Published online May 7, 2015. doi: 10.3748/wjg.v21.i17.5287
Peer-review started: October 30, 2014
First decision: November 26, 2014
Revised: December 9, 2014
Accepted: January 30, 2015
Article in press: January 30, 2015
Published online: May 7, 2015
Processing time: 195 Days and 10.6 Hours
AIM: To evaluate long-term outcomes of radiofrequency (RF) ablation as first-line therapy for single hepatocellular carcinoma (HCC) ≤ 3 cm and to determine survival and prognostic factors.
METHODS: We included all 184 patients who underwent RF ablation as a first-line treatment for single HCC ≤ 3 cm between April 2005 and December 2013. According to the criteria of Livraghi, the 184 patients were divided into two groups: those suitable for surgical resection (84 cases) and those unsuitable for surgical resection (100 cases). The primary endpoints were the overall survival (OS) rate and safety; the secondary endpoints were primary technique effectiveness and recurrence rate.
RESULTS: There were 19 (10.3%) cases of ablation related minor complications. The complete tumor ablation rate after one RF session was 97.8% (180/184). The rate of local tumor progression, extrahepatic metastases and intrahepatic distant recurrence were 4.9% (9/184), 9.8% (18/184) and 37.5% (69/184), respectively. In the 184 patients, the 1-, 3-, and 5-year OS rates were 99.5%, 81.0%, and 62.5%, respectively. The 1-, 3-, and 5-year OS rates were 100%, 86.9%, and 71.4%, respectively, in those suitable for surgical resection and 99.0%, 76.0%, and 55.0%, respectively, in those unsuitable for surgical resection (P = 0.021). On univariate and multivariate analyses, poorer OS was associated with Child-Pugh B class and portal hypertension (P < 0.05).
CONCLUSION: RF ablation is a safe and effective treatment for single HCC ≤ 3 cm. The OS rate of patients suitable for surgical resection was similar to those reported in surgical series.
Core tip: The argument against the role of radiofrequency (RF) ablation as a first treatment option for patients with small hepatocellular carcinoma (HCC) is represented by the lack of adequate evidence proving that its effectiveness is comparable to that of surgical resection (SR). The study provides evidence that RF ablation is a safe and effective first-line treatment for single HCC 3 cm or less, even when SR is possible. Furthermore, we also induced the systemic technical measures to promote the efficacy of RF ablation for HCC from the surgeon’s perspective.
- Citation: Gao J, Wang SH, Ding XM, Sun WB, Li XL, Xin ZH, Ning CM, Guo SG. Radiofrequency ablation for single hepatocellular carcinoma 3 cm or less as first-line treatment. World J Gastroenterol 2015; 21(17): 5287-5294
- URL: https://www.wjgnet.com/1007-9327/full/v21/i17/5287.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i17.5287
Hepatocellular carcinoma (HCC) is the third most common global cause of cancer-related death[1]. Nowadays, HCC is diagnosed at an early stage with increasing frequency, improving the prospects of radical treatment by means of liver transplantation, surgical resection (SR), or radiofrequency (RF) ablation[2,3]. Liver transplantation is considered the best option, as it allows eliminating both tumor and cirrhosis at the same time, but lack of liver donors represents a major limitation[2]. SR can provide a 5-year-survival rate of over 50%, and therefore it is considered the first choice of treatment for patients with early-stage HCC[2,3].
Reportedly, optimal efficacy of treatment for small HCC can be achieved by means of RF ablation[4-10]. The advantage of minimal invasiveness has made this method into the first-line treatment for small HCC in patients with compromised liver function or associated severe medical conditions. Previous studies comparing the clinical effectiveness of RF ablation with that of SR suggested that SR was more effective than RF ablation for early-stage HCC because local tumor progression (LTP) and intrahepatic distant recurrence (IDR) were lower with SR than with RF ablation[5,6]. However, other studies reported conflicting results in this regard[4,7-9]. Hence, whether RF ablation or SR is the better choice for early-stage HCC has long been debated[11].
The aim of this study was to evaluate from the surgeon’s perspective on the technical and clinical outcomes of RF ablation as the first-line treatment in a cohort of patients with a single HCC 3 cm or less.
The following hospitals in China participated in the study: Beijing Chaoyang Hospital affiliated to Capital Medical University, Beijing, China; Affiliated Hospital of Chifeng University, Inner Mongolia, China; Zhanhua People’s Hospital, Shandong, China; Chaoyang Central Hospital, Liaoning, China. From April 2005 to December 2013, a total of 1008 patients with HCC received RF ablation in the four hospitals. Of these, we retrospectively reviewed the records of 198 consecutive patients diagnosed with a single HCC 3 cm or less.
Each patient provided a medical history and underwent physical examination, screening for hepatitis B and C, serum laboratory tests assessing liver function, hemostasis, renal function, and determination of serum a-fetoprotein level prior to the RF treatment. The diagnosis of HCC was made according to the American Association for the Study of Liver Disease guidelines[12]. Abdominal computed tomography (CT) and/or magnetic resonance imaging (MRI) were used to assess local tumor extension, and thoracic CT was systematically performed to detect the presence of pulmonary metastases.
Among the 198 HCC patients, 184 met the criteria and were enrolled in this study. The inclusion criteria were (1) a single HCC nodule with a diameter of 3 cm or less; (2) no previous treatment for HCC; (3) follow-up of at least 6 mo; and (4) complete follow-up data. The exclusion criteria were (1) extrahepatic metastasis; (2) unfeasible for RF ablation because of concurrent severe comorbidities; and (3) follow-up period less than 6 mo.
Patients were analyzed as a whole population but also grouped on the basis of surgical operability to perform a hypothetic comparison between RF ablation and SR. The theoretical operability criteria for each patient were those reported in the previous paper of Livraghi[8]: age younger than 75 years, Child-Pugh class A, total bilirubin level less than 1.5 mg/dL and absence of portal hypertension signs.
Local Review Boards approved the study according to the standards of the Declaration of Helsinki. Written informed consent was obtained from each patient before the treatment.
The strategies of RF ablation from the surgeon’s perspective used to treat the study patients were the following: (1) during RF ablative procedure, all patients were on mechanical ventilation, with tracheal tube or laryngeal mask airway under intravenous anesthesia for respiratory control; (2) all ablative procedures were performed via a percutaneous or laparoscopic approach. The selection of percutaneous or laparoscopic approach was based mainly on the location of the tumor. No open-access RF ablation was performed in this study. Subcapsular HCC, which was located near the capsule of the liver (< 1.0 cm) on axial CT scans, was treated by use of laparoscopy and ultrasonic guidance of electrode placement; whereas HCC located in the liver parenchyma was managed by CT-guided percutaneous placement of electrodes; (3) the treatment aimed to obtain an ablative margin (AM) of at least 1.0 cm of normal hepatic tissue surrounding the tumor as a tumor free margin; (4) for the tumor less than 2.0 cm, a single RF probe position with one to two cycles was adopted for ablation. For the tumor larger than 2.0 cm, we repositioned the RF probe to target areas using an overlapping ablation method; and (5) to prevent bleeding and tumor seeding, track ablation was performed as the RF electrode was withdrawn.
All RF procedures in this study were performed using either a 15-gauge multitined electrode (Starburst XL; RITA Medical Systems, Manchester, GA, United States), Cool-tip ACT 2030 or ACT 1530 electrodes and an RF generator (RITA 1500; RITA Medical Systems Inc, Manchester, GA, United States or Covidien Healthcare, Ireland), according to their respective manufacturers’ protocols.
After induction of general anesthesia, patients were placed in a supine position. Grounding was achieved by attaching two pads to the patient’s thighs. Two 10-mm trocars were placed in the abdomen, and initial laparoscopic exploration of the peritoneal cavity was performed. Under ultrasound guidance, the RF probe was introduced into the peritoneal cavity through the subcostal abdominal wall using laparoscopic visualization and deployed into the tumor. The ablation procedures were described in detail in our previous publication[10]. The RF process was monitored intraoperatively by ultrasound. The ablated lesion became hyperechoic because of outgassing from heated tissues. For the patients whose lesions were encroaching on the gallbladder fossa, laparoscopic cholecystectomy was also performed before ablation to avoid thermal injury to the gallbladder.
After induction of general anesthesia, the skin entrance point of the RF probe was chosen in the CT scanning plane containing the tumor. With CT monitoring, the RF probe was inserted through the chest wall to the liver, finally reaching the targeted tumor. After the position of the probe was confirmed to be appropriate by CT, RF procedures were performed in a manner similar to that used for the laparoscopic procedures.
All patients underwent postoperative evaluation by enhanced CT or MRI scans 1 mo post-ablation. Complete ablation was defined as absence of nodular or irregular enhancement adjacent to the ablation zone on the enhanced CT or MRI. Incomplete ablation was defined as irregular peripheral-enhanced foci in the ablation zone on the enhanced CT or MRI. In the case of incomplete ablation, repeated RF ablation procedures were performed to achieve complete necrosis.
The follow-up protocol mainly included routine physical examination, laboratory tests, and measurement of a-fetoprotein levels every month, as well as enhanced CT studies every 2 or 3 mo.
LTP was defined as the presence of a nodular lesion that was enhanced during the hepatic arterial phase and washed out by the delayed phase that was found along the peripheral margin of the low-attenuated ablative zone. IDR was defined as the lesion with similar characteristics, but not in contact with the original ablation zone in the liver. OS was defined as the interval between date of initial therapy and date of death or the last follow-up examination for surviving patients.
In cases of LTP or IDR, other supplemental examinations like CT of the chest and lower abdomen and bone scintigraphy were performed to detect other potential tumor nodules. When LTP or IDR was confirmed, patients were hospitalized as soon as possible. Basically, repeated RF ablation treatment cycles were administered for LTP and IDR of less than four nodules. Five or more IDR nodules were treated by transarterial chemoembolization. When extrahepatic metastasis was confirmed, the patient was advised to undergo treatment with sorafenib.
All the definitions are based on the standardization by the International Working Group on Image-Guided Tumor Ablation[13].
The primary endpoints of the study were the OS rate and safety (complications related to ablation). The secondary endpoints were the primary technique effectiveness and recurrence rate.
Values are expressed as mean ± SD. Continuous variables between groups were compared using the Student’s t-test and analysis of variance. Differences in the categorical data were analyzed by use of the χ2 test or Fisher’s exact test. OS rates were calculated by the Kaplan-Meier method and compared using the log-rank test. Risk factors for OS were evaluated by univariate analysis using Cox regression tests. If multiple risk factors were shown to be significant by this test, multivariate analysis was performed using Cox regression tests to identify independent prognostic factors for OS. All statistical analyses were performed using the SPSS 15.0 statistical software (SPSS Inc., Chicago, IL, United States). All reported P-values were 2-sided. P < 0.05 was considered statistically significant.
In 36 of the 184 patients with a single HCC 3 cm or less, preoperative diagnosis of HCC was histologically confirmed by needle biopsy under CT guidance. In the remaining 148 patients, HCC was established on the basis of compatible radiological features in enhanced CT and enhanced MRI.
According to the criteria of Livraghi[8], 184 cases were divided into two groups: those suitable for surgical resection (84 cases) and those unsuitable for surgical resection (100 cases). Of the 184 patients, 74 subcapsular HCCs were treated by laparoscopic RF ablation, and 110 HCCs located deep in the liver parenchyma were treated by CT-guided percutaneous RF ablation. Nine patients underwent laparoscopic cholecystectomy because their tumors were adjacent to the gallbladder. The baseline patient characteristics are shown in Table 1.
| Variable | Non suitable for surgical resection(n = 100) | Suitable for surgical resection(n = 84) | P value |
| Age (yr) | 76 (36-88) | 52 (32-74) | 0.015 |
| Gender | |||
| Male/female | 70 (70.0)/30 (30.0) | 58 (69.0)/26 (31.0) | 0.889 |
| Pre-existing hepatitis | |||
| Hepatitis B | 87 (87.0) | 66 (78.5) | 0.128 |
| Hepatitis C | 9 (9.0) | 6 (7.1) | 0.647 |
| Hepatitis B and C | 2 (2.0) | 1 (1.2) | 1.000 |
| Others | 2 (2.0) | 1 (1.2) | 1.000 |
| Child-Pugh grade | 0.000 | ||
| Class A/class B | 51 (51.0)/49 (49.0) | 84(100)/ 0 (0) | |
| Portal hypertension | 84 (84.0) | 0 (0) | 0.000 |
| AFP (ng/mL) | 50.8 (2-240) | 68.7 (4-300) | 0.188 |
| Tumor diameter (cm) | 2.5 (1.1-3.0) | 2.6 (1.0-3.0) | 0.608 |
| Location of tumor (S1/S2/S3/S4/S5/S6/S7/S8) | 1/9/5/12/15/18/21/19 | 2/7/5/9/13/16/18/14 | 0.997 |
| Subcapsular location | 0.401 | ||
| Yes/no | 43 (43.0)/57 (57.0) | 31 (36.9)/53 (63.1) | |
| Approach of the first ablation session | 0.401 | ||
| Percutanous | 57 (57.0) | 53 (63.1) | |
| Laparoscopic | 43 (43.0) | 31 (36.9) |
The RF ablation treatment was performed successfully for all patients. There were no technical failures. Complications related to the ablation developed in 19 (10.3%) patients, including nine (10.7%, 9/84) patients in the suitable for surgical resection group and 10 (10.0%, 10/100) patients in the unsuitable for surgical resection group (P > 0.05). According to the Dindo-Clavien classification[14], two complications were Grade IIIA and all the other complications were Grade I.
Eleven patients had right shoulder pain post-ablation with a duration ranging between 2 and 7 (median, 3.5) d. Four patients developed asymptomatic pneumothorax and received only conservative treatment. Two patients developed pleural effusion and underwent drainage via a chest tube. Shallow second-degree skin burns occurred at the edge of grounding pads in two patients, which healed spontaneously. There was no perioperative mortality. No severe complications developed (liver failure, thoracic hemorrhage, abdominal hemorrhage, destructive biliary damage, adjacent viscera perforation, or liver abscess).
Complete ablation was achieved in 97.8% (180/184) of the 184 patients treated by RF ablation. Moreover, the complete ablation rate was 98.8% (83/84) for the suitable for surgical resection group and 97.0% (97/100) for the unsuitable for surgical resection group (P > 0.05). Four patients who presented incomplete ablation received repeated RF ablation, which resulted in complete ablation of the tumors.
During follow-up, the LTP rate was 4.9% (9/184). LTP was found in four (4.8%) of 84 patients in the suitable for surgical resection group and in five (5.0%) of 100 patients in the unsuitable for surgical resection group (P > 0.05). IDR rate was 37.5% (69/184). IDR was found in 24 (28.6%) of 84 patients in the suitable for surgical resection group and in 45 (45.0%) of 100 patients in the unsuitable for surgical resection group (P = 0.022). Extrahepatic metastasis rate was 9.8% (18/184). Extrahepatic metastasis was found in six (7.1%) of 84 patients in the suitable for surgical resection group and in 11 (11.0%) of 100 patients in the unsuitable for surgical resection group (P > 0.05).
Nine patients with LTP received RF ablation. Of the 65 patients who had IDR, 54 underwent RF ablation, and 15 underwent transarterial chemoembolization. Of the 18 patients who had extrahepatic metastasis, eight underwent treatment with sorafenib, and 10 refused symptomatic treatment.
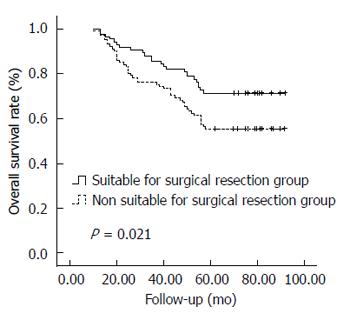
As of December 2013 (with a median follow-up of 65.0 mo), 115 patients (62.5%) remained alive, and 69 (37.5%) had died, including 24 patients in the suitable for surgical resection group and 45 patients in the unsuitable for surgical resection group. The cause of death was HCC in 52 patients (75.4%), liver failure in nine (13.0%), upper gastrointestinal bleeding in four (5.8%), causes unrelated to liver disease in four (including three patients who died of cardiovascular disease and one of pulmonary embolism; 5.8%). In the 184 patients, the 1-, 3-, and 5-year OS rates were 99.5%, 81.0%, and 62.5%, respectively. The 1-, 3-, and 5-year OS rates were 100%, 86.9%, and 71.4%, respectively, in the suitable for surgical resection group and 99.0%, 76.0%, and 55.0%, respectively, in the unsuitable for surgical resection group (Figure 1); the two groups differed significantly (P = 0.021, log-rank test).
Table 2 lists the results of univariate and multivariate analyses using Cox regression tests to identify the independent prognostic factors for OS. Factors that significantly predicted OS by univariate analysis were age, portal hypertension, and total bilirubin. Furthermore, multivariate analysis revealed that the factors associated with OS included portal hypertension (OR = 2.089; 95%CI: 1.387-2.958; P = 0.027) and total bilirubin (OR = 1.556; 95%CI: 1.827-2.965; P = 0.028) (Table 2).
| Significant variable | Univariate analysis | Multivariate analysis | ||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Age (> 65 yr), yes/no | 1.141 (0.735-1.724) | 0.034 | 1.057 (0.787-1.587) | 0.097 |
| Gender (male), yes/no | 0.980 (0.545-1.763) | 0.947 | ||
| Tumor size (> 2.0 cm/ ≤ 2.0 cm) | 1.001 (0.754-1.328) | 0.994 | ||
| Child-Pugh grade (class A/B) | 0.976 (0.438-2.177) | 0.954 | ||
| AFP (> 20 ng/mL/ ≤ 20 ng/mL) | 1.031 (0.726-2.041) | 0.342 | ||
| Hepatitis B (yes/no) | 1.070 (0.557-2.054) | 0.839 | ||
| Hepatitis C (yes/no) | 0.596 (0.252-1.412 | 0.240 | ||
| Hepatitis B and C (yes/no) | 0.596 (0.252-1.412) | 0.240 | ||
| Portal hypertension (yes/no) | 2.847 (1.819-4.458) | 0.015 | 2.089(1.387-2.958) | 0.027 |
| Total bilirubin (< 1.5 mg/dL/≥ 1.5 mg/dL) | 3.023 (1.632-5.600) | 0.006 | 1.556 (1.827-2.965) | 0.028 |
| Approach of the first ablation session (laparoscopic) (yes/no) | 1.205 (0.678-2.231) | 0.068 | ||
This study aimed to evaluate the efficacy of RF ablation from the surgeon’s perspective as the first-line treatment in a cohort of patients with a single HCC 3 cm or less. Our data suggest that the high level of safety and technical effectiveness, with a satisfactory 5-year OS rate, is comparable to the rates reported by most studies on SR of HCCs at a similar stage[4-7], suggesting that RF ablation could be considered the first treatment of choice for early-stage HCC less than 3 cm, even when SR is possible.
RF ablation is accepted as a potentially curative treatment modality for HCC at an early stage when transplantation and resection are precluded[4-10]. However, the main argument against the role of RF ablation as a first treatment option for patients with small HCC is the lack of adequate evidence proving that its effectiveness is comparable to that of SR[4-10]. In a prospective randomized trial on 180 patients with solitary HCC less than 5 cm, percutaneous RF ablation was as effective as SR in terms of 1-, 2-, 3-, and 4-year OS rates and recurrence-free survival rates. Further, the results of a large, single-institution retrospective study suggested that RF ablation could be used as first-line treatment for early-stage HCC[4]. Conversely, Huang et al[5] showed that SR resulted in better survival and less recurrence than RF ablation for patients with HCC according to the Milan criteria.
RF ablation is less expensive and less invasive, and it is associated with lower complication rates and shorter hospital stay than SR[4-10]. However, tumor recurrence after RF ablation, including LTP and IDR, occurs frequently, affecting patient prognosis[5,6]. Furthermore, rapid tumor progression after RF ablation, which may mostly be associated with the progression of residual HCC, has been gaining increasing attention[15-17]. These experimental data indicated that any residual HCC tumors post-ablation might be the main obstacle to achieving a satisfactory effect.
In our study, complete ablation was achieved in 97.8% of the patients. The LTP rate was 4.9% and IDR, 37.5%. Additionally, the 5-year OS rate was 62.5% for the entire sample. In the suitable for surgical resection group, the 5-year OS rate was much better (71.4%). These results are obviously better than those of 209 similar patients reported by Brunello et al[9]. We induced the systemic technical measures to promote the efficacy of RF ablation for HCC from the surgeon’s perspective as follows. (1) General anesthesia should be recommended to prevent pain and discomfort during the RF procedure[18]. Furthermore, controlled ventilation would reduce ablation attempts and increase the rate of success in patients undergoing RF ablation under general anesthesia[19,20]; (2) We thought an AM of at least 1.0 cm could reduce the possibility of recurrence, making RF ablation a suitable treatment for HCC with a diameter of 3 cm or less[10]. For HCCs less than 3.0 cm, an AM of at least 1.0 cm is likely to remove microvascular invasions and satellite micronodules around the main tumor, which can decrease the likelihood of residual tumor, the incidence rates of LTP, IDR, and rapid tumor progression. Current technologies allow RF ablation to produce a necrotic area with a diameter of 5 cm or more in one treatment session, thus allowing full ablation of a 3-cm tumor plus a 1.0-cm margin[7]; (3) We preferred a laparoscopic approach to ablate subcapsular HCC to avoid adjacent organ injury and facilitate more aggressive ablation. Moreover, intraoperative ultrasonography was used routinely in conjunction with the laparoscopic approach to increase the ability to determine real-time RF electrode placement and evaluate the efficacy of ablation[21,22]; (4) Use of the internally cooled cluster electrode would increase efficacy of HCC located close to inferior venacava or portal vein. One advantage of internally cooled electrodes, such as Cool-tip, is that they keep a steady high temperature in the tumor while limiting vascular cooling. This characteristic increases the effectiveness of perivascular ablation[23]; and (5) Repeated RF ablation sessions for the control of recurrent HCC were more feasible because RF ablation was as effective as repeated SR for the treatment of small recurrent HCCs, and it did not affect liver function of patients or cause portal hypertension[24-26].
The major limitations of our study include its retrospective nature, the lack of a control group, and the relatively small number of patients. Feasibility for RF ablation is largely dependent on the operator’s technique, the experience, and the instrumental equipment of the center. The present patients were managed based on the treating surgeon’s perspective as well as by a team of surgeons, making the results less applicable to nonsurgical clinics. Nevertheless, our data may be helpful for clinicians who treat HCC by RF ablation and may also be useful as a basis for the design of future trials. Again, more long-term outcomes and prospective randomized control trials are needed to define the role of RF ablation in the treatment of small HCC, especially in comparison to SR.
In conclusion, RF ablation is an effective, minimally invasive, and safe first-line treatment for single HCC 3 cm or less. Furthermore, the OS rate of the patients suitable for surgical resection was similar to those reported in previous surgical series.
Hepatocellular carcinoma (HCC) is the third most common global cause of cancer-related death. Radiofrequency (RF) ablation is accepted as a potentially curative treatment modality for HCC at an early stage when transplantation and resection are precluded. However, the main argument against the role of RF ablation as a first treatment option for patients with small HCC is represented by the lack of adequate evidence proving that its effectiveness is comparable to that of surgical resection (SR).
Reportedly, optimal efficacy of treatment for small HCC can be achieved by means of RF ablation. The advantage of minimal invasiveness has made this method into the first-line treatment for small HCC in patients with compromised liver function or associated severe medical conditions. Previous studies comparing the clinical effectiveness of RF ablation with that of SR suggested that SR was more effective than RF ablation for early-stage HCC because local tumor progression (LTP) and intrahepatic distant recurrence (IDR) were lower with SR than with RF ablation. However, other studies reported conflicting results in this regard. Hence, whether RF ablation or SR is the better choice for early-stage HCC has long been debated.
Present data suggest that the high level of safety and technical effectiveness, with a satisfactory 5-year overall survival (OS) rate, is comparable to the rates reported by most studies on SR of HCCs at a similar stage, suggesting that RF ablation could be considered the first treatment of choice for early-stage HCC less than 3 cm, even when SR is possible. Furthermore, the authors induced the systemic technical measures to promote the efficacy of RF ablation for HCC from the surgeon’s perspective.
The study results provide evidence that, RF ablation could be considered the first treatment of choice for early stage HCC ≤ 3 cm, even when SR is possible.
LTP was defined as the presence of a nodular lesion that was enhanced during the hepatic arterial phase and washed out by the delayed phase that was found along the peripheral margin of the low-attenuated ablative zone. IDR was defined as the lesion with similar characteristics, but not in contact with the original ablation zone in the liver. OS was defined as the interval between date of initial therapy and date of death or the last follow-up examination for surviving patients.
This is an excellent retrospective study in which the authors used data of 4 institutions to evaluate long-term outcomes of RF ablation as first-line therapy for single HCC ≤ 3 cm and determine survival and prognostic factors. The results are interesting and suggest that RF ablation could be considered the first treatment of choice for early-stage HCC ≤ 3 cm, even when SR is possible.
P- Reviewer: Berkane S, Kim SH, Perini MV S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Fong ZV, Tanabe KK. The clinical management of hepatocellular carcinoma in the United States, Europe, and Asia: a comprehensive and evidence-based comparison and review. Cancer. 2014;120:2824-2838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 205] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 2. | Ravaioli M, Ercolani G, Neri F, Cescon M, Stacchini G, Del Gaudio M, Cucchetti A, Pinna AD. Liver transplantation for hepatic tumors: a systematic review. World J Gastroenterol. 2014;20:5345-5352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Squires MH, Hanish SI, Fisher SB, Garrett C, Kooby DA, Sarmiento JM, Cardona K, Adams AB, Russell MC, Magliocca JF. Transplant versus resection for the management of hepatocellular carcinoma meeting Milan Criteria in the MELD exception era at a single institution in a UNOS region with short wait times. J Surg Oncol. 2014;109:533-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, Lin XJ, Lau WY. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1100] [Cited by in RCA: 1103] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 5. | Huang J, Yan L, Cheng Z, Wu H, Du L, Wang J, Xu Y, Zeng Y. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg. 2010;252:903-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 580] [Cited by in RCA: 641] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 6. | Imai K, Beppu T, Chikamoto A, Doi K, Okabe H, Hayashi H, Nitta H, Ishiko T, Takamori H, Baba H. Comparison between hepatic resection and radiofrequency ablation as first-line treatment for solitary small-sized hepatocellular carcinoma of 3 cm or less. Hepatol Res. 2013;43:853-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | Peng ZW, Liu FR, Ye S, Xu L, Zhang YJ, Liang HH, Lin XJ, Lau WY, Chen MS. Radiofrequency ablation versus open hepatic resection for elderly patients (& gt; 65 years) with very early or early hepatocellular carcinoma. Cancer. 2013;119:3812-3820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C, Rossi S. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology. 2008;47:82-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 850] [Cited by in RCA: 825] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 9. | Brunello F, Cantamessa A, Gaia S, Carucci P, Rolle E, Castiglione A, Ciccone G, Rizzetto M. Radiofrequency ablation: technical and clinical long-term outcomes for single hepatocellular carcinoma up to 30 mm. Eur J Gastroenterol Hepatol. 2013;25:842-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Ke S, Ding XM, Qian XJ, Zhou YM, Cao BX, Gao K, Sun WB. Radiofrequency ablation of hepatocellular carcinoma sized & gt; 3 and ≤ 5 cm: is ablative margin of more than 1 cm justified? World J Gastroenterol. 2013;19:7389-7398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Tombesi P, Di Vece F, Sartori S. Resection vs thermal ablation of small hepatocellular carcinoma: What’s the first choice? World J Radiol. 2013;5:1-4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4333] [Cited by in RCA: 4507] [Article Influence: 225.4] [Reference Citation Analysis (0)] |
| 13. | Ahmed M, Solbiati L, Brace CL, Breen DJ, Callstrom MR, Charboneau JW, Chen MH, Choi BI, de Baère T, Dodd GD, Dupuy DE, Gervais DA, Gianfelice D, Gillams AR, Lee FT, Leen E, Lencioni R, Littrup PJ, Livraghi T, Lu DS, McGahan JP, Meloni MF, Nikolic B, Pereira PL, Liang P, Rhim H, Rose SC, Salem R, Sofocleous CT, Solomon SB, Soulen MC, Tanaka M, Vogl TJ, Wood BJ, Goldberg SN; International Working Group on Image-guided Tumor Ablation; Interventional Oncology Sans Fronti res Expert Panel; Technology Assessment Committee of the Society of Interventional Radiology,; Standard of Practice Committee of the Cardiovascular and Inter. Image-guided tumor ablation: standardization of terminology and reporting criteria--a 10-year update. Radiology. 2014;273:241-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 896] [Article Influence: 81.5] [Reference Citation Analysis (0)] |
| 14. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 24773] [Article Influence: 1179.7] [Reference Citation Analysis (0)] |
| 15. | Kong J, Kong J, Pan B, Ke S, Dong S, Li X, Zhou A, Zheng L, Sun WB. Insufficient radiofrequency ablation promotes angiogenesis of residual hepatocellular carcinoma via HIF-1α/VEGFA. PLoS One. 2012;7:e37266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 16. | Ke S, Ding XM, Kong J, Gao J, Wang SH, Cheng Y, Sun WB. Low temperature of radiofrequency ablation at the target sites can facilitate rapid progression of residual hepatic VX2 carcinoma. J Transl Med. 2010;8:73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | Kong J, Kong L, Kong J, Ke S, Gao J, Ding X, Zheng L, Sun H, Sun W. After insufficient radiofrequency ablation, tumor-associated endothelial cells exhibit enhanced angiogenesis and promote invasiveness of residual hepatocellular carcinoma. J Transl Med. 2012;10:230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 18. | Wang ZY, Sun WB, Li MY, Zhang XX, Ding XM. Percutaneous extrapulmonary radiofrequency ablation for tumors in the hepatic dome. Hepatogastroenterology. 2008;55:1164-1166. [PubMed] |
| 19. | Yang LL, Ji JS, Wu W, Lei LP, Zhao ZW, Shao GL, Zheng JP. [Clinical observation of remifentanyl and propofol injection in total intravenous anesthesia for percutaneous radiofrequency ablation]. Zhonghua Yi Xue Za Zhi. 2013;93:3623-3625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 20. | Yokoyama K, Ikeda O, Kawanaka K, Nakasone Y, Inoue S, Tamura Y, Yamashita Y. Pain control in patients with hepatocellular carcinoma treated by percutaneous radiofrequency ablation: comparison of the efficacy of one-shot and continuous intravenous fentanyl delivery. Acta Radiol. 2014;55:1219-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Gao J, Ke S, Ding XM, Zhou YM, Qian XJ, Sun WB. Radiofrequency ablation for large hepatic hemangiomas: initial experience and lessons. Surgery. 2013;153:78-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 22. | Herbold T, Wahba R, Bangard C, Demir M, Drebber U, Stippel DL. The laparoscopic approach for radiofrequency ablation of hepatocellular carcinoma--indication, technique and results. Langenbecks Arch Surg. 2013;398:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Gao J, Ding X, Ke S, Xin Z, Ning C, Sha Q, Sun W. Radiofrequency ablation in the treatment of large hepatic hemangiomas: a comparison of multitined and internally cooled electrodes. J Clin Gastroenterol. 2014;48:540-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Chen MS, Peng ZW, Xu L, Zhang YJ, Liang HH, Li JQ. Role of radiofrequency ablation in the treatment of hepatocellular carcinoma: experience of a cancer center in China. Oncology. 2011;81 Suppl 1:100-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Yokoyama K, Anan A, Iwata K, Nishizawa S, Morihara D, Ueda S, Sakurai K, Iwashita H, Hirano G, Sakamoto M. Limitation of repeated radiofrequency ablation in hepatocellular carcinoma: proposal of a three (times) × 3 (years) index. J Gastroenterol Hepatol. 2012;27:1044-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Lee S, Jeong WK, Rhim H. Repeated percutaneous radiofrequency ablation for hepatocellular carcinoma in patients with cirrhosis: assessment of safety based on liver function and portal hypertension parameters. J Vasc Interv Radiol. 2014;25:1573-1579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |