Published online May 7, 2015. doi: 10.3748/wjg.v21.i17.5271
Peer-review started: October 29, 2014
First decision: December 26, 2014
Revised: January 27, 2015
Accepted: February 11, 2015
Article in press: February 11, 2015
Published online: May 7, 2015
Processing time: 196 Days and 3.7 Hours
AIM: To investigate anti-apoptotic effects of glycyrrhizic acid (GA) against fibrosis in carbon tetrachloride (CCl4)-induced liver injury and its contributing factors.
METHODS: Liver fibrosis was induced by administration of CCl4 for 8 wk. Pathological changes in the liver of rats were examined by hematoxylin-eosin staining. Collagen fibers were detected by Sirius red staining. Hepatocyte apoptosis was determined by TUNEL assay and the expression levels of cleaved caspase-3, Bax, α-SMA, connective tissue growth factor (CTGF), matrix metalloproteinase (MMP) 2 and MMP9 proteins were evaluated by western blot analysis, and α-SMA mRNA, collagen type I and III mRNA were estimated by real-time PCR.
RESULTS: Treatment with GA significantly improved the pathological changes in the liver and markedly decreased the positive area of Sirius red compared with rats in the CCl4-treated group. TUNEL assay showed that GA significantly reduced the number of TUNEL-positive cells compared with the CCl4-treated group. The expression levels of cleaved caspase-3, Bax, α-SMA, CTGF, MMP2 and MMP9 proteins, and α-SMA mRNA, collagen type I and III mRNA were also significantly reduced by GA compared with the CCl4-treated group (P < 0.05).
CONCLUSION: GA treatment can ameliorate CCl4-induced liver fibrosis by inhibiting hepatocyte apoptosis and hepatic stellate cell activation.
Core tip: This study showed that glycyrrhizic acid (GA) had inhibitory effects on hepatocyte apoptosis and liver fibrosis, which were mainly associated with down-regulation of hepatic stellate cell (HSC) activation, thus regulating fibrotic-related factors, such as expression levels of connective tissue growth factor, MMP2 and MMP9 proteins, and collagen type I and III mRNA. Collectively, these results demonstrate that GA treatment significantly ameliorated CCl4-induced liver fibrosis by inhibiting hepatocyte apoptosis and HSC activation, which may provide potential therapeutic strategies for anti-fibrosis.
- Citation: Liang B, Guo XL, Jin J, Ma YC, Feng ZQ. Glycyrrhizic acid inhibits apoptosis and fibrosis in carbon-tetrachloride-induced rat liver injury. World J Gastroenterol 2015; 21(17): 5271-5280
- URL: https://www.wjgnet.com/1007-9327/full/v21/i17/5271.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i17.5271
Liver fibrosis, induced by various pathological factors, is commonly encountered in many chronic liver diseases, such as chronic viral hepatitis, nonalcoholic steatohepatitis, and alcoholic liver disease[1]. The progression of fibrosis is likely to lead to liver failure, portal hypertension, and even an increased risk of hepatic carcinoma[2].
Apoptosis, a stereotypical morphologic form of cell death, results in liver damage in a wide range of acute and chronic liver diseases[3]. Following liver injury, two distinct paths are involved in the repair process: one is a regenerative path, in which the same type of cells replace injured cells; and the other path is known as fibroplasia or fibrosis, in which normal parenchymal tissue is replaced by connective tissue in an uncontrolled manner[4]. Studies have shown that hepatocyte apoptosis can induce liver fibrosis[5-7], which is an excessive wound healing response to chronic liver injury. Liver fibrosis, a dynamic and reversible process[8-10], is characterized by an imbalance between synthesis and degradation of the extracellular matrix (ECM), which is rich in fibrillar collagens (mainly collagen I and III). It was demonstrated that activated hepatic stellate cells (HSCs) are the main fibrogenic cells in injured liver[10]. Therefore, focusing on HSC activation, signaling pathways activating HSCs and molecules that modulate fibrolysis and fibrogenesis may be effective strategies for the treatment and prevention of hepatic fibrosis[11].
Glycyrrhiza glabra, a perennial herb, has been widely used to cure diseases for thousands of years in China. In recent years, the efficacy of Chinese herbal medicine has been appraised by modern biological technology[12,13]. Glycyrrhizic acid (GA), extracted from the roots of G. glabra, is a major active component, and has been found to have numerous pharmacological effects, such as anti-inflammatory, anti-viral and hepatoprotective activities[14]. GA also exerts an anti-apoptotic effect by inhibiting hepatocyte apoptosis[15,16]. As shown in our previous study[17], GA can inhibit CCl4-induced hepatocyte apoptosis via a p53-dependent mitochondrial pathway to retard the progress of liver fibrosis in rats. However, the mechanism how GA exerts its anti-apoptotic effect against fibrosis in CCl4-induced liver injury and its contributing factors are unknown.
GA and α-smooth muscle actin (SMA) antibody were purchased from Sigma-Aldrich (St Louis, MO, United States). MMP2 and MMP9 antibodies were bought from Abcam (Cambridge, MA, United States), CTGF antibody was bought from Santa Cruz Biotechnology (Santa Cruz, CA, United States), caspase-3 and Bax antibodies were purchased from Cell Signaling Technology (Beverly, MA, United States). GAPDH and tubulin antibodies were bought from Beyotime Biotechnology (Haimen, Jiangsu, China), horseradish peroxidase (HRP)-conjugated anti-mouse, anti-goat and anti-rabbit IgG antibodies were purchased from Cell Signaling Technology. The chemiluminescence reaction kit (ECL Plus) was purchased from Millipore (Billerica, MA, United States).
Male Sprague-Dawley rats weighing 150-200 g were supplied by the Experimental Animal Center of Zhongshan Hospital, Fudan University. The animals were acclimatized to laboratory conditions (23 °C, 12 h/12 h light/dark, 50% humidity, ad libitum access to food and water) for 2 wk prior to experimentation. Forty-five rats were randomly and equally divided into the control group, CCl4 group and GA treatment group. In order to induce the liver fibrosis model, rats were given a 40% solution of CCl4 in olive oil by hypodermic injection at a dose of 3 mL/kg biweekly for 8 wk, while the rats in the control group were given the same dose of olive oil by subcutaneous injection, with an initial double-dose injection. In the GA group, rats were also treated with a 40% solution of CCl4 by hypodermic injection at a dose of 3 mL/kg plus 0.2% GA solution in water (3 mL) by intraperitoneal injection three times weekly, beginning at the first week, following a previously published method[17]. Rats in the control group were treated with the same isovolumetric dose of olive oil and water. Animals were sacrificed 24 h after the last injection. All animals were euthanized by barbiturate overdose (intravenous injection, 150 mg/kg pentobarbital sodium) for tissue collection. Liver tissues were removed and rinsed with 0.9% saline, some were fixed in 10% buffered formaldehyde and embedded in paraffin for hematoxylin-eosin (HE) and Sirius-red staining, and the remaining sections were stored at -70 °C for analysis.
Liver tissues were embedded in paraffin and 5-μm-thick slices were cut and placed on glass slides, stained with HE, and examined under a light microscope (Olympus, Tokyo, Japan). HE staining was performed to assess pathological changes in the liver. Sirius-red staining was performed to detect collagen deposition and observed under a light microscope.
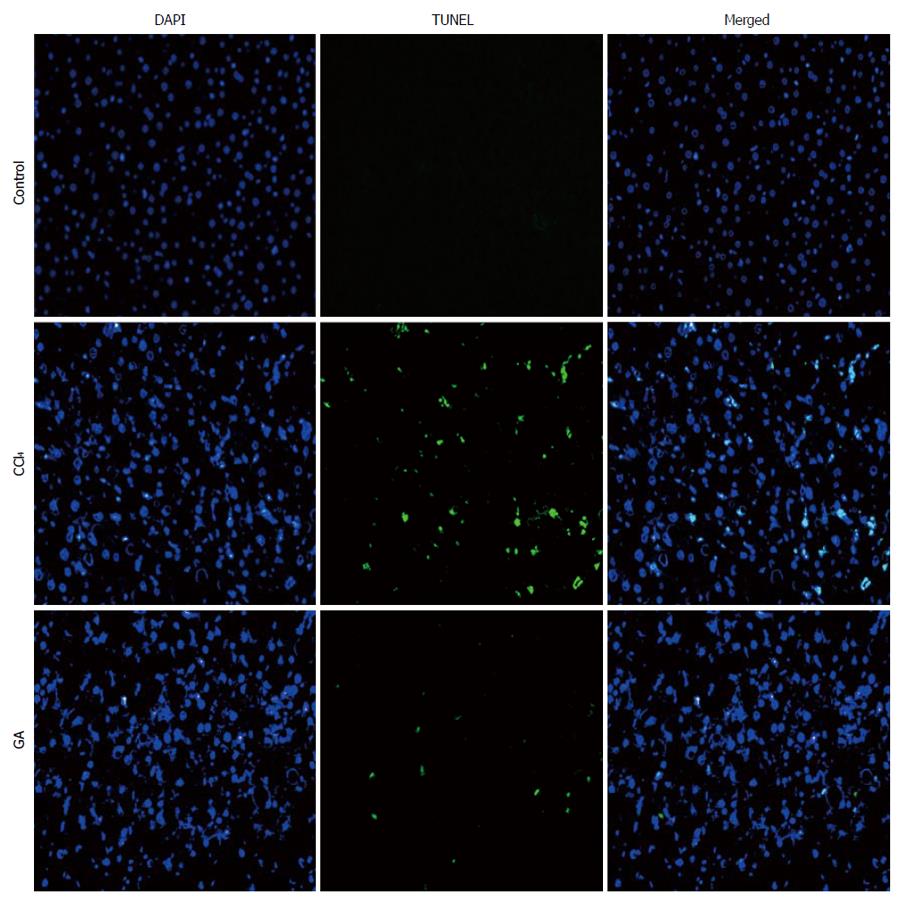
The deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay (Roche, Germany) was performed following the manufacturer’s protocol. Nuclei were respectively counterstained with 4, 6-diamidino-2-phenylindole (DAPI) in the same sections. Cells stained by TUNEL were evaluated using fluorescence microscopy (Olympus).
Total proteins in liver tissue were extracted and quantified using the bicinchoninic acid protein concentration assay kit (Beyotime Biotechnology). Sample proteins were separated by 10% SDS-PAGE and then transferred to polyvinylidene difluoride membranes (Millipore). The membranes were blocked with 5% bovine serum albumin for 2 h, and then incubated overnight at 4 °C with primary antibodies rabbit caspase-3, Bcl-2, Bax, MMP2 and MMP9 antibodies, mouse α-SMA, GAPDH and tubulin antibodies, and goat CTGF antibody. On the next day, the membranes were incubated with the secondary antibodies conjugated with horseradish-peroxidase goat anti-rabbit IgG, goat anti-mouse IgG and rabbit anti-goat IgG (1:5000 dilution) at room temperature for 2 h, and then washed three times with Tris-buffered saline with 0.1% Tween-20 (TBST) , and detected by enhanced chemiluminescence. The intensities of the bands were analyzed by Image J software.
Total RNA in liver tissue was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, United States), subsequently converted to cDNA, which was carried out using PrimeScript reagent kit with gDNA Eraser (Takara Bio,. Dalian, China), and subjected to real-time PCR using SYBR Premix Ex TaqII (Takara Bio). The results were calculated in line with the dissociation curves and normalized against a housekeeping gene (GAPDH). This was performed using a 7300HT Fast Real-time polymerase chain reaction (PCR) System. Primer sequences used for real-time PCR were as follows: α-SMA: sense 5’-AGGGAGTGATGGTTGGAATG-3’, antisense 5’-GATGATGCCGTGTTCTATCG’; collagen I: sense 5’-TCAAGATGGTGGCCGTTACT-3’, antisense 5’-GCGGATGTTCTCAATCTGCT-3’; collagen III: sense 5’-ACCTCCTGGTGCTATTGGTC-3’, antisense 5’-TCTCTCCATTGCGTCCATC-3’, GAPDH: sense 5’-GACATGCCGCCTGGAGAAAC-3’, antisense 5’-AGCCCAGGATGCCCTTTAGT-3’. The mRNA expression levels of samples relative to the control groups were analyzed by the comparative CT (2-∆∆CT) method[18].
All results were expressed as mean ± SD of three independent experiments. Data were analyzed using one-way analysis of variance. Differences were considered statistically significant if P was < 0.05. All analyses in the study were carried out using SPSS for Windows version 18.0.
Under fluorescence microscopy, no staining was observed and non-apoptotic nuclei were found in normal liver tissue slices using the TUNEL assay. High quantities of TUNEL cells were observed in the liver tissue slices from the CCl4 group, and numerous condensed and fragmented nuclei were seen. In the GA-treated group, few TUNEL cells were observed in the liver tissue sections, and fewer condensed and fragmented nuclei, in the same slice, were observed. In the same view, co-location of green and blue staining indicated TUNEL-positive cells, and numerous TUNEL-positive cells were found in the CCl4 group, while a significant reduction in TUNEL-positive cells was observed in the GA-treated group (Figure 1). Overall, these results demonstrated that GA treatment reduced apoptosis during the process of liver injury.
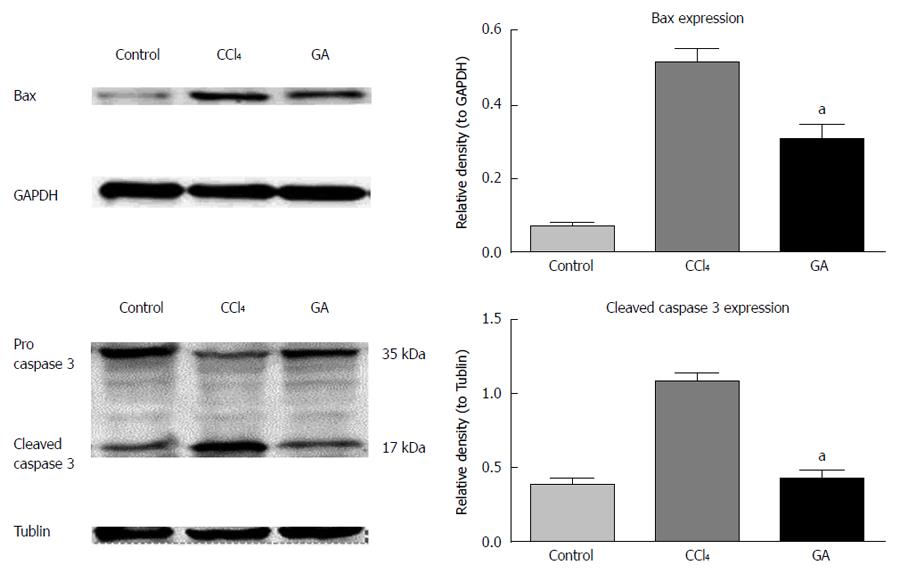
Expression of apoptosis-related proteins in liver tissue from the different treatment groups was evaluated by western blot analysis. As shown in Figure 2, the expression level of Bax, the pro-apoptotic protein, was markedly increased in the CCl4-induced hepatic injury group, whereas GA treatment significantly decreased the expression level of Bax (Figure 2). Caspase activation plays a vital role in apoptosis, and cleaved caspase-3 is a typical feature of apoptosis[19]. In the present study, cleaved caspase-3 (17 kDa) was increased in the CCl4 group, which suggested severe apoptosis. In addition, the expression level of cleaved caspase-3 significantly decreased in the GA treatment group (Figure 2).
These findings indicated that GA suppressed CCl4-induced hepatocyte apoptosis by inhibiting the activation of caspase-3. Thus, GA treatment ameliorated CCl4-induced hepatocyte apoptosis.
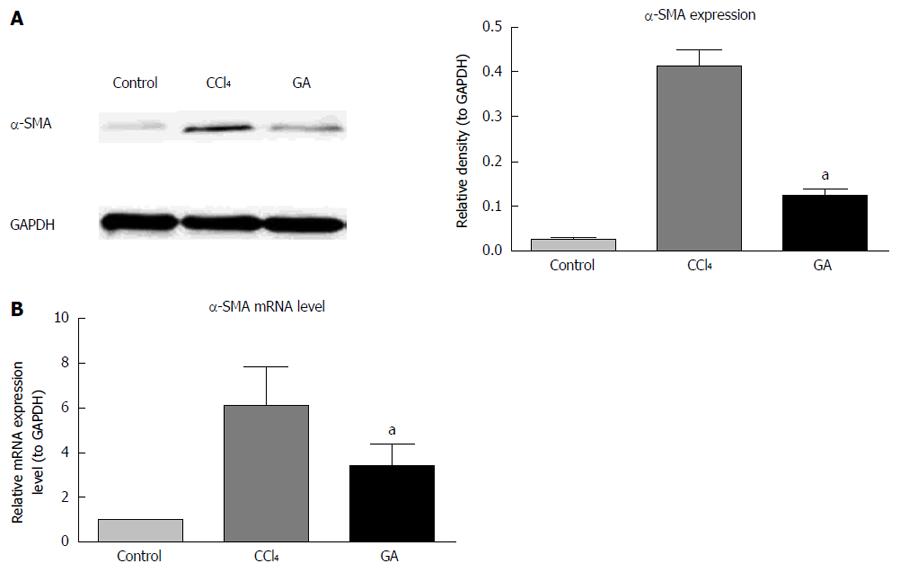
We examined the effects of GA on α-SMA protein and mRNA expression in CCl4-induced liver injury using western blotting and real-time PCR. As shown in Figure 3A, the expression level of α-SMA protein was maintained at a low level in the control group, and was up-regulated in the CCl4-induced hepatic injury group. In contrast, GA significantly down-regulated expression of α-SMA protein. The trend in mRNA expression of α-SMA was in accordance with the results of Western blotting (Figure 3B).
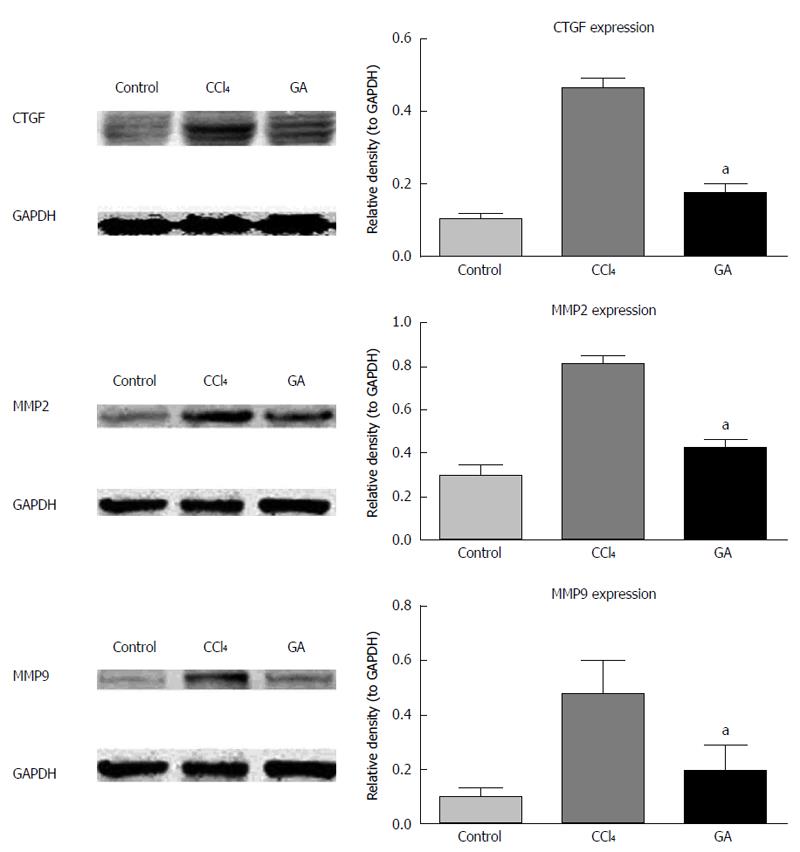
Using Western blotting, we also examined the effects of GA on CTGF, MMP2 and MMP9 protein expression levels in CCl4-induced liver injury. As indicated in Figure 4, expression of CTGF, MMP2 and MMP9 proteins was up-regulated in CCl4-induced hepatic injury. In contrast, following GA treatment, expression of CTGF, MMP2 and MMP9 proteins was down-regulated compared to that in the CCl4 group.
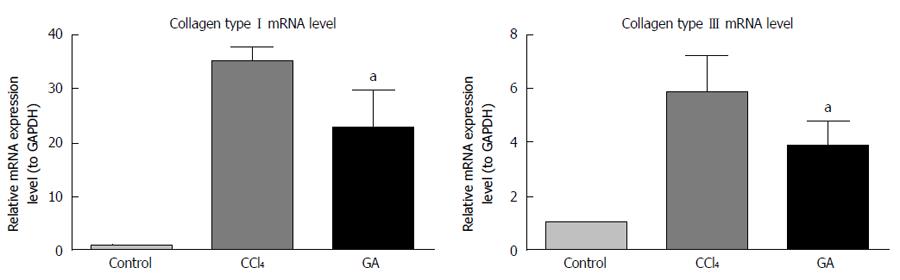
We evaluated the expression levels of type I and III collagen mRNA using real-time PCR. In the CCl4-treated group, expression of type I and III collagen mRNA was enhanced, however, the pattern and degree of change clearly differed between the two transcripts (Figure 5). Type I collagen mRNA expression level increased in parallel with worsening liver disease. In the CCl4-induced liver injury group, this enhancement was > 30 times higher than that in the control group, and type III collagen mRNA expression was about 6 times higher than that in the control group. Moreover, GA significantly decreased expression of type I and III collagen mRNA, which was about 20 times and about 4 times that in the control group, respectively.
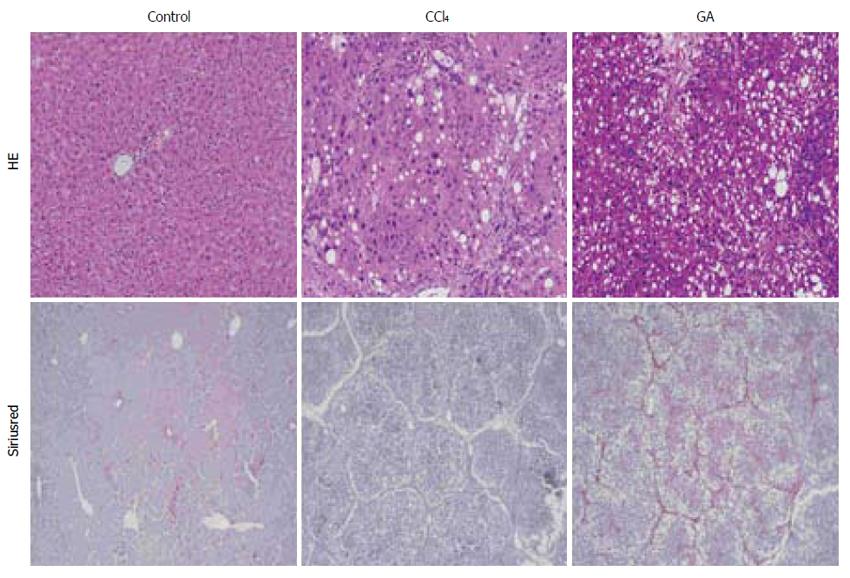
After 8 wk CCl4 administration, HE and Sirius red staining showed an integrated lobular structure with central venous and hepatic cord radiation in liver slices from the control group (Figure 6), in which there were few positive areas of Sirius red staining around small central venous walls. These disorders of lobular structure, including wide fibrous tissue hyperplasia and fibrous septa formation, were identified in the CCl4-treated group, and some pseudolobuli were present (Figure 6). Many positive areas of Sirius red staining were seen in the boundaries of the hepatic lobules in the CCl4-treated group. Less fibrous tissue hyperplasia and fibrous septa formation were observed in the GA treatment group compared with the CCl4-treated group, and positive areas of Sirius red staining were significantly reduced in the GA treatment group.
GA is a major active component of G. glabra roots and is commonly used in Asia to treat patients with chronic hepatitis[20,21], and has satisfactory therapeutic effects in many other diseases. GA has been reported to have numerous pharmacological effects, such as anti-inflammatory, anti-viral and hepatoprotective activity. In the present study, we demonstrated that GA significantly ameliorated liver fibrosis induced by CCl4. GA exerted a beneficial anti-apoptotic effect and inhibited fibrosis-related factors.
In animal models, CCl4 can induce hepatocyte apoptosis and liver fibrosis[22-26]. Responses to CCl4-induced damage in rat and mouse models are similar to responses to liver cirrhosis in humans[27], and these models can be used to screen for anti-hepatotoxic and/or hepatoprotective drugs[28].
Hepatocyte apoptosis, a cardinal feature of many liver diseases, is considered to play a part in initiating and maintaining HSC activation[29]. Apoptosis of parenchymal cells, an important inflammatory stimulus, activates HSCs, which display a surprising capacity to phagocytize apoptotic bodies, rather than being a silent consequence of liver injury[30]. Thus, factors that affect apoptosis may be used to modulate liver fibrosis[26], and may be developed as a potential anti-fibrotic strategy.
Although the incidence of hepatic fibrosis is high worldwide, few effective and accepted antifibrogenic therapies are available. However, our previous study found that GA could inhibit CCl4-induced hepatocyte apoptosis and retard the progression of liver fibrosis in rats[17]. Similar to our previous research, DNA fragmentation of hepatocyte apoptosis was assessed using the TUNEL assay. In the CCl4-treated group, TUNEL-positive cells were increased compared to the control group and GA treatment group, whereas GA treatment significantly decreased the number of TUNEL-positive cells, and these results agree with previous findings that GA reduced the number of TUNEL-labeled cells[16]. However, the TUNEL assay is not a specific marker of apoptosis, thus Western blotting was conducted and found that GA treatment inhibited hepatocyte apoptosis by down-regulating expression of cleaved caspase-3 and Bax proteins. These results coincided with those from the TUNEL assay showed that GA reduced hepatocyte apoptosis.
Necrosis and apoptosis are involved in the process of liver fibrosis[26]. In our previous study, we found that hepatocyte apoptosis could induce liver fibrosis. However, the mechanism how GA exerts its anti-apoptotic effect against fibrosis in CCl4-induced liver injury and its contributing factors are unknown. HSC activation, characterized by a high rate of proliferation, expression of fibrotic cell markers and production of ECM[29], plays a key role in liver fibrogenesis[30,31], and a positive relationship between the degree of fibrosis and HSC activation in damaged livers has been observed in animal and human fibrogenesis[32]. α-SMA, a marker of activated HSCs, was evaluated by western blotting and real-time PCR in the present study. Expression of α-SMA protein and mRNA was significantly up-regulated following CCl4 administration and down-regulated by GA. These results indicated that HSCs were activated in CCl4-induced liver fibrosis, and significantly inhibited by GA treatment.
An imbalance between ECM degradation and production existed during fibrosis, and both collagen type I and III exhibited a significant increase. Activation of HSCs, involved in the conversion of quiescent, vitamin-A-storing cells into proliferative, fibrogenic and contractile myofibroblasts, can synthesize and secrete a large number of fibril-forming collagens, especially collagen type I and III[33,34]. During liver fibrosis, the main components of the scar matrix are interstitial collagen type I and III, which replace the basal membrane of the subendothelial space of Disse and sinusoids[35,36]. In the present study, using real-time PCR, we found that expression of collagen type I and III mRNA was enhanced in CCl4-induced liver fibrosis, while expression in the liver of GA-treated rats was significantly reduced, thus, GA significantly decreased accumulation of ECM.
Matrix degradation is dependent on the role of the MMP family in the extracellular space. Activation of HSCs also generates MMP2, MMP9 and MMP3, which destroy the basement membrane, leading to recruitment of inflammatory cells to the site of injury[37-39]. The activity of MMP2 and MMP9 in liver fibrosis progression increases as HSCs become activated both in humans and animals[1,10]. The present study showed that expression of MMP2 and MMP9 significantly increased in the CCl4-treated group, while GA treatment decreased expression of MMP2 and MMP9.
CTGF, another important fibrogenic factor, is synthesized by hepatocytes and HSCs[40,41]. CTGF is a general mediator of the interactions between fiber-fiber, fiber-matrix and matrix-matrix. During liver fibrosis, CTGF is a hepatic fibrogenic master switch in the epithelial to mesenchymal transition, and plays a pivotal role in the increase of ECM-producing fibroblasts[41]. In the present study, expression of CTGF was up-regulated in the CCl4-treated group, while GA treatment significantly reversed this increase. We also found that hepatic injury in the CCl4 group was more serious than that in the GA treatment group on the basis of histological observation and Sirius red staining.
In the present study, we found that GA has inhibitory effects on hepatocyte apoptosis and liver fibrosis, which are mainly associated with down-regulation of HSC activation, thus regulating fibrosis-related factors, such as expression of CTGF, MMP2 and MMP9 proteins, and collagen type I and III mRNA. Collectively, these results demonstrated that GA significantly ameliorates CCl4-induced liver fibrosis by inhibiting hepatocyte apoptosis and HSC activation, which may provide potential therapeutic strategies for fibrosis.
Glycyrrhizic acid (GA), a major active component of Glycyrrhiza glabra roots, is commonly used in Asia to treat patients with chronic hepatitis. Liver fibrosis is a common outcome in many chronic liver diseases. Necrosis and apoptosis are involved in the development of liver fibrosis. In a previous study, the authors found that hepatocyte apoptosis could induce liver fibrosis. In this study, the authors investigated the mechanism how GA exerts its antiapoptotic effect against fibrosis in carbon tetrachloride (CCl4)-induced liver injury, and its contributing factors in this process.
In the present study, the authors found that GA treatment significantly ameliorated CCl4-induced liver fibrosis by inhibiting hepatocyte apoptosis and hepatic stellate cell (HSC) activation, which may provide potential therapeutic strategies for fibrosis.
This study investigated the mechanism how GA exerts its anti-apoptotic effect against fibrosis in CCl4-induced liver injury, and its contributing factors in this process. The results of this study demonstrated that GA treatment significantly ameliorated CCl4-induced liver fibrosis by inhibiting hepatocyte apoptosis and HSC activation.
The results of this study may provide potential therapeutic strategies for fibrosis.
Hepatocyte apoptosis, a cardinal feature of many liver diseases, is considered to play a part in initiating and maintaining HSC activation. Apoptosis of parenchymal cells, an important inflammatory stimulus, activates HSCs, which display a surprising capacity to phagocytize apoptotic bodies, rather than being a silent consequence of liver injury. Thus, factors which affect apoptosis may be used to modulate liver fibrosis.
In this study, the authors investigated how GA exerted its anti-apoptotic effect against fibrosis in CCl4-induced liver injury and its contributing factors. The study is well designed and presented. The results shown in the manuscript is interesting and GA is a potential therapeutic agent for liver cirrhosis.
P- Reviewer: Alisi A, Kayadibi H, Miura K S- Editor: Ma YJ L- Editor: Kerr C E- Editor: Wang CH
| 1. | Iredale JP. Models of liver fibrosis: exploring the dynamic nature of inflammation and repair in a solid organ. J Clin Invest. 2007;117:539-548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 698] [Cited by in RCA: 699] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 2. | Ginès P, Cárdenas A, Arroyo V, Rodés J. Management of cirrhosis and ascites. N Engl J Med. 2004;350:1646-1654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 546] [Cited by in RCA: 481] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 3. | Yoon JH, Gores GJ. Death receptor-mediated apoptosis and the liver. J Hepatol. 2002;37:400-410. [PubMed] |
| 4. | Liu T, Wang X, Karsdal MA, Leeming DJ, Genovese F. Molecular serum markers of liver fibrosis. Biomark Insights. 2012;7:105-117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 5. | Canbay A, Higuchi H, Bronk SF, Taniai M, Sebo TJ, Gores GJ. Fas enhances fibrogenesis in the bile duct ligated mouse: a link between apoptosis and fibrosis. Gastroenterology. 2002;123:1323-1330. [PubMed] |
| 6. | Canbay A, Friedman S, Gores GJ. Apoptosis: the nexus of liver injury and fibrosis. Hepatology. 2004;39:273-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 397] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 7. | Kodama T, Takehara T, Hikita H, Shimizu S, Shigekawa M, Tsunematsu H, Li W, Miyagi T, Hosui A, Tatsumi T. Increases in p53 expression induce CTGF synthesis by mouse and human hepatocytes and result in liver fibrosis in mice. J Clin Invest. 2011;121:3343-3356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 139] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 8. | Friedman SL, Bansal MB. Reversal of hepatic fibrosis -- fact or fantasy? Hepatology. 2006;43:S82-S88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 283] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 9. | Arthur MJ. Reversibility of liver fibrosis and cirrhosis following treatment for hepatitis C. Gastroenterology. 2002;122:1525-1528. [PubMed] |
| 10. | Cohen-Naftaly M, Friedman SL. Current status of novel antifibrotic therapies in patients with chronic liver disease. Therap Adv Gastroenterol. 2011;4:391-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 150] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 11. | Popov Y, Schuppan D. Targeting liver fibrosis: strategies for development and validation of antifibrotic therapies. Hepatology. 2009;50:1294-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 254] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 12. | Liu JP, McIntosh H, Lin H. Chinese medicinal herbs for chronic hepatitis B. Cochrane Database Syst Rev. 2001;CD001940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Dang SS, Zhang X, Jia XL, Cheng YA, Song P, Liu EQ, He Q, Li ZF. Protective effects of emodin and astragalus polysaccharides on chronic hepatic injury in rats. Chin Med J (Engl). 2008;121:1010-1014. [PubMed] |
| 14. | Sato H, Goto W, Yamamura J, Kurokawa M, Kageyama S, Takahara T, Watanabe A, Shiraki K. Therapeutic basis of glycyrrhizin on chronic hepatitis B. Antiviral Res. 1996;30:171-177. [PubMed] |
| 15. | Gwak GY, Moon TG, Lee DH, Yoo BC. Glycyrrhizin attenuates HMGB1-induced hepatocyte apoptosis by inhibiting the p38-dependent mitochondrial pathway. World J Gastroenterol. 2012;18:679-684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 40] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Ikeda T, Abe K, Kuroda N, Kida Y, Inoue H, Wake K, Morito M, Sato T. The inhibition of apoptosis by glycyrrhizin in hepatic injury induced by injection of lipopolysaccharide / D-galactosamine in mice. Arch Histol Cytol. 2008;71:163-178. [PubMed] |
| 17. | Guo XL, Liang B, Wang XW, Fan FG, Jin J, Lan R, Yang JH, Wang XC, Jin L, Cao Q. Glycyrrhizic acid attenuates CCl4-induced hepatocyte apoptosis in rats via a p53-mediated pathway. World J Gastroenterol. 2013;19:3781-3791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 63] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 18. | Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101-1108. [PubMed] |
| 19. | Mantena SK, Sharma SD, Katiyar SK. Berberine inhibits growth, induces G1 arrest and apoptosis in human epidermoid carcinoma A431 cells by regulating Cdki-Cdk-cyclin cascade, disruption of mitochondrial membrane potential and cleavage of caspase 3 and PARP. Carcinogenesis. 2006;27:2018-2027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 137] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 20. | Arase Y, Ikeda K, Murashima N, Chayama K, Tsubota A, Koida I, Suzuki Y, Saitoh S, Kobayashi M, Kumada H. The long term efficacy of glycyrrhizin in chronic hepatitis C patients. Cancer. 1997;79:1494-1500. [PubMed] |
| 21. | van Rossum TG, Vulto AG, Hop WC, Schalm SW. Glycyrrhizin-induced reduction of ALT in European patients with chronic hepatitis C. Am J Gastroenterol. 2001;96:2432-2437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 45] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Shi J, Aisaki K, Ikawa Y, Wake K. Evidence of hepatocyte apoptosis in rat liver after the administration of carbon tetrachloride. Am J Pathol. 1998;153:515-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 190] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 23. | Patella S, Phillips DJ, Tchongue J, de Kretser DM, Sievert W. Follistatin attenuates early liver fibrosis: effects on hepatic stellate cell activation and hepatocyte apoptosis. Am J Physiol Gastrointest Liver Physiol. 2006;290:G137-G144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 90] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 24. | Aram G, Potter JJ, Liu X, Wang L, Torbenson MS, Mezey E. Deficiency of nicotinamide adenine dinucleotide phosphate, reduced form oxidase enhances hepatocellular injury but attenuates fibrosis after chronic carbon tetrachloride administration. Hepatology. 2009;49:911-919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Kuwahata M, Kubota H, Kanouchi H, Ito S, Ogawa A, Kobayashi Y, Kido Y. Supplementation with branched-chain amino acids attenuates hepatic apoptosis in rats with chronic liver disease. Nutr Res. 2012;32:522-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Lee TY, Chang HH, Wang GJ, Chiu JH, Yang YY, Lin HC. Water-soluble extract of Salvia miltiorrhiza ameliorates carbon tetrachloride-mediated hepatic apoptosis in rats. J Pharm Pharmacol. 2006;58:659-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Weiler-Normann C, Herkel J, Lohse AW. Mouse models of liver fibrosis. Z Gastroenterol. 2007;45:43-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 82] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 28. | Weber LW, Boll M, Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit Rev Toxicol. 2003;33:105-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 1149] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 29. | Liu C, Wang G, Chen G, Mu Y, Zhang L, Hu X, Sun M, Liu C, Liu P. Huangqi decoction inhibits apoptosis and fibrosis, but promotes Kupffer cell activation in dimethylnitrosamine-induced rat liver fibrosis. BMC Complement Altern Med. 2012;12:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 30. | Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655-1669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2139] [Cited by in RCA: 2161] [Article Influence: 127.1] [Reference Citation Analysis (0)] |
| 31. | Friedman SL. Mechanisms of disease: Mechanisms of hepatic fibrosis and therapeutic implications. Nat Clin Pract Gastroenterol Hepatol. 2004;1:98-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 386] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 32. | Reeves HL, Burt AD, Wood S, Day CP. Hepatic stellate cell activation occurs in the absence of hepatitis in alcoholic liver disease and correlates with the severity of steatosis. J Hepatol. 1996;25:677-683. [PubMed] |
| 33. | Friedman SL. Liver fibrosis -- from bench to bedside. J Hepatol. 2003;38 Suppl 1:S38-S53. [PubMed] |
| 34. | Schuppan D, Ruehl M, Somasundaram R, Hahn EG. Matrix as a modulator of hepatic fibrogenesis. Semin Liver Dis. 2001;21:351-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 389] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 35. | Rojkind M, Giambrone MA, Biempica L. Collagen types in normal and cirrhotic liver. Gastroenterology. 1979;76:710-719. [PubMed] |
| 36. | Nakatsukasa H, Nagy P, Evarts RP, Hsia CC, Marsden E, Thorgeirsson SS. Cellular distribution of transforming growth factor-beta 1 and procollagen types I, III, and IV transcripts in carbon tetrachloride-induced rat liver fibrosis. J Clin Invest. 1990;85:1833-1843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 272] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 37. | Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3533] [Cited by in RCA: 3357] [Article Influence: 197.5] [Reference Citation Analysis (0)] |
| 38. | Brenner DA. Molecular pathogenesis of liver fibrosis. Trans Am Clin Climatol Assoc. 2009;120:361-368. [PubMed] |
| 39. | Kisseleva T, Brenner DA. Mechanisms of fibrogenesis. Exp Biol Med (Maywood). 2008;233:109-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 392] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 40. | Gressner OA, Lahme B, Demirci I, Gressner AM, Weiskirchen R. Differential effects of TGF-beta on connective tissue growth factor (CTGF/CCN2) expression in hepatic stellate cells and hepatocytes. J Hepatol. 2007;47:699-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 125] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 41. | Gressner OA, Gressner AM. Connective tissue growth factor: a fibrogenic master switch in fibrotic liver diseases. Liver Int. 2008;28:1065-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 213] [Article Influence: 12.5] [Reference Citation Analysis (0)] |