Published online Jan 14, 2015. doi: 10.3748/wjg.v21.i2.465
Peer-review started: April 15, 2014
First decision: May 13, 2014
Revised: June 8, 2014
Accepted: July 11, 2014
Article in press: July 11, 2014
Published online: January 14, 2015
Processing time: 278 Days and 18.5 Hours
AIM: To evaluate the effects of butein on inflammatory cytokines, matrix metalloproteinase-9 (MMP-9), and colitis in interleukin (IL)-10-/- mice.
METHODS: To synchronize colitis, 8- to 10-wk-old IL-10-/- mice were fed pellet-chow containing piroxicam for 2 wk. Subsequently, phosphate-buffered saline or butein (1 mg/kg per day, ip) was injected for 4 wk. Histologic scores, inflammatory cytokines, MMP-9 and phosphorylated signal transducer and activator of transcription 3 (pSTAT3) expressions were analyzed in IL-10-/- mice and in Colo 205 cells.
RESULTS: Butein reduced the colonic inflammatory score by > 50%. Expression levels of IL-6, IL-1β, interferon (IFN)-γ and MMP-9 were decreased in the colons of mice exposed to butein, whereas other inflammatory cytokines (IL-17A, IL-21 and IL-22) were unchanged. Immunohistochemical staining for pSTAT3 and MMP-9 was significantly decreased in the butein-treated groups compared with the controls. Butein inhibited IL-6-induced activation of STAT3 in Colo 205 cells.
CONCLUSION: Butein ameliorated colitis in IL-10-/- mice by regulating IL-6/STAT3 and MMP-9 activation.
Core tip: This study examined if butein, a naturally derived substance, has therapeutic effects in an animal model of inflammatory bowel disease, interleukin (IL)-10-/- mice. The results show that butein suppressed bowel inflammation and interfered with the IL-6/signal transducer and activator of transcription 3 and matrix metalloproteinase-9 pathways, suggesting that butein should be used to treat bowel inflammation-induced colon cancer. Although there have been several in vitro studies on tumor cells, there have been no in vivo studies examining the effect of butein on colitis in mice.
- Citation: Lee SD, Choe JW, Lee BJ, Kang MH, Joo MK, Kim JH, Yeon JE, Park JJ, Kim JS, Bak YT. Butein effects in colitis and interleukin-6/signal transducer and activator of transcription 3 expression. World J Gastroenterol 2015; 21(2): 465-474
- URL: https://www.wjgnet.com/1007-9327/full/v21/i2/465.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i2.465
Inflammatory bowel diseases (IBDs), including Crohn’s disease (CD) and ulcerative colitis, are chronic diseases that are occasionally complicated by bowel perforations, strictures and fistulas[1,2]. The overall risk of colorectal cancer among patients with ulcerative colitis is about ten times higher than that of the general population[3,4]. In Asia, including South Korea, the occurrence of colon cancer and IBD has recently increased due to environmental factors such as the influence of Westernized lifestyles. This incidence pattern is expected to continue[2]. Immunosuppressive agents and 5-aminosalicylic acid have classically been used as treatments for IBD. Inhibition of tumor necrosis factor (TNF), an inflammatory cytokine, has been considered as a therapeutic target. Many studies have focused on diverse biologic agents to treat IBD, but a completely efficient treatment agent has not yet been discovered. Research continues for the development of new alternative drugs and in clinical trials[5,6].
IBD results from immune modulation abnormalities; helper T cells, in particular, play a critical role in the development of disease. CD is largely associated with abnormal activation of Th1-related cytokines [interleukin (IL)-1β, interferon (IFN)-γ, TNF-α, IL-6 and IL-22]; in addition, the importance of Th17-related cytokines in the emergence of CD has recently been highlighted[7]. IL-10-/- mice are known to be an appropriate animal model for CD due to the similarity of their condition to CD morbidity. In these mice, the manifestation of bowel inflammation is mediated by Th1 cytokines (IL-1β, IFN-γ, TNF-α and IL-6), and the ulcerative lesions are found in the proximal portion of the colon. In IL-10-/- mice, chronic bowel inflammation can occur without any other stimuli or specific pathogens. Making use of the fact that bowel inflammation occurs after a certain period of time, it is possible to promote inflammation and inflammation-induced tumors with piroxicam, a non-steroidal anti-inflammatory drug. Therefore, the IL-10-/- mouse can be a useful animal model for the occurrence of bowel inflammation and inflammation-induced tumors[8,9].
The therapeutic effects of natural substances on inflammation and tumors are being widely studied[10,11]. It has been reported that a number of chemical substances obtained from edible plants with botanical and antioxidant characteristics interfere with tumorigenesis through suppression of the inflammatory reaction[12]. However, there has been no agreement in results between actual clinical applications and a large-scale prospective study[13]. In addition, numerous mechanisms have been suggested for the anti-inflammatory and anti-tumor effects of such natural substances. Cell signal transmission systems have been actively studied, but it has been difficult to elucidate the mechanism of the therapeutic effects due to the structural diversity[13]. Toxicodendron vernicifluum, a deciduous tree from the Anacardiaceae family, both grows natively in various places and is cultivated in South Korea. Urushiol, the major constituent, is primarily responsible for the toxicity. Other constituents, such as butein (3,4,2’,4’-tetrahydroxychalcone), have been found in in vitro studies to have antioxidant and anti-inflammatory effects and to suppress tumor cell proliferation and angiogenesis[14-18]. One in vitro study demonstrated that butein inhibits the activation of nuclear factor kappa B (NF-κB) through inhibition of TNF-α, IL-6 and IL-8 in human mast cells[19].
Matrix metalloproteinase (MMP), an enzyme that degrades zinc-dependent gelatin matrices, serves an important role in inflammatory cell infiltration, cytokine activation and tissue injury, reformation and recovery. MMP-9 is specifically known to be closely associated with rheumatoid arthritis, atherosclerosis, colon cancer and IBD. The suppressive effects that butein has on MMP-9 activation have been reported in an in vitro study using prostate cancer cells[20,21]. However, to our knowledge, no study has yet been conducted that examines the therapeutic effects of butein on bowel inflammation and colon cancer. This study, therefore, aims to evaluate the interfering effects of butein on inflammatory cytokines and MMP-9 in IL-10-/- mice and ultimately to examine the therapeutic effects of butein.
IL-10-/- mice were purchased from Jackson Laboratories (Bar Harbor, ME, United States). All mice were housed and bred in the animal care facility of Korea University Guro Hospital.
Butein and piroxicam were purchased from Sigma-Aldrich Inc. (St. Louis, MO, United States). Butein was dissolved in dimethylsulfoxide and was diluted in phosphate-buffered saline (PBS) before use. Antibodies against phosphorylated signal transducer and activator of transcription 3 (pSTAT3)/STAT3 and β-actin were purchase from Cell Signaling Co. (Beverly, MA, United States), anti-MMP-9 was purchased from Abcam Inc. (Cambridge, United Kingdom), anti-BrdU was purchased from Accurate Chemical (Westbury, NY, United States), GAPDH and donkey anti-rabbit antibodies were purchased from Santa Cruz Biotechnology Inc. (Dallas, TX, United States), and anti-rabbit and mouse-HRP-labeled polymers were purchased from Dako of Agilent Technologies (Glostrup, Denmark).
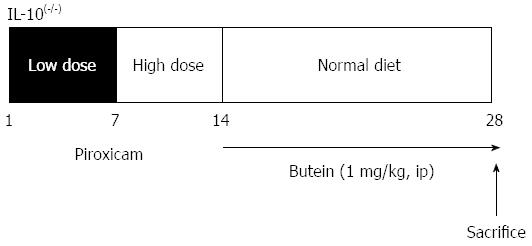
To synchronize and accelerate colitis, 8-wk-old IL-10-/- mice were treated with piroxicam as previously described[22,23]. In brief, a lower dose of piroxicam (60 mg/250 g chow) was administrated for 7 d followed by a higher dose of piroxicam (80 mg/250 g chow) for 7 d. Mice were then placed on normal chow for the remainder of the experimental period. On days 15-28, 1 mg/kg of butein or PBS was administered daily to mice, and mice were sacrificed on the 28th day (Figure 1). This experiment was performed in accordance with the guidelines of the Korea University Animal Ethics Committee.
Entire colons were dissected longitudinally and made into Swiss rolls. The tissues were fixed in 10% formalin for 14-16 h, embedded in paraffin and 4-μm sections were cut and stained with hematoxylin and eosin. The degree of colitis was assessed using the inflammation scoring system as described previously with slight modifications[24] (Table 1).
| Grade 0 | Normal tissue |
| Grade 1 | One or a few multifocal mononuclear cell infiltrates in the lamina propria |
| Minimal epithelial hyperplasia | |
| Slight to no depletion of mucus from goblet cells | |
| Grade 2 | Lesions tended to involve more of the intestine than grade 1 lesions |
| Several multifocal, mild inflammatory cell infiltrates in the lamina propria | |
| Mild epithelial hyperplasia and mucin depletion | |
| Small epithelial erosions | |
| Grade 3 | Lesions involved a large area of the mucosa or were more frequent than grade 2 lesions |
| Moderate inflammation with the involvement of submucosa | |
| Mixture of mononuclear cells as well as neutrophils, and crypt abscesses | |
| Moderate epithelial hyperplasia and mucin depletion | |
| Ulcers were occasionally observed | |
| Grade 4 | Lesions usually involved most of the intestinal section and were more severe than grade 3 lesions |
| Severe inflammation including mononuclear cells and neutrophils with transmural involvement | |
| Marked epithelial hyperplasia | |
| Crypt abscesses and ulcers |
The human colon cancer cell line, Colo 205, was purchased from the American Type Culture Collection (Manassas, VA, United States). Cells were cultured in RPMI media containing 1% penicillin-streptomycin (Sigma) and 10% heat-inactivated fetal bovine serum in 5% CO2 at 37 °C. Cells were seeded at 9 × 105 cells/mL in 60-mm dishes and then treated with IL-6 (25 ng/mL) and the indicated concentrations of butein.
Colonic epithelial cells were isolated as previously described[25]. Mouse colonic tissues were washed using cold PBS and opened longitudinally. Colons were irrigated with cold Ca2+- and Mg+-free Hank’s balanced salt solution (CMF-HBSS). The tissue was then transferred to 5 mL CMF-HBSS containing 10 mmol/L dithiothreitol (1:100; Sigma-Aldrich Inc.) and 50 nmol/L calyculin A (1:200; Wako, Richmond, VA, United States) and incubated in rotator for 30 min at 4 °C. The tissue was then transferred to another 5 mL CMF-HBSS solution containing 1 mmol/L ethylenediaminetetraacetic acid and 50 nmol/L calyculin A, and incubated at 4 °C for 1 h. Colonic tissues were removed from the tube, and epithelial cells were isolated by centrifugation at 300 rpm for 5 min. The supernatant was removed, and the remaining cells were snap-frozen in liquid nitrogen and maintained at -70 °C for future analysis.
For the proliferation assay, 1 mg 5-Bromo-2’-Deoxyuridine (BrdU; Sigma-Aldrich Inc.) was injected into mice 2 h before sacrifice. Formalin-fixed, paraffin-embedded sections were processed for immunohistochemistry. Paraffin-embedded slides were deparaffinized and hydrated. Antigen retrieval was performed using Target retrieval solution in a decloaking chamber followed by staining with antibodies against pSTAT3 (1:100), BrdU (1:200) or MMP-9 (1:00) followed by anti-rabbit or anti-mouse-HRP-labeled polymers. Sections were developed using 3,3’-diaminobenzidine tetrahydrochloride and counterstained with hematoxylin.
Protein was extracted from isolated colonic epithelial cells and Colo 205 cells using protein extraction buffer (Fisher Thermo Scientific Inc., Waltham, MA, United States) as described by the manufacturer. Extracted protein concentrations were measured using the BCA method, and 30 μg protein samples were separated on a 10% sodium dodecyl sulfate-polyacrylamide gel, transferred to nitrocellulose membranes and blocked with Tris-buffered saline containing 0.05% Tween-20, 5% nonfat milk and 5% fetal bovine serum. The primary antibodies were applied overnight at 4 °C. Antibodies against STAT3 (1:1000) and GAPDH served as the sample loading controls. After extensive washing, the membranes were incubated for 1 h in corresponding horseradish peroxidase-coupled secondary antibodies (donkey anti-rabbit 1:1000). The chemiluminescence reaction was developed using a West Pico reagent (Pierce, Rockford, IL, United States).
Quantitative real-time polymerase chain reaction (qRT-PCR) was performed to quantify the expression of mRNA for cytokines and MMP-9 with the expression of GAPDH for endogenous control. Total RNA from the proximal colon of each mouse was extracted using Trizol (Invitrogen of Thermo Fisher Scientific Inc.), and cDNA was synthesized using the iScript™ cDNA synthesis Kit (Roche, Basel, Germany). qRT-PCR was performed using the LightCycler 480 SYBR Green I Master kit (Roche) according to the manufacturer’s instructions with the primers summarized in Table 2.
| Name | Sequence | |
| IL-6 | F | AGT TGC CTT CTT GGG ACT GA |
| R | TCC ACG ATT TCC CAG AGA AC | |
| IL-1b | F | GCC CAT CCT CTG TGA CTC AT |
| R | AGG CCA CAG GTA TTT TGT CG | |
| IL-22 | F | CAA CTT CCA GCA GCC ATA CA |
| R | GTT GAG CAC CTG CTT CAT CA | |
| IL-17A | F | TCC AGA AGG CCC TCA GAC TA |
| R | AGC ATC TTC TCG ACC CTG AA | |
| IFN-γ | F | ACT GGC AAA AGG ATG GTG AC |
| R | TGA GCT CAT TGA ATG CTT GG | |
| MMP-2 | F | CAG ACT TGT CCC GTT TCC AT |
| R | GGT GCT GAC TGC ATC AAA GA | |
| MMP-9 | F | CGT CGT GAT CCC CAC TTA CT |
| R | AAC ACA CAG GGT TTG CCT TC | |
| IL-21 | F | GGC AAC ATG GAG AGG ATT GT |
| R | AAG CAG GAA AAA GCT GAC CA | |
| IL-23R | F | CAT GAC TTG CAC CTG GAA TG |
| R | GCT TGG ACC CAA ACC AAG TA | |
| IL-12β (P.40) | F | AGG TCA CAC TGG ACC AAA GG |
| R | TGG TTT GAT GAT GTC CCT GA |
Statistical analysis was performed using Student’s t-test or the Mann-Whitney U test. Results are expressed as mean ± SE.
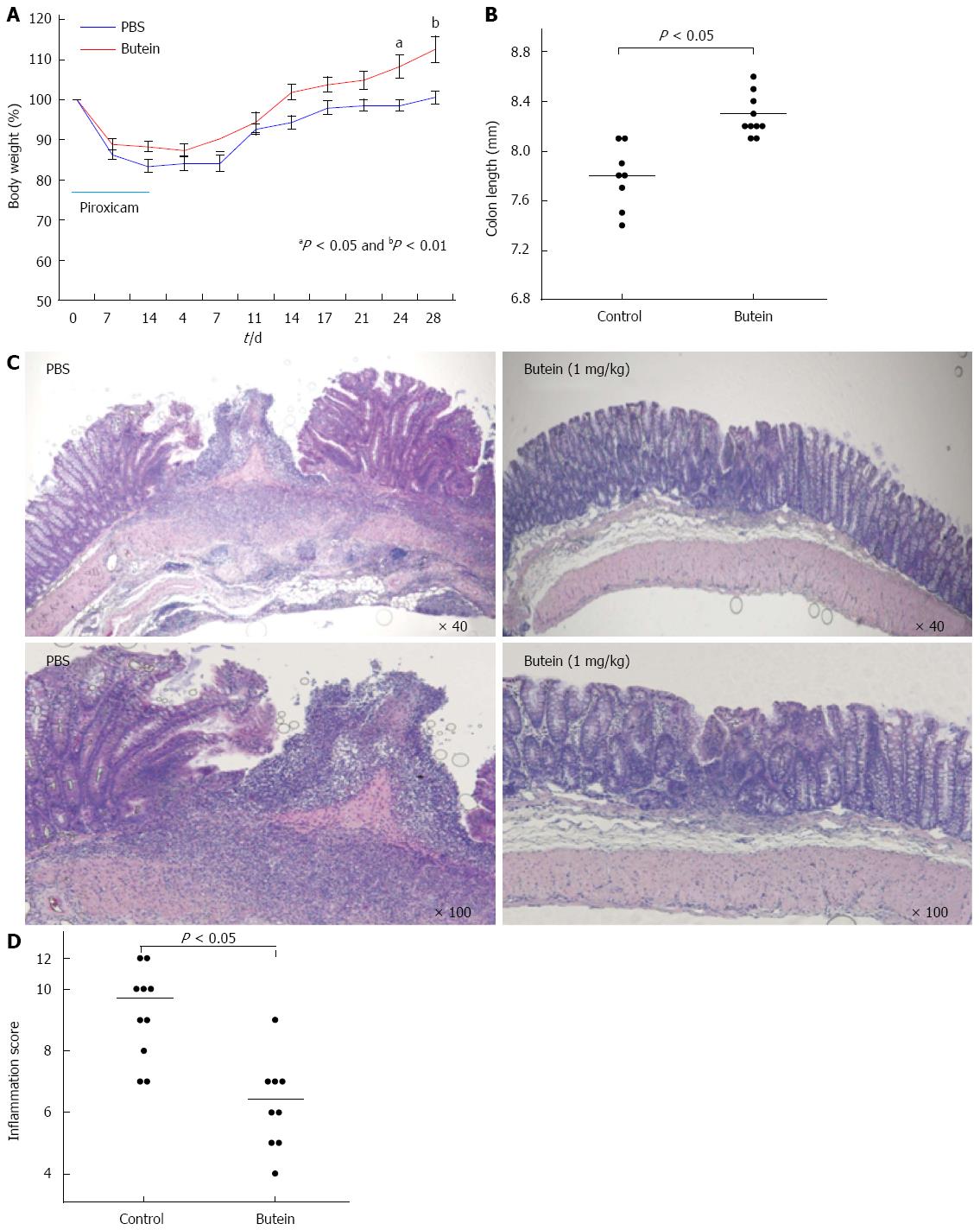
It has been established that induction with piroxicam for 2 wk accelerates and synchronizes the onset and severity of colitis in IL-10-/- mice with marked transmural inflammation and ulceration in the proximal colon[8,23]. During the colitis experiment, mice treated with butein exhibited a greater body weight gain and longer colonic length (Figure 2A and B). Histologic analysis demonstrated that treatment with butein (1 mg/kg) for 2 wk after cessation of the 2-wk piroxicam administration significantly reduced the inflammatory score with no visible deep ulceration and lessened inflammatory cell infiltrates (Figure 2C and D).
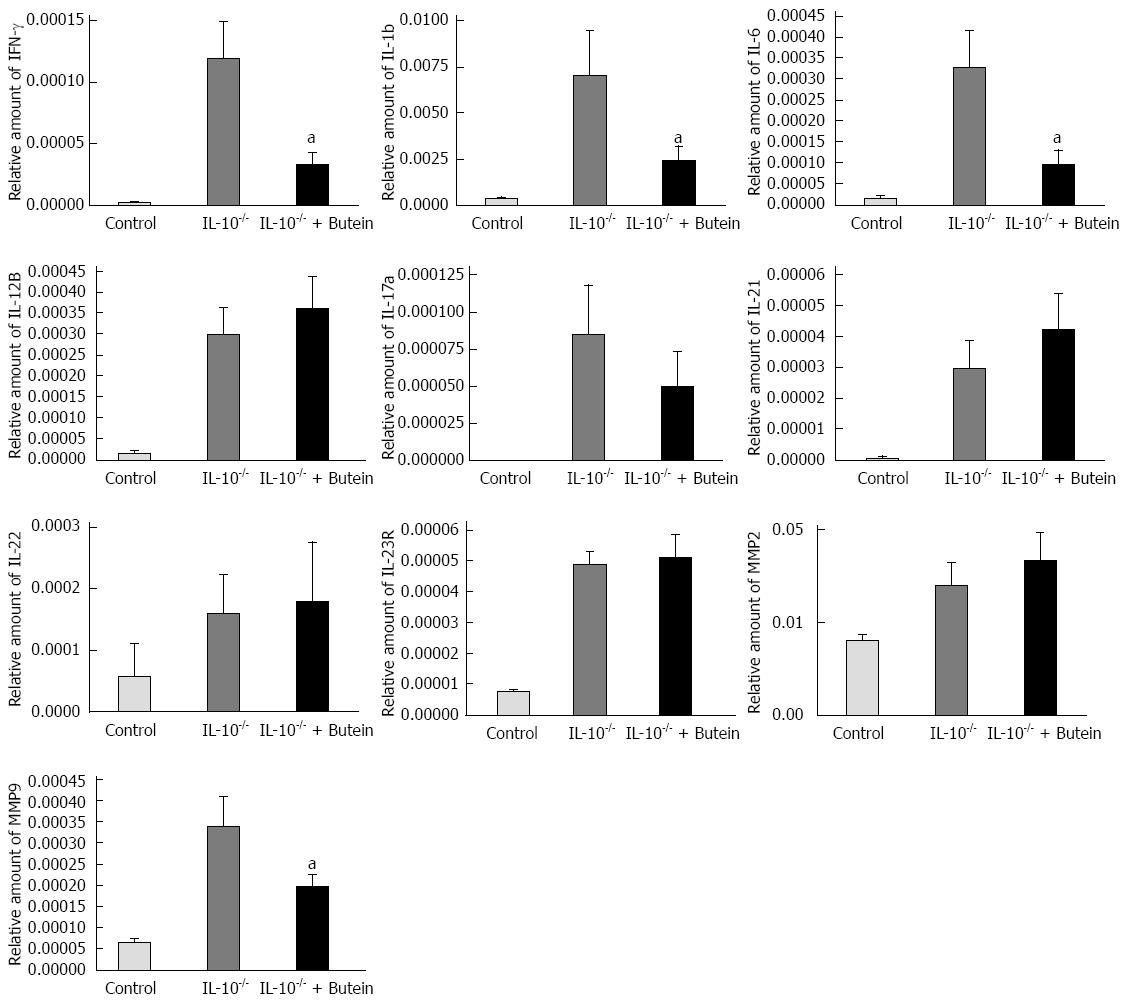
Previous studies have shown that colitis in IL-10-/- mice is induced by dysregulation of Th1-mediated cytokines, including IL-1β, IFN-γ and IL-6. The Th1 cytokines, IFN-γ, IL-1β, IL-6, IL-21, IL-23 and IL-12β were heavily upregulated in IL-10-/- mice, together with significantly increased MMP-9, two weeks after the last administration of piroxicam. IL-10-/- mice that received 2-wk-treatment with butein demonstrated significantly reduced expression of IFN-γ, IL-1β, IL-6 and MMP-9 mRNA in the proximal colon, while there was no effect on the expression of IL-17a, IL-21, IL-22 and MMP-2 (Figure 3).
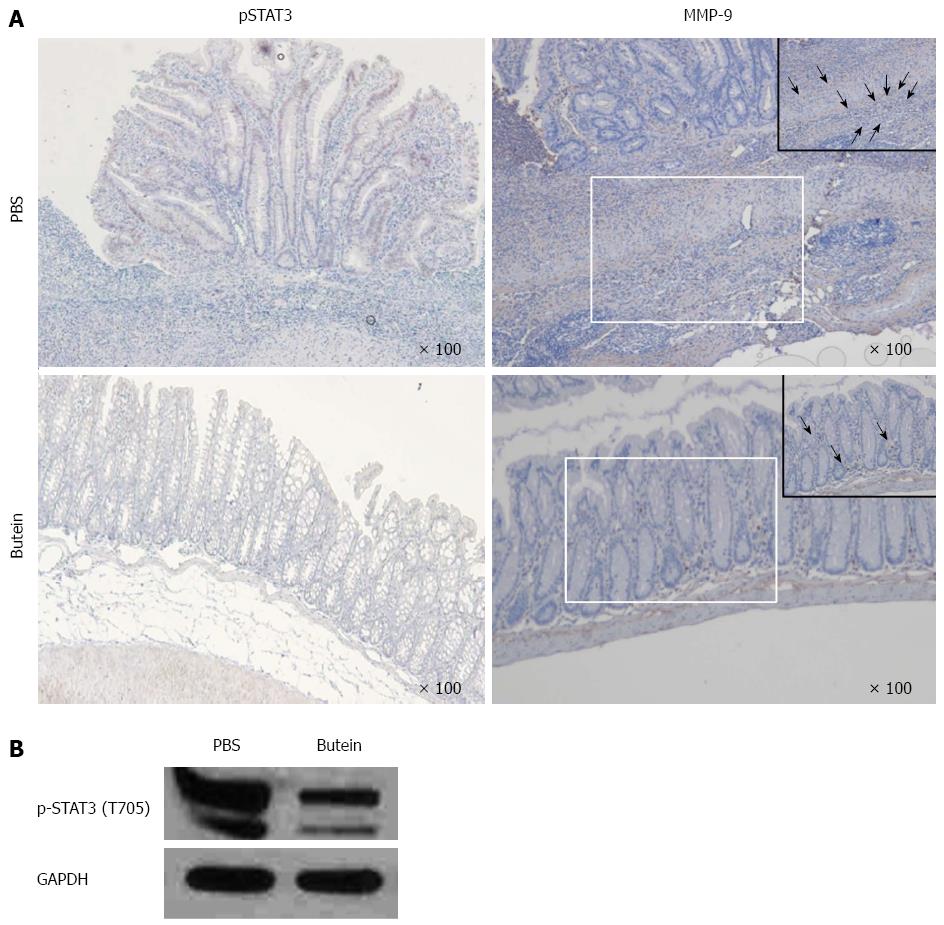
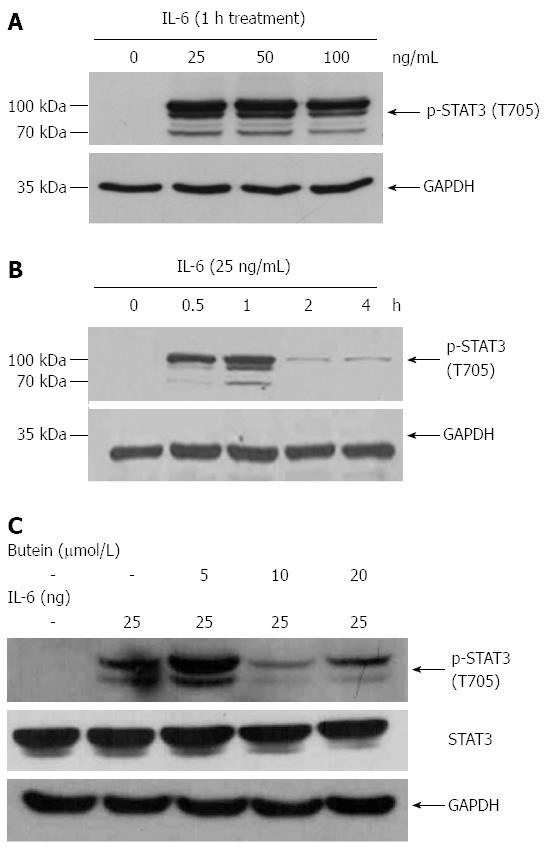
Knowing that STAT3 is part of a major intrinsic pathway for inflammation and inflammation-associated cancers that are mediated and activated by cytokines, chemokines and other mediators including IL-6, IL-1β and macrophage colony-stimulating factor, we investigated STAT3 activity in the colons of IL-10-/- mice. Increased STAT3 activity was noted in inflamed colonic epithelial cells, which was inhibited by butein treatment, as noted both in immunohistochemistry and Western blots (Figure 4).
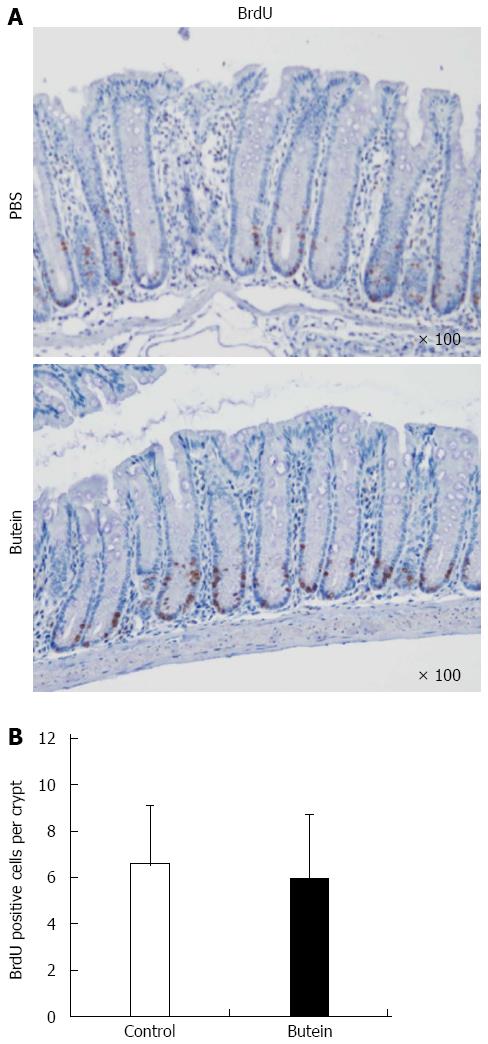
Butein treatment suppressed MMP-9 expression, which was noted in adjacent inflammatory cells in mice with colitis and ulcerations in control mice. To evaluate proliferation, a BrdU incorporation assay was performed by immunohistochemical analysis. There was no significant difference between the control and butein treatment groups (Figure 5).
We investigated the effects of butein on the modulation of IL-6/STAT3 activation in vitro using Colo 205 cells. Exposure to IL-6 for different times and at different concentrations increased phosphorylation of STAT3. Butein treatment suppressed the phosphorylation of STAT3 induced by IL-6 (25 ng/mL) at a concentration of 10 μmol/L (Figure 6).
The objective of this study was to examine whether butein, a naturally derived substance, had therapeutic effects in IL-10-/- mice, an IBD model. The occurrence of IBD significantly decreased in mice injected with butein; butein blocked the IL-6/STAT3 signal transmission pathway and suppressed MMP-9 activation. Butein, a major constituent of Toxicodendron vernicifluum, can also be found in the stems of Semecarpus anacardium and in the heartwood of Dalbergia odorifera, as well as other plants. Previous studies have reported that butein has anti-oxidant, anti-inflammatory and antitumor effects, and that it suppresses angiogenesis[26-29]. Butein is known to suppress cell proliferation and promote apoptosis in both solid and hematologic tumors; it is also less toxic than urushiol, another constituent of Toxicodendron vernicifluum[30,31]. Butein’s effects on tumor cells arise as a result of suppressing c-Src and JAK1/JAK2 activation, thus inhibiting the IL-6/STAT3 pathway. Butein also directly inhibits the expression of Bcl-xL, Bcl-2 and cyclin D1, the target genes of STAT3[31]. STAT3 plays an important role in cytokine receptor transmission, which is a system that connects a membrane receptor to nuclear transcription and is principally activated by gp130-related cytokines, the most representative of which is IL-6.
STAT3, a principal mechanism of the inflammatory reaction and inflammation-related malignant tumors, is involved in the inflammatory reaction and inflammation-related malignant changes from the initial stage to tumor progression. STAT3 modulates the activity of NF-κB and is activated by the IL-6/JAK signal pathway[32-34]. The IL-6/JAK/STAT3 signal system promotes tumorigenesis by inducing cellular or epigenetic changes that follow intracellular inflammation. IL-6/JAK/STAT3, activated by gp130-related IL-6, IL-22, cytokines, and other growth factors, is found to be active in multiple malignant tumors, such as multiple myeloma, lymphoma, hematologic malignancies, breast cancer and prostate cancer.
In this study, IL-6 mRNA expression was increased in IL-10-/- mice with bowel inflammation, and STAT3 activation was also observed in colonic epithelial cells with inflammation and ulcers following the inflammation. Butein inhibited the expression of STAT3 in epithelial cells, which was demonstrated by immunohistochemical staining and Western blot analysis. We also found that MMP-9 expression in the colonic tissues was blocked by butein. In vitro experiments showed that IL-6-activated MMP-9 was highly concentrated, and this activity was blocked by butein. MMP-9 is closely connected with tissue remodeling and tumor metastasis, and is secreted with MMP-2 from tumor cells, inflammatory cells and cell matrix cells[35]. In a previous in vitro study, butein was reported to inhibit the activation of MMP-9[36]. Similarly, we found that butein blocked the increased expression of MMP-9 in inflammatory cells, and infiltration of the muscle layer in bowel inflammation or inflammation-induced ulcers.
Here, we present the first in vivo study examining the therapeutic effects of butein in an animal model of bowel inflammation. There were a few limitations to our study. First, there was no confirmatory analysis of MMP-9 with zymography for analyzing MMP-9 activation after butein treatment. Second, we did not determine whether the protein expression of IL-6 matched mRNA levels. Third, we had other limitations related to our experiment methods. These shortcomings will be further modified in future studies. Finally, no quantitative analysis of inflammatory cytokine activation was undertaken, and the analysis of the signal transmission system was limited to epithelial cells. It is reasonable to state that IL-10-/- mice are not an appropriate model to study the proliferation and recovery of epithelial cells, as they are a model principally defined by the degree of inflammatory cytokine expression. This limitation could be overcome by conducting additional experiments with other study models. In doing so, the therapeutic effects of butein could be evaluated more accurately.
The results of this study regarding the interfering effects of butein on the IL-6/STAT3-MMP-9 pathway are similar to those of an in vitro study that used established malignant tumor cells. Considering that the suppression of bowel inflammation and the recovery capability of the injured mucous membrane are critical for IBD treatment, the findings obtained from the BrdU analysis indicating that the cell proliferation necessary for the recovery of the mucous is not affected by butein treatment further supports the clinical applicability of butein in treating IBD.
Chronic inflammation is a major mechanism of malignant tumors. The incidence of malignant tumors in the colon and the small intestine is significantly increased with IBD such as ulcerative colitis and CD. The IL-6/STAT3 pathway is an important pathway for the initiation of inflammation-mediated malignant tumors, and MMP-9 is an essential signal transmission system related to the metastasis of malignant tumors. In IBD patients, the expression of IL-6 is increased, and the expression of MMP-9 is known to serve a critical role in pathogenesis.
Our results point to the possibility of applying butein to bowel inflammation-induced colon cancer, as butein suppressed bowel inflammation and interfered with the IL-6/STAT3 and MMP-9 pathways in IL-10-/- mice. It is therefore important to further investigate the effects of butein on the occurrence of bowel inflammation-related colon cancer.
The therapeutic effects of natural substances on inflammation and tumors are being widely studied. Butein has been found in in vitro studies to have antioxidant and anti-inflammatory effects and to suppress tumor cell proliferation and angiogenesis.
The suppressive effects that butein has on matrix metalloproteinase-9 (MMP-9) activation have been reported in an in vitro study using several cancer cells. However, no study has yet to be conducted that examines the therapeutic effects of butein on bowel inflammation and colon cancer. This study, therefore, aims to evaluate the interfering effects of butein on inflammatory cytokines and MMP-9 in interleukin (IL)-10-/- mice, an inflammatory bowel disease model, and ultimately to examine the therapeutic effects of butein.
The results suggest that butein could be used to treat bowel inflammation-induced colon cancer, as it suppressed bowel inflammation and interfered with the IL-6/signal transducer and activator of transcription 3 (STAT3) and MMP-9 pathways in IL-10-/- mice. To our knowledge, while there have been several in vitro studies on tumor cells, this is the first in vivo study to show the effect of butein in mice with colitis.
The study results suggest that it is important to represent the inhibitory effects of butein on the occurrence of bowel inflammation-related colon cancer by regulating IL-6/STAT3 and MMP-9 activation.
The authors examined whether butein, a naturally derived substance, had therapeutic effects in IL-10-/- mice, a model of inflammatory bowel disease. Butein blocked the IL-6/STAT3 signal transmission pathway and suppressed MMP-9 activation. The results are interesting and may represent the effects of butein on the occurrence of bowel inflammation-related colon cancer.
P- Reviewer: Harmanci O, Lakatos PT S- Editor: Nan J L- Editor: AmEditor E- Editor: Ma S
| 1. | Karlén P, Löfberg R, Broström O, Leijonmarck CE, Hellers G, Persson PG. Increased risk of cancer in ulcerative colitis: a population-based cohort study. Am J Gastroenterol. 1999;94:1047-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 144] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 2. | Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785-1794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1390] [Cited by in RCA: 1554] [Article Influence: 111.0] [Reference Citation Analysis (1)] |
| 3. | Ekbom A, Helmick C, Zack M, Adami HO. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med. 1990;323:1228-1233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1294] [Cited by in RCA: 1197] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 4. | Bernstein CN, Blanchard JF, Kliewer E, Wajda A. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer. 2001;91:854-862. [PubMed] |
| 5. | Plevy SE, Targan SR. Future therapeutic approaches for inflammatory bowel diseases. Gastroenterology. 2011;140:1838-1846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 6. | Rutgeerts P, Vermeire S, Van Assche G. Biological therapies for inflammatory bowel diseases. Gastroenterology. 2009;136:1182-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 278] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 7. | Strober W, Fuss IJ. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011;140:1756-1767. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 887] [Cited by in RCA: 851] [Article Influence: 60.8] [Reference Citation Analysis (0)] |
| 8. | Hale LP, Gottfried MR, Swidsinski A. Piroxicam treatment of IL-10-deficient mice enhances colonic epithelial apoptosis and mucosal exposure to intestinal bacteria. Inflamm Bowel Dis. 2005;11:1060-1069. [PubMed] |
| 9. | Brown JB, Lee G, Grimm GR, Barrett TA. Therapeutic benefit of pentostatin in severe IL-10(-/-) colitis. Inflamm Bowel Dis. 2008;14:880-887. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2044] [Cited by in RCA: 1899] [Article Influence: 86.3] [Reference Citation Analysis (0)] |
| 11. | Tan AC, Konczak I, Sze DM, Ramzan I. Molecular pathways for cancer chemoprevention by dietary phytochemicals. Nutr Cancer. 2011;63:495-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 12. | Neergheen VS, Bahorun T, Taylor EW, Jen LS, Aruoma OI. Targeting specific cell signaling transduction pathways by dietary and medicinal phytochemicals in cancer chemoprevention. Toxicology. 2010;278:229-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 13. | Russo GL. Ins and outs of dietary phytochemicals in cancer chemoprevention. Biochem Pharmacol. 2007;74:533-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 217] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 14. | Ma CY, Ji WT, Chueh FS, Yang JS, Chen PY, Yu CC, Chung JG. Butein inhibits the migration and invasion of SK-HEP-1 human hepatocarcinoma cells through suppressing the ERK, JNK, p38, and uPA signaling multiple pathways. J Agric Food Chem. 2011;59:9032-9038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Kim N. Butein sensitizes human leukemia cells to apoptosis induced by tumor necrosis factor-related apoptosis inducing ligand (TRAIL). Arch Pharm Res. 2008;31:1179-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Chua AW, Hay HS, Rajendran P, Shanmugam MK, Li F, Bist P, Koay ES, Lim LH, Kumar AP, Sethi G. Butein downregulates chemokine receptor CXCR4 expression and function through suppression of NF-κB activation in breast and pancreatic tumor cells. Biochem Pharmacol. 2010;80:1553-1562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 122] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 17. | Lau GT, Huang H, Lin SM, Leung LK. Butein downregulates phorbol 12-myristate 13-acetate-induced COX-2 transcriptional activity in cancerous and non-cancerous breast cells. Eur J Pharmacol. 2010;648:24-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Chen YH, Yeh CW, Lo HC, Su SL, Hseu YC, Hsu LS. Generation of reactive oxygen species mediates butein-induced apoptosis in neuroblastoma cells. Oncol Rep. 2012;27:1233-1237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Rasheed Z, Akhtar N, Khan A, Khan KA, Haqqi TM. Butrin, isobutrin, and butein from medicinal plant Butea monosperma selectively inhibit nuclear factor-kappaB in activated human mast cells: suppression of tumor necrosis factor-alpha, interleukin (IL)-6, and IL-8. J Pharmacol Exp Ther. 2010;333:354-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Garg P, Vijay-Kumar M, Wang L, Gewirtz AT, Merlin D, Sitaraman SV. Matrix metalloproteinase-9-mediated tissue injury overrides the protective effect of matrix metalloproteinase-2 during colitis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G175-G184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 126] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 21. | Lakatos G, Sipos F, Miheller P, Hritz I, Varga MZ, Juhász M, Molnár B, Tulassay Z, Herszényi L. The behavior of matrix metalloproteinase-9 in lymphocytic colitis, collagenous colitis and ulcerative colitis. Pathol Oncol Res. 2012;18:85-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Berg DJ, Zhang J, Weinstock JV, Ismail HF, Earle KA, Alila H, Pamukcu R, Moore S, Lynch RG. Rapid development of colitis in NSAID-treated IL-10-deficient mice. Gastroenterology. 2002;123:1527-1542. [PubMed] |
| 23. | Brown JB, Lee G, Grimm GR, Barrett TA. Therapeutic benefit of pentostatin in severe IL-10-/- colitis. Inflamm Bowel Dis. 2008;14:880-887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Berg DJ, Davidson N, Kühn R, Müller W, Menon S, Holland G, Thompson-Snipes L, Leach MW, Rennick D. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J Clin Invest. 1996;98:1010-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 870] [Cited by in RCA: 894] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 25. | Dirisina R, Katzman RB, Goretsky T, Managlia E, Mittal N, Williams DB, Qiu W, Yu J, Chandel NS, Zhang L. p53 and PUMA independently regulate apoptosis of intestinal epithelial cells in patients and mice with colitis. Gastroenterology. 2011;141:1036-1045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 26. | Jeong GS, Lee DS, Song MY, Park BH, Kang DG, Lee HS, Kwon KB, Kim YC. Butein from Rhus verniciflua protects pancreatic β cells against cytokine-induced toxicity mediated by inhibition of nitric oxide formation. Biol Pharm Bull. 2011;34:97-102. [PubMed] |
| 27. | Jang JH, Yang ES, Min KJ, Kwon TK. Inhibitory effect of butein on tumor necrosis factor-α-induced expression of cell adhesion molecules in human lung epithelial cells via inhibition of reactive oxygen species generation, NF-κB activation and Akt phosphorylation. Int J Mol Med. 2012;30:1357-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Sehrawat A, Kumar V. Butein imparts free radical scavenging, anti-oxidative and proapoptotic properties in the flower extracts of Butea monosperma. Biocell. 2012;36:63-71. [PubMed] |
| 29. | Chung CH, Chang CH, Chen SS, Wang HH, Yen JY, Hsiao CJ, Wu NL, Chen YL, Huang TF, Wang PC. Butein Inhibits Angiogenesis of Human Endothelial Progenitor Cells via the Translation Dependent Signaling Pathway. Evid Based Complement Alternat Med. 2013;2013:943187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Cui Z, Song E, Hu DN, Chen M, Rosen R, McCormick SA. Butein induces apoptosis in human uveal melanoma cells through mitochondrial apoptosis pathway. Curr Eye Res. 2012;37:730-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Rajendran P, Ong TH, Chen L, Li F, Shanmugam MK, Vali S, Abbasi T, Kapoor S, Sharma A, Kumar AP. Suppression of signal transducer and activator of transcription 3 activation by butein inhibits growth of human hepatocellular carcinoma in vivo. Clin Cancer Res. 2011;17:1425-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 121] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 32. | Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2935] [Cited by in RCA: 3384] [Article Influence: 211.5] [Reference Citation Analysis (0)] |
| 33. | Kato T. Stat3-driven cancer-related inflammation as a key therapeutic target for cancer immunotherapy. Immunotherapy. 2011;3:587-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 34. | Hodge DR, Hurt EM, Farrar WL. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer. 2005;41:2502-2512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 665] [Cited by in RCA: 716] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 35. | Zheng H, Takahashi H, Murai Y, Cui Z, Nomoto K, Niwa H, Tsuneyama K, Takano Y. Expressions of MMP-2, MMP-9 and VEGF are closely linked to growth, invasion, metastasis and angiogenesis of gastric carcinoma. Anticancer Res. 2006;26:3579-3583. [PubMed] |
| 36. | Lee SH, Seo GS, Jin XY, Ko G, Sohn DH. Butein blocks tumor necrosis factor alpha-induced interleukin 8 and matrix metalloproteinase 7 production by inhibiting p38 kinase and osteopontin mediated signaling events in HT-29 cells. Life Sci. 2007;81:1535-1543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |