanisms through which GLP-2 produces these actions are complex, involving unique signaling mechanisms and multiple indirect mediators. However, few studies have investigated its function in rats or humans with obstructive jaundice. This study was designed to fill this gap in knowledge.
This paper demonstrates the usefulness of GLP-2 in rats with obstructive jaundice. The study is well designed, and the manuscript is interesting and clearly described.
P- Reviewer: Crenn PP, Fujino Y, Kayadibi H S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Liu XM
| 1. | Drucker DJ, Erlich P, Asa SL, Brubaker PL. Induction of intestinal epithelial proliferation by glucagon-like peptide 2. Proc Natl Acad Sci USA. 1996;93:7911-7916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 643] [Cited by in RCA: 672] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 2. | Drucker DJ. Biological actions and therapeutic potential of the glucagon-like peptides. Gastroenterology. 2002;122:531-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 364] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 3. | Dubé PE, Forse CL, Bahrami J, Brubaker PL. The essential role of insulin-like growth factor-1 in the intestinal tropic effects of glucagon-like peptide-2 in mice. Gastroenterology. 2006;131:589-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 150] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 4. | Martin GR, Wallace LE, Sigalet DL. Glucagon-like peptide-2 induces intestinal adaptation in parenterally fed rats with short bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2004;286:G964-G972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 111] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Evans R, Kamdar SJ. Stability of RNA isolated from macrophages depends on the removal of an RNA-degrading activity early in the extraction procedure. Biotechniques. 1990;8:357-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Halaçlar B, Ağaç Ay A, Akcan AC, Ay A, Öz B, Arslan E. Effects of glucagon-like peptide-2 on bacterial translocation in rat models of colitis. Turk J Gastroenterol. 2012;23:691-698. [PubMed] |
| 7. | Li H, Wu GH, Chen J. [Effect of glucagon-like peptide 2 on the intestinal mucosal immunity and correlative cytokines in mice with gut ischemia/reperfusion injury]. Zhonghua Weichang Waike Zazhi. 2006;9:67-70. [PubMed] |
| 8. | Walsh NA, Yusta B, DaCambra MP, Anini Y, Drucker DJ, Brubaker PL. Glucagon-like peptide-2 receptor activation in the rat intestinal mucosa. Endocrinology. 2003;144:4385-4392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Tsai CH, Hill M, Asa SL, Brubaker PL, Drucker DJ. Intestinal growth-promoting properties of glucagon-like peptide-2 in mice. Am J Physiol. 1997;273:E77-E84. [PubMed] |
| 10. | Ghatei MA, Goodlad RA, Taheri S, Mandir N, Brynes AE, Jordinson M, Bloom SR. Proglucagon-derived peptides in intestinal epithelial proliferation: glucagon-like peptide-2 is a major mediator of intestinal epithelial proliferation in rats. Dig Dis Sci. 2001;46:1255-1263. [PubMed] |
| 11. | Kononenko SN, Limonchikov SV. [The diagnostics of the obstructive jaundice and possibilities to improve the efficacy of its miniinvasive treatment]. Khirurgiia (Mosk). 2011;4-10. [PubMed] |
| 12. | Iida A, Yoshidome H, Shida T, Kimura F, Shimizu H, Ohtsuka M, Morita Y, Takeuchi D, Miyazaki M. Does prolonged biliary obstructive jaundice sensitize the liver to endotoxemia? Shock. 2009;31:397-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Jones C, Badger SA, Black JM, McFerran NV, Hoper M, Diamond T, Parks RW, Taylor MA. The use of antiendotoxin peptides in obstructive jaundice endotoxemia. Eur J Gastroenterol Hepatol. 2012;24:248-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Assimakopoulos SF, Scopa CD, Zervoudakis G, Mylonas PG, Georgiou C, Nikolopoulou V, Vagianos CE. Bombesin and neurotensin reduce endotoxemia, intestinal oxidative stress, and apoptosis in experimental obstructive jaundice. Ann Surg. 2005;241:159-167. [PubMed] |
| 15. | Margaritis VG, Filos KS, Michalaki MA, Scopa CD, Spiliopoulou I, Nikolopoulou VN, Vagianos CE. Effect of oral glutamine administration on bacterial tanslocation, endotoxemia, liver and ileal morphology, and apoptosis in rats with obstructive jaundice. World J Surg. 2005;29:1329-1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Zulfikaroglu B, Zulfikaroglu E, Ozmen MM, Ozalp N, Berkem R, Erdogan S, Besler HT, Koc M, Korkmaz A. The effect of immunonutrition on bacterial translocation, and intestinal villus atrophy in experimental obstructive jaundice. Clin Nutr. 2003;22:277-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Ogata Y, Nishi M, Nakayama H, Kuwahara T, Ohnishi Y, Tashiro S. Role of bile in intestinal barrier function and its inhibitory effect on bacterial translocation in obstructive jaundice in rats. J Surg Res. 2003;115:18-23. [PubMed] |
| 18. | Muto M, Kaji T, Mukai M, Nakame K, Yoshioka T, Tanimoto A, Matsufuji H. Ghrelin and glucagon-like peptide-2 increase immediately following massive small bowel resection. Peptides. 2013;43:160-166. [PubMed] |
| 19. | Madsen KB, Askov-Hansen C, Naimi RM, Brandt CF, Hartmann B, Holst JJ, Mortensen PB, Jeppesen PB. Acute effects of continuous infusions of glucagon-like peptide (GLP)-1, GLP-2 and the combination (GLP-1+GLP-2) on intestinal absorption in short bowel syndrome (SBS) patients. A placebo-controlled study. Regul Pept. 2013;184:30-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 20. | Lee BW, Kim MH, Chae HY, Hwang HJ, Kang D, Ihm SH. Enhanced gene transfer to pancreatic islets using glucagon-like peptide-1. Transplant Proc. 2013;45:591-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Zhang XP, Jiang J, Yu YP, Cheng QH, Chen B. Effect of Danshen on apoptosis and NF-κB protein expression of the intestinal mucosa of rats with severe acute pancreatitis or obstructive jaundice. Hepatobiliary Pancreat Dis Int. 2010;9:537-546. [PubMed] |
| 22. | de Boer D, de Jong EG, van Rossum JM, Maes RA. Doping control of testosterone and human chorionic gonadotrophin: a case study. Int J Sports Med. 1991;12:46-51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Qiao SF, Lu TJ, Sun JB, Li F. Alterations of intestinal immune function and regulatory effects of L-arginine in experimental severe acute pancreatitis rats. World J Gastroenterol. 2005;11:6216-6218. [PubMed] |
| 24. | Qi KK, Wu J, Xu ZW. Effects of PEGylated porcine glucagon-like peptide-2 therapy in weaning piglets challenged with lipopolysaccharide. Peptides. 2014;58:7-13. [PubMed] |
| 25. | Rotondo A, Amato A, Baldassano S, Lentini L, Mulè F. Gastric relaxation induced by glucagon-like peptide-2 in mice fed a high-fat diet or fasted. Peptides. 2011;32:1587-1592. [PubMed] |
| 26. | Taylor-Edwards CC, Burrin DG, Holst JJ, McLeod KR, Harmon DL. Glucagon-like peptide-2 (GLP-2) increases small intestinal blood flow and mucosal growth in ruminating calves. J Dairy Sci. 2011;94:888-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 27. | Martin GR, Beck PL, Sigalet DL. Gut hormones, and short bowel syndrome: the enigmatic role of glucagon-like peptide-2 in the regulation of intestinal adaptation. World J Gastroenterol. 2006;12:4117-4129. [PubMed] |
| 28. | Yusta B, Huang L, Munroe D, Wolff G, Fantaske R, Sharma S, Demchyshyn L, Asa SL, Drucker DJ. Enteroendocrine localization of GLP-2 receptor expression in humans and rodents. Gastroenterology. 2000;119:744-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 263] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 29. | Guan X, Karpen HE, Stephens J, Bukowski JT, Niu S, Zhang G, Stoll B, Finegold MJ, Holst JJ, Hadsell D. GLP-2 receptor localizes to enteric neurons and endocrine cells expressing vasoactive peptides and mediates increased blood flow. Gastroenterology. 2006;130:150-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 213] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 30. | Ørskov C, Hartmann B, Poulsen SS, Thulesen J, Hare KJ, Holst JJ. GLP-2 stimulates colonic growth via KGF, released by subepithelial myofibroblasts with GLP-2 receptors. Regul Pept. 2005;124:105-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 166] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 31. | Jasleen J, Ashley SW, Shimoda N, Zinner MJ, Whang EE. Glucagon-like peptide 2 stimulates intestinal epithelial proliferation in vitro. Dig Dis Sci. 2002;47:1135-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Rocha FG, Shen KR, Jasleen J, Tavakkolizadeh A, Zinner MJ, Whang EE, Ashley SW. Glucagon-like peptide-2: divergent signaling pathways. J Surg Res. 2004;121:5-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 33. | Koehler JA, Yusta B, Drucker DJ. The HeLa cell glucagon-like peptide-2 receptor is coupled to regulation of apoptosis and ERK1/2 activation through divergent signaling pathways. Mol Endocrinol. 2005;19:459-473. [PubMed] |
| 34. | Hartmann B, Thulesen J, Hare KJ, Kissow H, Orskov C, Poulsen SS, Holst JJ. Immunoneutralization of endogenous glucagon-like peptide-2 reduces adaptive intestinal growth in diabetic rats. Regul Pept. 2002;105:173-179. [PubMed] |
| 35. | Leen JL, Izzo A, Upadhyay C, Rowland KJ, Dubé PE, Gu S, Heximer SP, Rhodes CJ, Storm DR, Lund PK. Mechanism of action of glucagon-like peptide-2 to increase IGF-I mRNA in intestinal subepithelial fibroblasts. Endocrinology. 2011;152:436-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |