Published online May 7, 2015. doi: 10.3748/wjg.v21.i17.5382
Peer-review started: September 29, 2014
First decision: October 14, 2014
Revised: December 25, 2014
Accepted: January 30, 2015
Article in press: January 30, 2015
Published online: May 7, 2015
Processing time: 228 Days and 14 Hours
AIM: To determine the preventive effect and safety of proton pump inhibitors (PPIs) in low-dose aspirin (LDA)-associated gastrointestinal (GI) ulcers and bleeding.
METHODS: We searched MEDLINE, EMBASE and the Cochrane Controlled Trials Register from inception to December 2013, and checked conference abstracts of randomized controlled trials (RCTs) on the effect of PPIs in reducing adverse GI events (hemorrhage, ulcer, perforation, or obstruction) in patients taking LDA. The preventive effects of PPIs were compared with the control group [taking placebo, a cytoprotective agent, or an H2 receptor antagonist (H2RA)] in LDA-associated upper GI injuries. The meta-analysis was performed using RevMan 5.1 software.
RESULTS: We evaluated 8780 participants in 10 RCTs. The meta-analysis showed that PPIs decreased the risk of LDA-associated upper GI ulcers (OR = 0.16; 95%CI: 0.12-0.23) and bleeding (OR = 0.27; 95%CI: 0.16-0.43) compared with control. For patients treated with dual anti-platelet therapy of LDA and clopidogrel, PPIs were able to prevent the LDA-associated GI bleeding (OR = 0.36; 95%CI: 0.15-0.87) without increasing the risk of major adverse cardiovascular events (MACE) (OR = 1.00; 95%CI: 0.76-1.31). PPIs were superior to H2RA in prevention of LDA-associated GI ulcers (OR = 0.12; 95%CI: 0.02-0.65) and bleeding (OR = 0.32; 95%CI: 0.13-0.79).
CONCLUSION: PPIs are effective in preventing LDA-associated upper GI ulcers and bleeding. Concomitant use of PPI, LDA and clopidogrel did not increase the risk of MACE.
Core tip: Ten randomized controlled trials on the preventive effect and safety of proton pump inhibitors (PPIs) in low-dose aspirin (LDA)-associated gastrointestinal tract injuries were included in this meta-analysis. Based on the data collected and presented, the authors conclude that PPIs are effective in preventing LDA-associated upper gastrointestinal tract ulcers and bleeding, without increasing the risk of major adverse cardiovascular events. The findings further confirm and extend the observations already published.
- Citation: Mo C, Sun G, Lu ML, Zhang L, Wang YZ, Sun X, Yang YS. Proton pump inhibitors in prevention of low-dose aspirin-associated upper gastrointestinal injuries. World J Gastroenterol 2015; 21(17): 5382-5392
- URL: https://www.wjgnet.com/1007-9327/full/v21/i17/5382.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i17.5382
With the wide use of low-dose acetylsalicylic acid (LDA) for the primary and secondary prevention of cardiovascular and cerebrovascular diseases, the incidence of LDA-associated upper gastrointestinal (GI) injuries, including gastric mucosal erosions, peptic ulcers, and bleeding has been increasing. The American College of Cardiology Foundation (ACCF), American College of Gastroenterology (ACG), and American Heart Association (AHA) established an expert consensus document on reducing the GI risks of anti-platelet therapy and NSAID use in 2008, and indicated that proton pump inhibitors (PPIs) were the preferred agents for the treatment and prophylaxis of NSAID- and LDA-associated GI injuries[1].
There have been several randomized controlled trials (RCTs) and observational studies to verify the effect of PPIs on the prevention of LDA-associated GI injuries, but there has been no meta-analysis on this subject to date. This meta-analysis aims to determine the preventive effect and safety of PPIs against LDA-associated GI ulcers and bleeding, and to provide the best evidence for clinical practice.
This systematic review was performed based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement revised in 2009[2].
Patients eligible for inclusion were adults (aged ≥ 18 years) who used LDA for at least 2 continuous weeks. RCTs were included regardless of the combined medication used, medical condition, and comorbidities in the patients. Oral PPIs were used in the experimental group and placebo, cytoprotective agents or histamine 2 receptor antagonists (H2RA) were used as the controls. The incidences of LDA-related peptic ulcer and upper gastrointestinal bleeding, and the incidences of the major adverse cardiovascular events (MACE) and diarrhea in the 2 groups were observed. Only studies published in English were included.
Non-RCTs, cohort studies, case-control studies, pharmacokinetic experiments and case reports were excluded from this study.
We conducted a comprehensive literature search of MEDLINE and EMBASE databases, and the Cochrane Central Register of Controlled Trials (CENTRAL) from their inception to December 31, 2013. The following keywords were used: aspirin, acetylsalicylic, low-dose aspirin, LDA, proton pump inhibitor, PPI, esomeprazole, pantoprazole, omeprazole, rabeprazole, lansoprazole, and randomized controlled trial. The search strategy for MEDLINE was as follows: (1) aspirin OR acetylsalicylic OR low-dose aspirin OR LDA; (2) proton pump inhibitor OR PPI OR omeprazole OR esomeprazole OR lansoprazole OR pantoprazole OR rabeprazole; and (3) controlled trial; and (4) #1 AND #2 AND #3.
In this meta-analysis, we aimed to evaluate the preventive effect of PPIs against GI injuries in long-term LDA users, therefore, we did not include pharmacokinetic studies, and studies with short-term or intermittent use of LDA. Two independent reviewers (Mo C and Lu ML) used a predefined relevance criteria form to screen the studies. After scrutinized the title and abstract, papers not meeting the inclusion criteria and duplicate papers were eliminated. The remaining full-text papers were screened for inclusion. Discrepancies were resolved through discussion with a third reviewer (SG).
Data were extracted after reading the full-text. Two independent reviewers (Mo C and Lu ML) extracted the data. A third independent reviewer (Sur G) reviewed the data abstraction and resolved any discrepancies. When multiple publications reported the data from the same population, the trial reporting the primary outcome of interest was considered the major publication.
The extracted data included the following items: authors and publication year, the country or region of the study, medical condition or risk factor, sample size, intervention measures, GI ulcer or bleeding events, adverse events including cardiovascular events and diarrhea, and statistical methods.
Risks of bias in individual studies were assessed using the Cochrane Risk of Bias tool. This tool assesses six domains of bias: sequence generation (low risk, high risk, and unclear risk of bias), allocation concealment (low risk, high risk, and unclear risk of bias), blinding of outcome assessment (low risk, high risk, and unclear risk of bias), incomplete outcome data (low risk, high risk, and unclear risk of bias), selective outcome reporting (low risk, high risk, and unclear risk of bias), and other sources of bias (low risk, high risk, and unclear risk of bias). The two reviewers (Mo C and Lu ML) assessed study quality independently and the assessments were verified by the third reviewer (SG).
For dichotomous data, summary statistics are expressed as an odds ratio (OR) with 95%CI. A statistically significant level was considered as α = 0.05. Statistical heterogeneity in the included studies was examined using I2 statistics. If P≥ 0.10 in the heterogeneity test, a fixed effects model was used for the meta-analysis; if P < 0.10, the sources of heterogeneity were further investigated. If no obvious clinical heterogeneity and no clear statistical heterogeneity occurred, a random effects model was used for the meta-analysis. If the clinical heterogeneity was too large, data synthesis was abandoned and a single analysis used instead. All analyses were conducted using Review Manager Version 5.1.
Publication bias was determined by the funnel plot.
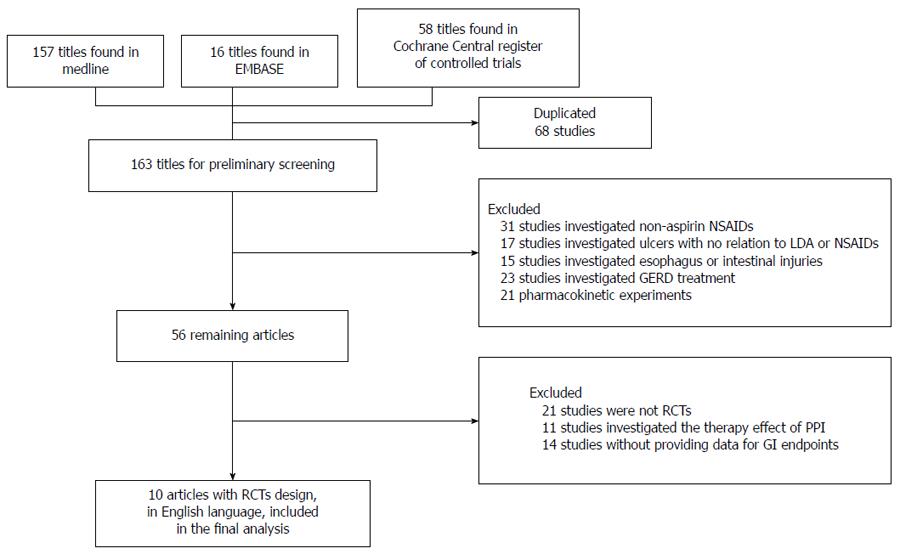
The literature search identified 58 articles in the Cochrane Controlled Trial Register, 16 articles in EMBASE and 157 articles in MEDLINE that met the search criteria. Figure 1 shows the flow chart of the retrieved studies and studies excluded, with the reasons for exclusion. Finally, 10 RCTs published in English were included[3-12]. Of these, 5 RCTs compared the preventive effect of PPIs with placebo[3-6,8]; 2 compared PPIs with gefarnate[7,9], and 3 compared PPIs with famotidine[10-12].
All the included studies were published in the United States or Japan between 2002 and 2012. Demographic and clinical characteristics of the studies included in this meta-analysis are summarized in Table 1. The number of participants in the experimental group ranged from 62 to 1876, and the duration of follow-up from 4 to 52 wk. The PPIs used were esomeprazole, pantoprazole, omeprazole, rabeprazole and lansoprazole, at doses ranging from 10 to 40 mg/d. The number of participants in the control group ranged from 61 to 1885 and the duration of follow-up from 4 to 52 wk. The drugs used in the control group included placebo, cytoprotective agents (gefarnate 100 mg/d) and H2RA (famotidine 20-80 mg/d). The populations varied across the included RCTs, but all had a high risk of gastrointestinal bleeding. Of these studies, 4 RCTs[3,7,9,12] included patients who suffered from ulcer/erosion or with a history of peptic ulcer, 3 RCTs[3,8,12] included Helicobacter pylori (H. pylori)-negative patients or patients whose infection had been eradicated, 4 RCTs[4,5,7,9] performed hierarchical analysis according to the infection status of H. pylori, 4 RCTs[5,6,10,11] included patients with acute coronary syndrome and myocardial infarction who were treated with dual anti-platelet therapy of PPIs and clopidogrel, 4 RCTs[3,4,7,12] performed endoscopy in the patients before and after treatment, and 4 RCTs[5,8,9,11] only conducted endoscopy after treatment.
| Ref. | Country/region | Risk factors | Ppis | Control | Peptic ulcer | UGIB | MACE | Diarrhea | ||||||||
| Drug | n | Dose (mg/d) | Drug | n | Dose (mg/d) | PPI | Control | PPI | Control | PPI | Control | PPI | Control | |||
| Lai et al[3] | Hong Kong | ADA-induced ulcers | Lansoprazole | 62 | 30 | Placebo | 61 | - | 1 | 9 | 0 | 8 | - | - | - | - |
| H. pylori eradicated | ||||||||||||||||
| Yeomans et al[4] | 10 countries | Aged ≥ 60, without | Esomeprazole | 493 | 20 | Placebo | 498 | - | 8 | 27 | 2 | 4 | 8 | 14 | - | - |
| ulcer | ||||||||||||||||
| Bhatt et al[5] | 15 countries | Combined with clopidogrel | Omeprazole | 1876 | 20 | Placebo | 1885 | - | 2 | 6 | 8 | 26 | 55 | 54 | 56 | 34 |
| Ren et al[6] | China | Combined with clopidogrel | Omeprazole | 86 | 20 | Placebo | 86 | - | - | - | 0 | 2 | 22 | 22 | - | - |
| Scheiman et al[8] | 20 countries | H. pylori-negative, high risk | Esomeprazole | 1623 | 20-40 | Placebo | 804 | - | 19 | 53 | 1 | 3 | 1 | 1 | 48 | 18 |
| Sugano et al[9] | Japan | With a history of ulcer | Lansoprazole | 226 | 15 | Gefarnate | 235 | 100 | 6 | 53 | 2 | 9 | - | - | 19 | 2 |
| Sanuki et al[7] | Japan | With a history of ulcer | Rabeprazole | 176 | 10-20 | Gefarnate | 85 | 100 | 9 | 20 | 0 | 1 | - | - | - | - |
| Ng et al[12] | Hong Kong | Aspirin-related ulcers/erosions | Pantoprazole | 65 | 20 | Famotidine | 65 | 80 | 0 | 6 | 0 | 5 | - | - | - | - |
| Ng et al[11] | Hong Kong | Combined with clopidogrel | Esomeprazole | 163 | 20 | Famotidine | 148 | 40 | 1 | 5 | 3 | 12 | 7 | 5 | - | - |
| Yano et al[10] | Japan | Combined with clopidogrel | Omeprazole | 65 | 10 | Famotidine | 65 | 20 | - | - | 3 | 1 | 8 | 11 | - | - |
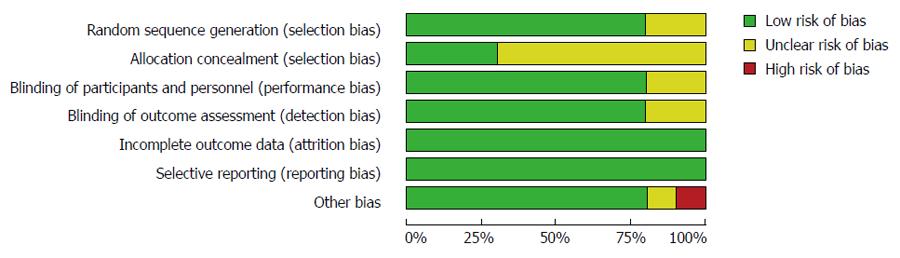
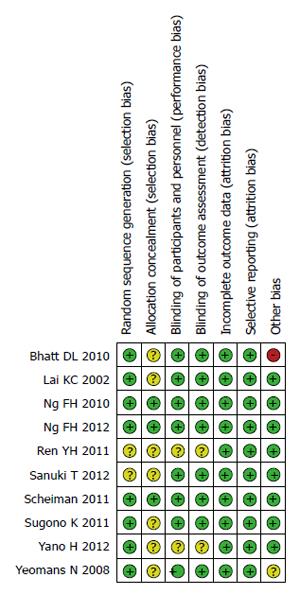
The risks of bias within the 10 studies included in this meta-analysis are summarized in Table 2 and Figures 2 and 3.
| Ref. | Random sequence generation | Allocation blinding | Blinding of participants and personnel | Blinding of outcome assessment | Imcomplete outcome data | Selective reporting | Other bias |
| Lai et al[3] | Low risk | Unclear risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Yeomans et al[4] | Low risk | Unclear risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Bhatt et al[5] | Low risk | Unclear risk | Low risk | Low risk | Low risk | Low risk | high risk |
| Ren et al[6] | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Low risk | Low risk |
| Scheiman et al[8] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Sugano et al[9] | Low risk | Unclear risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Sanuki et al[7] | Unclear risk | Unclear risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Ng et al[12] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Ng et al[11] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Yano et al[10] | Low risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Low risk | Low risk |
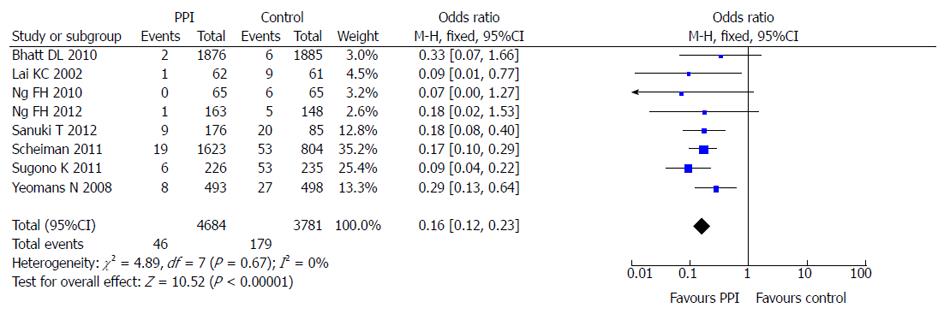
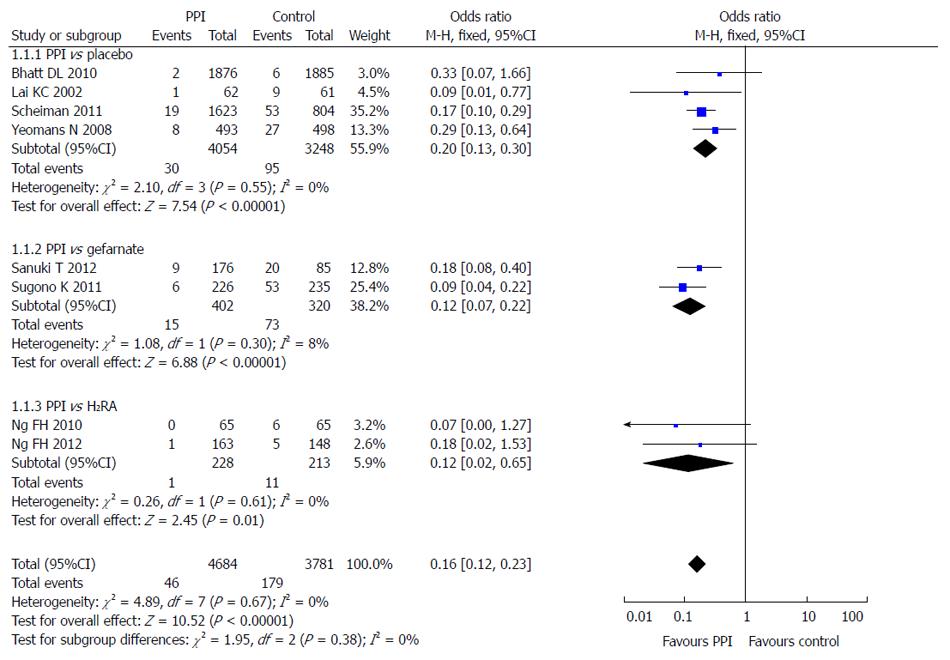
Eight of the 10 included studies reported the incidence of LDA-associated peptic ulcer in the PPI group and the control group (taking placebo, gefarnate and H2RA). There was no statistical heterogeneity among the research results (I2 = 0; P = 0.67), and the fixed effects model was used for the meta-analysis. The result showed that PPIs were superior to the control drugs (OR = 0.16; 95%CI: 0.12-0.23) in prevention of LDA-associated peptic ulcer (Figure 4).
Subgroup analysis was used in different control groups. Four RCTs compared the incidence of LDA-associated ulcer after a PPI and placebo, 2 after a PPI and gefarnate, and 2 after a PPI and famotidine. The results showed that PPIs were superior to placebo (OR = 0.20; 95%CI: 0.13-0.30), gefarnate (OR = 0.12; 95%CI: 0.07-0.22), and famotidine (OR = 0.12; 95%CI: 0.02-0.65) in prevention of LDA-associated peptic ulcer (Figure 5).
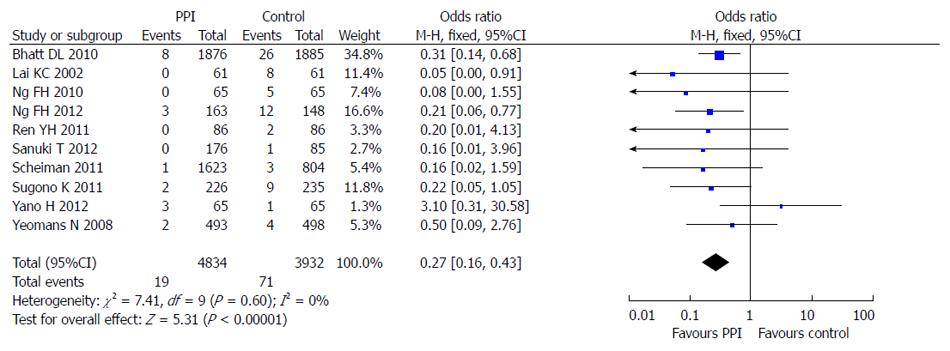
All 10 included studies reported the incidence of LDA-associated GI bleeding in a PPI group and a control group. There was no statistical heterogeneity among the research results (I2 = 0; P = 0.60), and the fixed effects model was used for the meta-analysis. The result showed that PPIs were superior to the control drugs (OR = 0.27; 95%CI: 0.16-0.43) in prevention of LDA-associated GI bleeding (Figure 6).
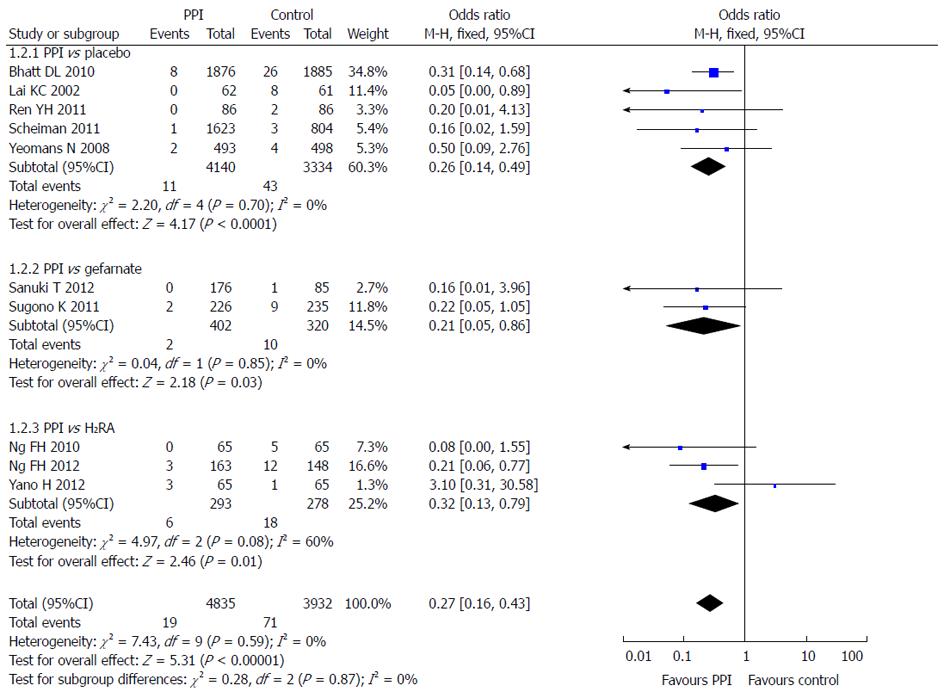
Subgroup analysis was also used according to the different control groups. Five RCTs compared the incidence of LDA-associated GI bleeding after PPI and placebo, 2 after PPI and gefarnate, and 3 after PPI and famotidine. The results showed that PPIs were superior to placebo (OR = 0.26; 95%CI: 0.14-0.49), gefarnate (OR = 0.21; 95%CI: 0.05-0.86), and famotidine (OR = 0.32; 95%CI: -0.13-0.79) in prevention of LDA-associated GI bleeding (Figure 7).
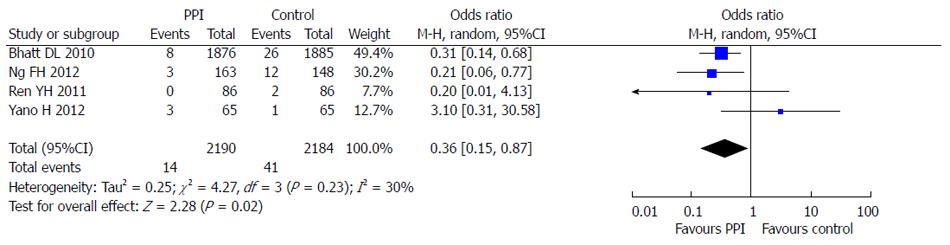
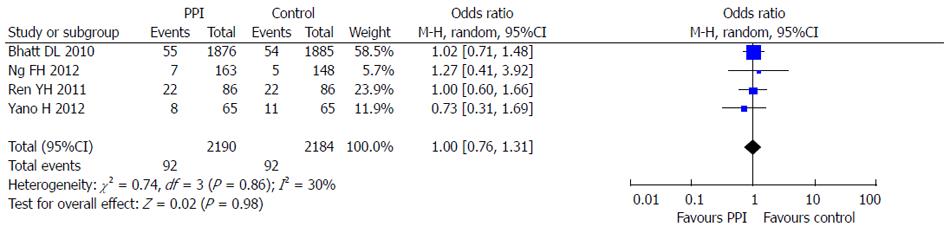
Four RCTs reporting dual anti-platelet therapy with LDA and clopidogrel were included in this meta-analysis[5,6,10,11]. There were 2190 patients in the PPI group treated with omeprazole or esomeprazole and 2184 patients in the control group. There was no statistical heterogeneity between the groups (I2 = 30%; P = 0.23) and the fixed effects model was used for the meta-analysis. The results showed that PPIs were superior to control drugs (OR = 0.36; 95%CI: 0.15-0.87) in prevention of dual anti-platelet drug-associated GI bleeding (Figure 8). At the same time, no significant difference (OR = 1.00; 95%CI: 0.76-1.31) in cardiovascular adverse events in the 4 RCTs was found between PPI and control drugs (Figure 9).
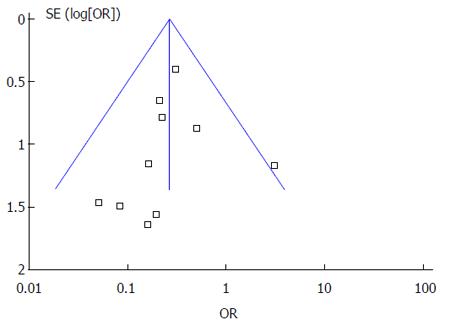
Funnel plot analysis of the 10 RCTs of PPIs and controls drugs in the prevention of LDA-associated GI bleeding indicated an asymmetric distribution that suggested the presence of publication bias (Figure 10).
It has been confirmed that long-term LDA use increases the risk of GI injury and bleeding[13]. The mechanism of LDA-associated GI injuries involves both topical and systemic effects, and the latter is the main cause[1]. Aspirin is a relatively soluble, weak acid which is deionized and becomes fat-soluble, diffusing back into the mucosal cells when pH < 3.5. On the other hand, LDA blocks production of prostaglandins via the COX-1 pathway. The inhibition of prostaglandins impairs protective factors such as gastric acid, pepsin, and bile salts, resulting in a gastric environment that is more susceptible to topical attack[1]. In theory, synthetic prostaglandin replacement therapy can reduce the GI toxicity of LDA. In addition, misoprostol has been shown to be superior to placebo in preventing the recurrence of gastric ulcers among patients with a history of gastric ulcer who were receiving LDA and NSAID[14]. However, misoprostol is associated with side effects such as diarrhea and abdominal pain that often restrict its clinical use. Acid suppressors are able to inhibit the acid secretion, and lower the pH in the stomach, thus reducing mucosal injury and bleeding complications.
H2RA can prevent LDA-associated GI injuries effectively, although data are limited. Nakashima[15] indicated in a retrospective study that H2RA were effective for the prevention of LDA-induced peptic ulcer, similar to the effects of PPIs, compared with cytoprotective anti-ulcer drugs. Lanas[16] discovered in a case control study that H2RA reduced the risk of GI bleeding induced by LDA and clopidogrel (relative risk: 0.65; 95%CI: 0.50-0.85). The FAMOUS trial evaluated the effect of a standard dose of famotidine in the prevention of ulcers and esophagitis induced by LDA, and concluded that famotidine was effective in the prevention of gastric and duodenal ulcers, and erosive esophagitis in patients taking LDA[17].
The studies in the prevention of LDA-associated GI injuries have focused on PPIs. The OITA-GF study indicated that about one-third of asymptomatic patients taking LDA had been found to have gastric and duodenal ulcers/erosions during the 3-mo follow-up, and PPI use was the only independent factor for gastroduodenal ulcers/erosions (OR = 0.35; 95%CI: 0.14-0.86; P = 0.02)[18]. Chin et al[19], Ng et al[20], and Yasuda et al[21] reported that PPI reduced upper GI bleeding after percutaneous coronary intervention. Lanas et al[13] included 3 RCTs in their meta-analysis evaluating the preventive effect of PPIs in long-time LDA users, and concluded that PPIs reduced the risk of major GI bleeding in patients given LDA (OR = 0.34; 95%CI: 0.21-0.57).
Five RCTs comparing the preventive effect of PPIs with placebo in LDA users were included in this meta-analysis, and the result indicated that PPIs were effective in preventing LDA-associated GI ulcers compared with placebo.
Gilard et al[22] first discovered in an in vitro experiment that PPI diminished the biological action of clopidogrel in coronary revascularization patients, and confirmed in the subsequent RCT that omprazole decreased the P2Y12 inhibition of clopidogrel significantly[23]. Ho et al[24] discovered in an retrospective cohort trial that patients with acute coronary syndromes who used clopidogrel and a PPI concomitantly had an increased risk of adverse cardiovascular outcomes than those who used clopidogrel without a PPI (15.5% vs 11.9%; OR = 1.49; 95%CI: 1.30-1.71), suggesting that use of a PPI may be associated with attenuation of the benefits of clopidogrel with acute coronary syndromes. In view of the results above, the FDA suggested in January 2009 that a combination of a PPI and clopidogrel should be avoided. However, subsequent clinical trials did not support this suggestion. Ray et al[25] found in a retrospective cohort trial of 20596 patients with coronary heart disease that concomitant use of a PPI and clopidogrel decreased the occurrence of GI bleeding (95%CI: 11.7-36.9), and did not increase MACE (95%CI: 0.82-1.19). GHOST and FAST-MI trials also concluded that concomitant use of a PPI and clopidogrel did not increase the risk of MACE[26,27]. Charlot et al[28] found that PPIs increased the risk of MACE in myocardial infarction patients after discharge whether clopidogrel was used or not, and indicated that a PPI was the independent risk factor of MACE. Kwok included 7 observational studies in his meta-analysis and found that either concomitant use of a PPI and clopidogrel or sole use of a PPI increased the risk of MACE. He concluded that a PPI was an important confounding factor and that the clinical hypothesis of a PPI-clopidogrel interaction remained to be further verified[29].
Our study included 4 RCTs investigating dual anti-platelet therapy with a PPI and clopidogrel and the preventive effect of PPIs against GI injuries and the incidence of MACE. The results showed that PPIs were able to prevent the dual anti-platelet therapy-associated GI bleeding, and at the same time, PPIs did not increase the risk of MACE. Our study aimed to evaluate the preventive effect of PPIs, so the number of studies we included may be insufficient, selection bias may exist and the interpretation of the results needs further verification.
Considering the debate regarding the PPI-clopidogrel interaction, the ACC/AHA/SCAI consensus indicated that H2RA may be a reasonable alternative for a lower risk of GI bleeding[30]. The FAMOUS trial[17] suggested that high dose H2RA may be an alternative to PPI to prevent LDA-associated GI bleeding. However, some scholars doubt the effects of H2RA and believe that H2RA are inferior to PPIs in the prevention of LDA-associated GI injuries. The OITA-GF2 study indicated that lansoprazole (15 mg/d) was superior to famotidine (40 mg/d) in the prevention of LDA-associated GI injuries[31]. Our meta-analysis included 3 RCTs to evaluate the preventive effects of PPI and H2RA in LDA-associated ulcers and bleeding, and the results indicated that PPIs were superior to H2RA in preventing both ulcers (OR = 0.12; 95%CI: 0.02-0.65) and bleeding (OR = 0.32; 95%CI: 0.13-0.79). Since the studies we included are all published in English, the number of studies is small and selection bias may exist, and better designed RCTs are needed to support our results.
Although this study is the first systematic review regarding the preventive effect of PPIs in LDA-associated GI injuries, there are some limitations as we did not include studies published in languages other than English. Furthermore, we searched for unpublished material, but were unable to identify any relevant papers. So there may be selection bias. We only included 4 RCTs related to the interactions of PPIs and clopidogrel because we focused on the adverse events but not the therapeutic effects, and studies which did not report GI endpoints events were not included in our meta-analysis. Publication bias was also found from the funnel plot with an asymmetric distribution.
In conclusion, PPIs are able to prevent LDA-associated upper GI ulcers and bleeding effectively. Concomitant use of a PPI, LDA and clopidogrel did not increase the risk of cardiovascular adverse events, so PPIs are safe in the prevention of LDA-associated GI injuries. Given the selection bias and publication bias, our results should be interpreted with caution.
With the widespread use of low-dose acetylsalicylic acid (LDA) in the primary and secondary prevention of cardiovascular and cerebrovascular diseases, the incidence of LDA-associated upper gastrointestinal injuries, including gastric mucosal erosions, peptic ulcers, and bleeding has been increasing. The aim of this meta-analysis is to verify that proton pump inhibitors (PPIs) are the preferred agents for the prophylaxis of LDA-associated gastrointestinal injuries.
The FDA suggested in January 2009 that combined use of PPI and clopidogrel should be avoided. However, subsequent clinical trials did not support this suggestion. Several randomized controlled trials concluded that concomitant use of a PPI and clopidogrel decreased the occurrence of GI bleeding, and did not increase the risk of major adverse cardiovascular events.
This meta-analysis aimed to determine the preventive effect and safety of PPIs in the LDA-associated GI ulcer and bleeding and this is the first meta-analysis on this subject.
This meta-analysis provided the best evidence for clinical practice that PPIs are able to prevent LDA-associated upper GI ulcers and bleeding effectively and safely.
LDA-associated upper gastrointestinal injury: Gastroduodenal mucosal erosions, peptic ulcers and upper gastrointestinal bleeding induced by the use of low-dose aspirin.
Based on the data collected and presented, the authors conclude that PPIs are effective in the prevention of LDA-associated upper gastrointestinal tract ulcers and bleeding, without increasing the major adverse cardiovascular events. In general, this is an interesting and well written review on an important topic. The data presented have confirmed and significantly extended the observations already published.
P- Reviewer: Nakajima N, Swierczynski JT, Tan HJ S- Editor: Ma YJ L- Editor: Cant MR E- Editor: Liu XM
| 1. | Bhatt DL, Scheiman J, Abraham NS, Antman EM, Chan FK, Furberg CD, Johnson DA, Mahaffey KW, Quigley EM. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation. 2008;118:1894-1909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 340] [Article Influence: 20.0] [Reference Citation Analysis (3)] |
| 2. | Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18665] [Cited by in RCA: 17544] [Article Influence: 1096.5] [Reference Citation Analysis (1)] |
| 3. | Lai KC, Lam SK, Chu KM, Wong BC, Hui WM, Hu WH, Lau GK, Wong WM, Yuen MF, Chan AO. Lansoprazole for the prevention of recurrences of ulcer complications from long-term low-dose aspirin use. N Engl J Med. 2002;346:2033-2038. [PubMed] |
| 4. | Yeomans N, Lanas A, Labenz J, van Zanten SV, van Rensburg C, Rácz I, Tchernev K, Karamanolis D, Roda E, Hawkey C. Efficacy of esomeprazole (20 mg once daily) for reducing the risk of gastroduodenal ulcers associated with continuous use of low-dose aspirin. Am J Gastroenterol. 2008;103:2465-2473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 163] [Article Influence: 9.6] [Reference Citation Analysis (1)] |
| 5. | Bhatt DL, Cryer BL, Contant CF, Cohen M, Lanas A, Schnitzer TJ, Shook TL, Lapuerta P, Goldsmith MA, Laine L. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med. 2010;363:1909-1917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 826] [Cited by in RCA: 843] [Article Influence: 56.2] [Reference Citation Analysis (0)] |
| 6. | Ren YH, Zhao M, Chen YD, Chen L, Liu HB, Wang Y, Sun ZJ, Chen JS, Huang TT, Guo YS. Omeprazole affects clopidogrel efficacy but not ischemic events in patients with acute coronary syndrome undergoing elective percutaneous coronary intervention. Chin Med J (Engl). 2011;124:856-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Sanuki T, Fujita T, Kutsumi H, Hayakumo T, Yoshida S, Inokuchi H, Murakami M, Matsubara Y, Kuwayama H, Kawai T. Rabeprazole reduces the recurrence risk of peptic ulcers associated with low-dose aspirin in patients with cardiovascular or cerebrovascular disease: a prospective randomized active-controlled trial. J Gastroenterol. 2012;47:1186-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Scheiman JM, Devereaux PJ, Herlitz J, Katelaris PH, Lanas A, Veldhuyzen van Zanten S, Nauclér E, Svedberg LE. Prevention of peptic ulcers with esomeprazole in patients at risk of ulcer development treated with low-dose acetylsalicylic acid: a randomised, controlled trial (OBERON). Heart. 2011;97:797-802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 9. | Sugano K, Matsumoto Y, Itabashi T, Abe S, Sakaki N, Ashida K, Mizokami Y, Chiba T, Matsui S, Kanto T. Lansoprazole for secondary prevention of gastric or duodenal ulcers associated with long-term low-dose aspirin therapy: results of a prospective, multicenter, double-blind, randomized, double-dummy, active-controlled trial. J Gastroenterol. 2011;46:724-735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Yano H, Tsukahara K, Morita S, Endo T, Sugano T, Hibi K, Himeno H, Fukui K, Umemura S, Kimura K. Influence of omeprazole and famotidine on the antiplatelet effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes: a prospective, randomized, multicenter study. Circ J. 2012;76:2673-2680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Ng FH, Tunggal P, Chu WM, Lam KF, Li A, Chan K, Lau YK, Kng C, Keung KK, Kwan A. Esomeprazole compared with famotidine in the prevention of upper gastrointestinal bleeding in patients with acute coronary syndrome or myocardial infarction. Am J Gastroenterol. 2012;107:389-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Ng FH, Wong SY, Lam KF, Chu WM, Chan P, Ling YH, Kng C, Yuen WC, Lau YK, Kwan A. Famotidine is inferior to pantoprazole in preventing recurrence of aspirin-related peptic ulcers or erosions. Gastroenterology. 2010;138:82-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 13. | Lanas A, Wu P, Medin J, Mills EJ. Low doses of acetylsalicylic acid increase risk of gastrointestinal bleeding in a meta-analysis. Clin Gastroenterol Hepatol. 2011;9:762-768.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 14. | Goldstein JL, Huang B, Amer F, Christopoulos NG. Ulcer recurrence in high-risk patients receiving nonsteroidalanti-inflammatory drugs plus low-dose aspirin: results of a post HOC subanalysis. Clin Ther. 2004;26:1637-1643. [PubMed] |
| 15. | Nakashima S, Ota S, Arai S, Yoshino K, Inao M, Ishikawa K, Nakayama N, Imai Y, Nagoshi S, Mochida S. Usefulness of anti-ulcer drugs for the prevention and treatment of peptic ulcers induced by low doses of aspirin. World J Gastroenterol. 2009;15:727-731. [PubMed] |
| 16. | Lanas A, García-Rodríguez LA, Arroyo MT, Bujanda L, Gomollón F, Forné M, Aleman S, Nicolas D, Feu F, González-Pérez A. Effect of antisecretory drugs and nitrates on the risk of ulcer bleeding associated with nonsteroidal anti-inflammatory drugs, antiplatelet agents, and anticoagulants. Am J Gastroenterol. 2007;102:507-515. [PubMed] |
| 17. | Taha AS, McCloskey C, Prasad R, Bezlyak V. Famotidine for the prevention of peptic ulcers and oesophagitis in patients taking low-dose aspirin (FAMOUS): a phase III, randomised, double-blind, placebo-controlled trial. Lancet. 2009;374:119-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 162] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 18. | Tamura A, Murakami K, Kadota J. Prevalence and independent factors for gastroduodenal ulcers/erosions in asymptomatic patients taking low-dose aspirin and gastroprotective agents: the OITA-GF study. QJM. 2011;104:133-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Chin MW, Yong G, Bulsara MK, Rankin J, Forbes GM. Predictive and protective factors associated with upper gastrointestinal bleeding after percutaneous coronary intervention: a case-control study. Am J Gastroenterol. 2007;102:2411-2416. [PubMed] |
| 20. | Ng FH, Wong SY, Lam KF, Chang CM, Lau YK, Chu WM, Wong BC. Gastrointestinal bleeding in patients receiving a combination of aspirin, clopidogrel, and enoxaparin in acute coronary syndrome. Am J Gastroenterol. 2008;103:865-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Yasuda H, Yamada M, Sawada S, Endo Y, Inoue K, Asano F, Takeyama Y, Yoshiba M. Upper gastrointestinal bleeding in patients receiving dual antiplatelet therapy after coronary stenting. Intern Med. 2009;48:1725-1730. [PubMed] |
| 22. | Gilard M, Arnaud B, Le Gal G, Abgrall JF, Boschat J. Influence of omeprazol on the antiplatelet action of clopidogrel associated to aspirin. J Thromb Haemost. 2006;4:2508-2509. [PubMed] |
| 23. | Gilard M, Arnaud B, Cornily JC, Le Gal G, Lacut K, Le Calvez G, Mansourati J, Mottier D, Abgrall JF, Boschat J. Influence of omeprazole on the antiplatelet action of clopidogrel associated with aspirin: the randomized, double-blind OCLA (Omeprazole CLopidogrel Aspirin) study. J Am Coll Cardiol. 2008;51:256-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 747] [Cited by in RCA: 726] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 24. | Ho PM, Maddox TM, Wang L, Fihn SD, Jesse RL, Peterson ED, Rumsfeld JS. Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome. JAMA. 2009;301:937-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 739] [Cited by in RCA: 709] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 25. | Ray WA, Murray KT, Griffin MR, Chung CP, Smalley WE, Hall K, Daugherty JR, Kaltenbach LA, Stein CM. Outcomes with concurrent use of clopidogrel and proton-pump inhibitors: a cohort study. Ann Intern Med. 2010;152:337-345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 163] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 26. | Harjai KJ, Shenoy C, Orshaw P, Usmani S, Boura J, Mehta RH. Clinical outcomes in patients with the concomitant use of clopidogrel and proton pump inhibitors after percutaneous coronary intervention: an analysis from the Guthrie Health Off-Label Stent (GHOST) investigators. Circ Cardiovasc Interv. 2011;4:162-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 27. | Simon T, Steg PG, Gilard M, Blanchard D, Bonello L, Hanssen M, Lardoux H, Coste P, Lefèvre T, Drouet E. Clinical events as a function of proton pump inhibitor use, clopidogrel use, and cytochrome P450 2C19 genotype in a large nationwide cohort of acute myocardial infarction: results from the French Registry of Acute ST-Elevation and Non-ST-Elevation Myocardial Infarction (FAST-MI) registry. Circulation. 2011;123:474-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 135] [Reference Citation Analysis (0)] |
| 28. | Charlot M, Ahlehoff O, Norgaard ML, Jørgensen CH, Sørensen R, Abildstrøm SZ, Hansen PR, Madsen JK, Køber L, Torp-Pedersen C. Proton-pump inhibitors are associated with increased cardiovascular risk independent of clopidogrel use: a nationwide cohort study. Ann Intern Med. 2010;153:378-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 210] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 29. | Kwok CS, Jeevanantham V, Dawn B, Loke YK. No consistent evidence of differential cardiovascular risk amongst proton-pump inhibitors when used with clopidogrel: meta-analysis. Int J Cardiol. 2013;167:965-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 30. | Abraham NS, Hlatky MA, Antman EM, Bhatt DL, Bjorkman DJ, Clark CB, Furberg CD, Johnson DA, Kahi CJ, Laine L. ACCF/ACG/AHA 2010 Expert Consensus Document on the concomitant use of proton pump inhibitors and thienopyridines: a focused update of the ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. Circulation. 2010;122:2619-2633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 196] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 31. | Tamura A, Murakami K, Kadota J. Prevalence of gastroduodenal ulcers/erosions in patients taking low-dose aspirin with either 15 mg/day of lansoprazole or 40 mg/day of famotidine: the OITA-GF study 2. BMC Res Notes. 2013;6:116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |