Published online May 7, 2015. doi: 10.3748/wjg.v21.i17.5149
Peer-review started: December 11, 2014
First decision: January 22, 2015
Revised: February 3, 2015
Accepted: March 19, 2015
Article in press: March 19, 2015
Published online: May 7, 2015
Processing time: 154 Days and 8.8 Hours
In the last years, an increasing interest has been raised on non-polypoid colorectal tumors (NPT) and in particular on large flat neoplastic lesions beyond 10 mm tending to grow laterally, called laterally spreading tumors (LST). LSTs and large sessile polyps have a greater frequency of high-grade dysplasia and local invasiveness as compared to pedunculated lesions of the same size and usually represent a technical challenge for the endoscopist in terms of either diagnosis and resection. According to the Paris classification, NPTs are distinguished in slightly elevated (0-IIa, less than 2.5 mm), flat (0-IIb) or slightly depressed (0-IIc). NPTs are usually flat or slightly elevated and tend to spread laterally while in case of depressed lesions, cell proliferation growth progresses in depth in the colonic wall, thus leading to an increased risk of submucosal invasion (SMI) even for smaller neoplasms. NPTs may be frequently missed by inexperienced endoscopists, thus a careful training and precise assessment of all suspected mucosal areas should be performed. Chromoendoscopy or, if possible, narrow-band imaging technique should be considered for the estimation of SMI risk of NPTs, and the characterization of pit pattern and vascular pattern may be useful to predict the risk of SMI and, therefore, to guide the therapeutic decision. Lesions suitable to endoscopic resection are those confined to the mucosa (or superficial layer of submucosa in selected cases) whereas deeper invasion makes endoscopic therapy infeasible. Endoscopic mucosal resection (EMR, piecemeal for LSTs > 20 mm, en bloc for smaller neoplasms) remains the first-line therapy for NPTs, whereas endoscopic submucosal dissection in high-volume centers or surgery should be considered for large LSTs for which en bloc resection is mandatory and cannot be achieved by means of EMR. After piecemeal EMR, follow-up colonoscopy should be performed at 3 mo to assess resection completeness. In case of en bloc resection, surveillance colonoscopy should be scheduled at 3 years for adenomatous lesions ≥ 1 cm, or in presence of villous features or high-grade dysplasia patients (regardless of the size), while less intensive surveillance (colonoscopy at 5-10 years) is needed in case of single (or two) NPT < 1 cm presenting tubular features or low-grade dysplasia at histology.
Core tip: Non polypoid tumors (NPTs) are distinguished in slightly elevated (0-IIa, less than 2.5 mm), flat (0-IIb) or slightly depressed (0-IIc). NPTs are usually flat or slightly elevated while depressed lesions show an increased risk of submucosal invasion (SMI). Chromoendoscopy or, if possible, narrow-band imaging technique should be considered for the estimation of SMI risk of NPTs, and the characterization of pit and vascular pattern may be useful to predict the risk of SMI. Endoscopic mucosal resection remains the first-line therapy for NPTs, whereas endoscopic submucosal dissection or surgery should be considered for larger neoplasms presenting SMI.
- Citation: Facciorusso A, Antonino M, Di Maso M, Barone M, Muscatiello N. Non-polypoid colorectal neoplasms: Classification, therapy and follow-up. World J Gastroenterol 2015; 21(17): 5149-5157
- URL: https://www.wjgnet.com/1007-9327/full/v21/i17/5149.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i17.5149
Colorectal cancer is a major health problem representing the second most commonly diagnosed cancer in women and the third in men[1]. Nowadays, colonoscopy is the most used tool for the early detection of colorectal cancer and the resection of pre-neoplastic lesions in order to prevent advanced and late stage neoplasms. In fact, it’s well known that more than 95% of colorectal cancers arise from adenomas (tumors of benign neoplastic epithelium with variable potential for malignancy) and the aim of colonoscopy surveillance is to timely interrupt the “adenoma-carcinoma sequence”[2-5].
In the last years, an increasing interest has been raised on non-polypoid colorectal tumor (NPT) and in particular on laterally spreading tumor (LST). LST is a large flat neoplastic lesion tending to grow laterally along the surface of the bowel[6,7]. By definition, LSTs show a diameter beyond 10 mm[8-10]. LSTs and large sessile polyps have a greater frequency of high-grade dysplasia (HGD) and local invasiveness as compared to pedunculated lesions of the same size and usually represent a technical challenge for the endoscopist either in terms of diagnosis and of resection. That is why the term “advanced mucosal neoplasia” (AMN) has been recently proposed for these two classes of lesions[11].
The morphology of colonic lesions depends on the direction of proliferation growth.
Following this, two main macroscopic types may be recognized: superficial lesions (type 0) and advanced cancers (type 1-5)[12].
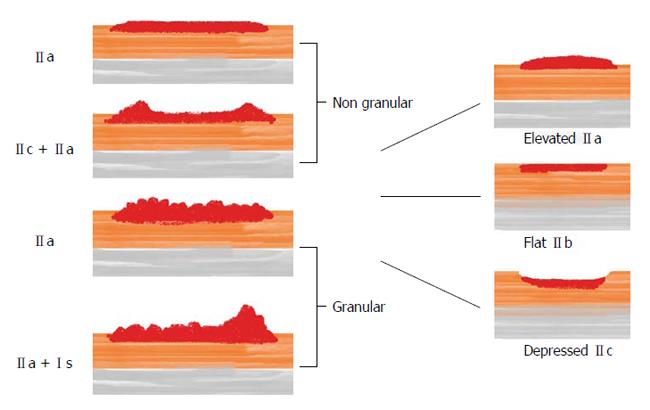
According to the Paris classification (Table 1), lesions with superficial appearance (category 0) are distinguished in: polypoid type [elevated more than 2.5 mm above the mucosal layer: pedunculated (0-1p), sessile (0-1s) or mixed (0-1sp)], non-polypoid [slightly elevated less than 2.5 mm (0-IIa), flat (0-IIb) or slightly depressed (0-IIc)] and mixed types.
| Polypoid type1 | Pedunculated (0-1p) |
| Sessile (0-1s) | |
| Mixed (0-1sp) | |
| Non-polypoid type | Slightly elevated (0-IIa) |
| Flat (0-IIb) | |
| Slightly depressed (0-IIc) | |
| Mixed types | Elevated and depressed (0-IIa + IIc) |
| Depressed and elevated (0-IIc + IIa) | |
| Sessile and depressed (0-1s + IIc) |
The threshold of 2.5 mm, which corresponds to the height of a closed biopsy forcep, is quite arbitrary and not really reliable because many flat lesions are not homogeneous in their whole surface.
Non-polypoid lesions are usually flat or slightly elevated and tend to spread laterally while in case of depressed lesions, cell proliferation growth progresses in depth in the colonic wall, thus leading to an increased risk of submucosal invasion (SMI) even for smaller lesions. In the colon and rectum, slightly elevated and flat NPTs are commonly classified together since they are not so easily distinguishable and because true flat masses (0-IIb) are rarely found in this intestinal tract. Among non-polypoid tumors, type 0-IIa (slightly elevated) is by far the most frequent[12].
NPTs (flat or depressed) may be found throughout the colon unlike polypoid cancers which are more frequent in the left part[13,14].
The distinction among the different subtypes is not always easy to capture, hence the local endoscopist and pathologist expertise plays a pivotal role in the diagnostic algorithm[15,16].
As aforementioned, flat colorectal neoplasms equal to or larger than 10 mm are called LSTs.
LSTs are divided into the granular (LST-G) and non-granular type (LST-NG) based on their detailed endoscopic appearance during chromoendoscopy with indigo carmine dye spraying[13,17]. The LST-G type is composed of conglomerates of nodules forming a flat broad-based mass (thus the term “granular”) while this characteristic is lacking in the latter group (LST-NG)[13,18].
NPTs have a higher risk of local invasiveness than polypoid tumors, regardless the size[13,14]. While the larger is the polypoid lesion the higher is the risk of SMI, not all the NPTs show a so strict correlation between size and local invasiveness.
LST-Gs with homogeneous surface have a low risk (< 2%) of SMI no matter their size is, whereas LST-Gs with mixed-size nodules have a higher risk of SMI (7.1% for lesions < 20 mm and 38% for those > 30 mm)[19]. Even higher is the risk of SMI for LST-NGs, particularly in those presenting a thinner center (LST-NGs with pseudo-depression): 12.5% in case of size < 20 mm and 83.3% for diameters > 30 mm[13].
Depressed lesions are rare (1%-6% of all NPTs) but present the highest overall risk of SMI: 27%-35.9%[13].
Figure 1 graphically describes the three main types of NPTs and the subclassification of LSTs.
The detection of a superficial lesion in asymptomatic patients undergoing complete colonoscopy is a frequent event ranging from 10% to 60%[12,20-22].
In a Japanese series, the rate of NPTs was 42% (10.948 out of 25.862 superficial neoplastic lesions identified)[23]. The proportion was lower, although still significant, in another Japanese series (27%: 2711/12811)[24]. In the United States and Western countries, the proportion of NPTs is highly variable ranging from 9.35% to 31.4%[25,26].
NPTs may be frequently missed by inexperienced endoscopists, thus a careful training and precise assessment of all suspected mucosal areas should be performed.
Chromoendoscopy or, if possible, narrow-band imaging (NBI) technique should be considered for the estimation of SMI risk of NPTs. NBI, by using light filters to narrow the bandwidth of the endoscope’s light aimed at selective evaluation of the area of interest, is able to recognize and better define the vascular and pit-pattern, both indicators of malignancy[27,28]. For instance, irregular and sparse vascularization and loss of epithelial crests are related to evolved lesions[29].
The microarchitecture of pits, epithelial crests, or ridges (so called “pit pattern”) has been extensively described by Kudo et al[29]: three main categories of pit patterns are described: (1) nonneoplastic (I and II); (2) neoplastic adenomatous (III and IV); or (3) neoplastic cancer (V)[29,30] (Table 2). Type IV is the most common among AMNs and is usually related to large NPT-Gs and implies tubule-villous histology.
| Non neoplastic | I: Normal mucosa |
| II: Enlarged regular stellar crypts | |
| Neoplastic, adenomatous | IIIL: Elongated, sinuous crests |
| IIIS: Narrowed round and irregular pits | |
| IV: Branched or gyrus-like crests | |
| Neoplastic, cancer | Vi: Irregular surface |
| VN: Amorphous surface |
With regard to vascularization, irregular multi-branched microvessels alternated to avascular areas are predictor of higher risk of SMI[31]. A more detailed description of vascular patterns detectable at the surface of colonic mucosa is summarized in Table 3.
| Non neoplastic | Normal: Well-delineated capillaries surrounding pits opening |
| Faint: Poor visibility of capillaries around enlarged pits | |
| Neoplastic, adenomatous | Network: Vessels organized in a large and regular mesh |
| Dense: Enlarged vessels of regular size at top of elongated crests | |
| Neoplastic, cancer | Irregular: Enlarged vessels of irregular diameter and diverging directions |
| Sparse: Poor distribution of irregular vessels with diverging directions |
Even if no single feature is absolutely specific for SMI, the presence of more than 1 high-risk characteristic correlates to more invasive lesions[32].
LST-Gs account for 60%-80% of the cases vs 20%-40% of LST-NGs, whereas depressed NPTs (those at higher risk of SMI) represent 1% to 6% of the total number of superficial colorectal lesions[7,14,33,34]. Most studies reported the majority of NPTs in the proximal colon (55.7%-80%)[33,34], which differs from two Chinese series wherein 65%-75% of non polypoid lesions were located in the distal colon[7,35].
It’s well known that patients with long-lasting inflammatory bowel disease (IBD) colitis have a higher risk of developing colorectal cancer than the general population[36-39].
Even if the vast majority of colitic dysplasia is endoscopically visible, colonoscopic surveillance remains challenging as the dysplasia can have a varied endoscopic appearance ranging from lesions appearing identical to sporadic adenomas to plaques, nodular mucosa, puckering of the mucosa, villiform mucosa, strictures, and broad-based masses with indistinct lateral margins. Dysplastic areas detected within inflamed or previously inflamed mucosa show a more aggressive behavior and tend to progress more rapidly than sporadic adenomas in non-inflamed mucosa[40-42]. Thus, all lesions suspected to be dysplastic should be removed promptly.
Random sampling is ineffective in detecting dysplastic areas, especially in the case of NPTs. Chromoendoscopy with targeted biopsy significantly improves surveillance efficacy (the increase was about 7% in a recent meta-analysis)[43], while NBI failed to show a clear superiority over high-definition white light colonoscopy in the detection of dysplasia in IBD patients[44-48].
Raised dysplastic lesions within an area of current or previous inflammation have been termed dysplasia-associated lesions/masses (DALMs). Until recently these have been considered an indication for colectomy because of their reported higher risk of cancer[49].
DALMs appear as well-circumscribed, sessile or pedunculated polyps and should be promptly and radically treated by means of endoscopic resection (contextually biopsies should be taken from the normal-looking mucosa surrounding the polypectomy margins in order to detect further areas of dysplasia). If a timely and appropriate endoscopic therapy is performed, the overall rate of progression to cancer of adenoma-like DALMs is very low (only 2.4% in a recent review)[50].
If the radical resection of the lesion is not feasible, or if dysplastic foci in the adjacent mucosa are detected, then colectomy is mandatory[51,52].
Although the polypoid aspect is predominant, however, as well as in the general population, some lesions are minimally elevated (less than 2.5 mm in height), completely flat or even depressed in morphology.
Non-polypoid lesions can be more difficult to detect and distinguish from the surrounding inflamed mucosa and a particularly careful endoscopic assessment is required.
Once a NPT is detected, the therapeutic approach is the same as that previously described for DALMs but en bloc resection is mandatory (when infeasible, surgery remains the sole option)[53-58].
According to the aforementioned characteristics (superficial aspect, pit and vascular pattern) and the predicted risk of SMI, the proper therapeutic indication should be considered.
Lesions suitable to endoscopic resection are those confined to the mucosa (or superficial layer of submucosa in selected cases) whereas deeper invasion makes endoscopic therapy infeasible[59].
In the last years, a number of resection techniques for the management of AMNs have been described; among them, inject-and-cut endoscopic mucosal resection (EMR) is the most common[59,60]. More recently, endoscopic submucosal dissection (ESD) has been developed to improve the “en bloc” resection rate of AMNs.
Saline is the most commonly used injection solution worldwide[59]. Submucosal epinephrine-saline solution injection has been shown to be an effective method for the complete endoscopic polypectomy, especially in flat or sessile lesions and is widely used because of its simplicity, low cost, and wide availability[61]. On the other hand, a number of studies have raised concerns about its efficacy in preventing post-procedural haemorrhage due to the short-lasting period of mucosal elevation following epinephrine injection[62].
Consequently, other substances (such as sodium hyaluronate, hydroxypropyl methylcellulose and glycerol), have been tested because of their ability to create a longer lasting submucosal cushion as a result of their viscous properties. In doing so, such substances enable lengthier procedures and increase the rate of en bloc resection, even for large lesions; however, despite the promising results of the aforementioned reports, their efficacy in preventing post-polypectomy bleeding (PPB) is still matter of debate[63,64].
An ideal submucosal injection solution should be inexpensive, readily available, non-toxic, easy to prepare and inject and should provide a long-lasting submucosal cushion.
Succinylated gelatin seems to fulfil these criteria but a recent randomized control trial, while showing a significant improvement of efficacy outcomes, failed to find a decreased PPB and perforation rate after gelatin submucosal injection as compared to saline[65]. Our group has recently published a retrospective propensity-score comparison of 306 patients treated with submucosal epinephrine injection and 306 with polidocanol injection for the endoscopic resection of LSTs or sessile lesions ≥ 20 mm, reporting a significantly lower PPB rate in the polidocanol group[66]. However, further confirms provided by randomized trials are warranted to identify the ideal injection solution.
The inject-and-cut EMR consists in simple predefined steps: at first, the solution is injected into the submucosa at one edge of the lesion with a disposable injection needle to create a submucosal cushion for safety purposes and better resection. While the assistant injects the solution, the endoscopist tangentially stabs the colonic wall. After the submucosal injection, a disposable electrosurgical snare is placed over the elevated tissue and gently pressed against the mucosa, while closing until resistance was felt. The lesion is then cut using an electrosurgical unit providing blended current[9,67-70].
Lesions larger than 20 mm are not usually amenable of en bloc resection, thus piecemeal resection is required[15]. In case of suspected residual tissue after resection, application of argon plasma coagulation (APC) may burn residual areas thus decreasing the risk of recurrence[71].
There is no unequivocal consensus on the prophylactic application of clips on the resection site after removal of AMNs: in fact, the promising efficacy in preventing PPB of this technique reported in a recent retrospective study still needs confirmation[72].
Recent refinement of ESD instruments and skills has lead to its application in the treatment of large colorectal lesions as an alternative to EMR. While in other fields of gastrointestinal endoscopy ESD has been proven superior to EMR[73], the indications for colorectal ESD, however, are relatively few even at experienced centers because most colorectal neoplasms are benign and can be resected using piecemeal EMR with minimal risk of recurrence[74].
During ESD, after submucosal injection, a marginal resection is performed to isolate the lesion with 3 or 4 mm surrounding normal mucosa. The submucosa under the lesion is injected further and then the ESD knife dissects through the submucosal layer to resect the lesion en bloc[74-80].
In conclusion, EMR (piecemeal for LSTs > 20 mm, en bloc for smaller neoplasms) remains the first-line therapy for NPTs. ESD in high-volume Centers or surgery should be considered for large LSTs for which en bloc resection is mandatory and cannot be achieved by means of EMR: namely, LST-G whole nodular type, LST-NG pseudo-depressed or other types with type V pit pattern areas which cannot be resected en bloc with a snare[81,82].
If the pathologic analysis of the specimen reveals the presence of adenocarcinoma and the lesion is limited to the mucosa (so called “carcinoma in situ”), there is no indication to surgery provided that the endoscopic resection has been radical. In contrast, adenocarcinomas with invasion into the submucosa show a 6% to 12% risk of lymph node metastasis[83,84], hence in Western countries they are commonly referred to surgery. As further studies have shown that well-differentiated adenocarcinomas with submucosal invasion within 10 mm without lymphatic or vascular involvement have small, if not nil, risk of lymph node metastasis[85], in Japan, NPTs with these features are treated by means of endoscopic resection.
Bleeding, abdominal pain and perforation are the most frequent and serious complications, in particular after endoscopic removal of AMNs[82]. All these adverse events are more frequently reported after ESD, hence this technique should be considered only in highly-experienced Centers.
Follow-up recommendations for NPTs are exactly the same as those proposed with regard to polypoid lesions[86-89].
After piecemeal EMR, follow-up colonoscopy should be performed at 3 mo to assess resection completeness and remove any residual or recurrent lesion[89]. If a residual or recurrent lesion is recognized, it should be treated accordingly. Once complete removal has been established, subsequent surveillance needs to be individualized based on the endoscopist’s judgment[89,90].
In case of en bloc resection, surveillance colonoscopy should be scheduled at 3 years for adenomatous lesions ≥ 1 cm, or in presence of villous features or high-grade dysplasia patients (regardless of the size)[90].
Usually, less intensive surveillance (colonoscopy at 5-10 years) is needed in case of single (or two) NPT < 1 cm presenting tubular features or low-grade dysplasia at histology[90].
However, in the real life, under certain circumstances (such as dubious radicality, need to treat margins with argon after resection, excised margin defined as not assessable by the pathologist), follow-up may be even stricter[90].
NPTs are commonly found during screening colonoscopy and usually represent a diagnostic and therapeutic challenge for the endoscopist, which should provide a careful characterization and classification of all diagnosed NPTs[50,91-94]. Chromoendoscopy and NBI are useful tools for the detection of non-polypoid lesions and should be routinely applied in the clinical practice. Pit pattern and vascular pattern features may accurately predict and assess the risk of SMI[95-99]. EMR represents the first line therapy in case of lesions confined into the mucosa, whereas patients with superficial SMI should be offered ESD (in highly-experienced Centers) or surgery.
P- Reviewer: Sali L, Tontini GE, Wang ZX S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH
| 1. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20516] [Article Influence: 2051.6] [Reference Citation Analysis (20)] |
| 2. | Bujanda L, Cosme A, Gil I, Arenas-Mirave JI. Malignant colorectal polyps. World J Gastroenterol. 2010;16:3103-3111. [PubMed] |
| 3. | Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383:1490-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1965] [Cited by in RCA: 2294] [Article Influence: 208.5] [Reference Citation Analysis (1)] |
| 4. | Brenner H, Chang-Claude J, Seiler CM, Rickert A, Hoffmeister M. Protection from colorectal cancer after colonoscopy: a population-based, case-control study. Ann Intern Med. 2011;154:22-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 612] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 5. | Elmunzer BJ, Hayward RA, Schoenfeld PS, Saini SD, Deshpande A, Waljee AK. Effect of flexible sigmoidoscopy-based screening on incidence and mortality of colorectal cancer: a systematic review and meta-analysis of randomized controlled trials. PLoS Med. 2012;9:e1001352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 153] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 6. | Lambert R, Tanaka S. Laterally spreading tumors in the colon and rectum. Eur J Gastroenterol Hepatol. 2012;24:1123-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Zhao X, Zhan Q, Xiang L, Wang Y, Wang X, Li A, Liu S. Clinicopathological characteristics of laterally spreading colorectal tumor. PLoS One. 2014;9:e94552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Endoscopic Classification Review Group. Update on the paris classification of superficial neoplastic lesions in the digestive tract. Endoscopy. 2005;37:570-578. [PubMed] |
| 9. | Xu MD, Wang XY, Li QL, Zhou PH, Zhang YQ, Zhong YS, Chen WF, Ma LL, Qin WZ, Hu JW. Colorectal lateral spreading tumor subtypes: clinicopathology and outcome of endoscopic submucosal dissection. Int J Colorectal Dis. 2013;28:63-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Iizuka H, Okamura S, Onozato Y, Ishihara H, Kakizaki S, Mori M. Endoscopic submucosal dissection for colorectal tumors. Gastroenterol Clin Biol. 2009;33:1004-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Holt BA, Bourke MJ. Wide field endoscopic resection for advanced colonic mucosal neoplasia: current status and future directions. Clin Gastroenterol Hepatol. 2012;10:969-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 12. | Bianco MA, Cipolletta L, Rotondano G, Buffoli F, Gizzi G, Tessari F; Flat Lesions Italian Network (FLIN). Prevalence of nonpolypoid colorectal neoplasia: an Italian multicenter observational study. Endoscopy. 2010;42:279-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Kudo Se, Lambert R, Allen JI, Fujii H, Fujii T, Kashida H, Matsuda T, Mori M, Saito H, Shimoda T. Nonpolypoid neoplastic lesions of the colorectal mucosa. Gastrointest Endosc. 2008;68:S3-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 364] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 14. | Lambert R, Kudo SE, Vieth M, Allen JI, Fujii H, Fujii T, Kashida H, Matsuda T, Mori M, Saito H. Pragmatic classification of superficial neoplastic colorectal lesions. Gastrointest Endosc. 2009;70:1182-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 15. | Kudo S, Tamura S, Hirota S, Sano Y, Yamano H, Serizawa M, Fukuoka T, Mitsuoka H, Nakajima T, Kusaka H. The problem of de novo colorectal carcinoma. Eur J Cancer. 1995;31A:1118-1120. [PubMed] |
| 16. | Kudo S, Kashida H, Tamura S, Nakajima T. The problem of “flat” colonic adenoma. Gastrointest Endosc Clin N Am. 1997;7:87-98. [PubMed] |
| 17. | Kudo S, Kashida H, Nakajima T, Tamura S, Nakajo K. Endoscopic diagnosis and treatment of early colorectal cancer. World J Surg. 1997;21:694-701. [PubMed] |
| 18. | Oka S, Tanaka S, Kanao H, Oba S, Chayama K. Therapeutic strategy for colorectal laterally spreading tumor. Dig Endosc. 2009;21 Suppl 1:S43-S46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 19. | Uraoka T, Saito Y, Matsuda T, Ikehara H, Gotoda T, Saito D, Fujii T. Endoscopic indications for endoscopic mucosal resection of laterally spreading tumours in the colorectum. Gut. 2006;55:1592-1597. [PubMed] |
| 20. | Brenner H, Altenhofen L, Stock C, Hoffmeister M. Prevention, early detection, and overdiagnosis of colorectal cancer within 10 years of screening colonoscopy in Germany. Clin Gastroenterol Hepatol. 2015;13:717-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 21. | The Japanese Society of Gastroenterological Cancer Screening. A nationwide totalling of mass screening for gastrointestinal cancers in 2005 [in Japanese]. J Gastroenterol Cancer Screening. 2008;46:53-76. |
| 22. | Imperiale TF, Wagner DR, Lin CY, Larkin GN, Rogge JD, Ransohoff DF. Results of screening colonoscopy among persons 40 to 49 years of age. N Engl J Med. 2002;346:1781-1785. [PubMed] |
| 23. | Okuno T, Sano Y, Ohkura Y. Incidence and clinicopathological characteristics of depressed type lesions: base line findings of multicenter retrospective cohort study [in Japanese]. Early Colorectal Cancer. 2004;8:21-27. |
| 24. | Togashi K, Konishi F, Koinuma K, Ishitsuka T, Kojima M, Okada M, Nagai H. Flat and depressed lesions of the colon and rectum: Pathogenesis and clinical management. Ann Acad Med Singapore. 2003;32:152-158. [PubMed] |
| 25. | O’brien MJ, Winawer SJ, Zauber AG, Bushey MT, Sternberg SS, Gottlieb LS, Bond JH, Waye JD, Schapiro M; National Polyp Study Workgroup. Flat adenomas in the National Polyp Study: is there increased risk for high-grade dysplasia initially or during surveillance? Clin Gastroenterol Hepatol. 2004;2:905-911. [PubMed] |
| 26. | Soetikno RM, Kaltenbach T, Rouse RV, Park W, Maheshwari A, Sato T, Matsui S, Friedland S. Prevalence of nonpolypoid (flat and depressed) colorectal neoplasms in asymptomatic and symptomatic adults. JAMA. 2008;299:1027-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 434] [Article Influence: 25.5] [Reference Citation Analysis (1)] |
| 27. | Gono K, Obi T, Yamaguchi M, Ohyama N, Machida H, Sano Y, Yoshida S, Hamamoto Y, Endo T. Appearance of enhanced tissue features in narrow-band endoscopic imaging. J Biomed Opt. 2004;9:568-577. [PubMed] |
| 28. | Ng SC, Lau JY. Narrow-band imaging in the colon: limitations and potentials. J Gastroenterol Hepatol. 2011;26:1589-1596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Kudo S, Hirota S, Nakajima T, Hosobe S, Kusaka H, Kobayashi T, Himori M, Yagyuu A. Colorectal tumours and pit pattern. J Clin Pathol. 1994;47:880-885. [PubMed] |
| 30. | Nagata S, Tanaka S, Haruma K, Yoshihara M, Sumii K, Kajiyama G, Shimamoto F. Pit pattern diagnosis of early colorectal carcinoma by magnifying colonoscopy: clinical and histological implications. Int J Oncol. 2000;16:927-934. [PubMed] |
| 31. | Katagiri A, Fu KI, Sano Y, Ikematsu H, Horimatsu T, Kaneko K, Muto M, Yoshida S. Narrow band imaging with magnifying colonoscopy as diagnostic tool for predicting histology of early colorectal neoplasia. Aliment Pharmacol Ther. 2008;27:1269-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 114] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 32. | Moss A, Bourke MJ, Williams SJ, Hourigan LF, Brown G, Tam W, Singh R, Zanati S, Chen RY, Byth K. Endoscopic mucosal resection outcomes and prediction of submucosal cancer from advanced colonic mucosal neoplasia. Gastroenterology. 2011;140:1909-1918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 439] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 33. | Rotondano G, Bianco MA, Buffoli F, Gizzi G, Tessari F, Cipolletta L. The Cooperative Italian FLIN Study Group: prevalence and clinico-pathological features of colorectal laterally spreading tumors. Endoscopy. 2011;43:856-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 34. | Kaku E, Oda Y, Murakami Y, Goto H, Tanaka T, Hasuda K, Yasunaga M, Ito K, Sakurai K, Fujimori T. Proportion of flat- and depressed-type and laterally spreading tumor among advanced colorectal neoplasia. Clin Gastroenterol Hepatol. 2011;9:503-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 35. | Miyamoto H, Oono Y, Fu KL, Ikematsu H, Fujii S, Kojima T, Yano T, Ochiai A, Sasaki Y, Kaneko K. Morphological change of a laterally spreading rectal tumor over a short period. BMC Gastroenterol. 2013;13:129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 36. | Singh H, Nugent Z, Targownik LE, El-Matary W, Brownell M, Bernstein CN. Health Care Use by a Population-Based Cohort of Children With Inflammatory Bowel Disease. Clin Gastroenterol Hepatol. 2015;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 37. | Parian A, Lazarev M. Who and how to screen for cancer in at-risk inflammatory bowel disease patients. Expert Rev Gastroenterol Hepatol. 2015;Epub ahead of print:1-16. [PubMed] |
| 38. | Herszényi L, Barabás L, Miheller P, Tulassay Z. Colorectal cancer in patients with inflammatory bowel disease: the true impact of the risk. Dig Dis. 2015;33:52-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 39. | Lee HS, Park SH, Yang SK, Ye BD, Kim JH, Kim SO, Soh JS, Lee S, Bae JH, Lee HJ. The risk of colorectal cancer in inflammatory bowel disease: a hospital-based cohort study from Korea. Scand J Gastroenterol. 2015;50:188-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 40. | Vieth M, Behrens H, Stolte M. Sporadic adenoma in ulcerative colitis: endoscopic resection is an adequate treatment. Gut. 2006;55:1151-1155. [PubMed] |
| 41. | Yashiro M. Ulcerative colitis-associated colorectal cancer. World J Gastroenterol. 2014;20:16389-16397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 153] [Cited by in RCA: 197] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 42. | Bjerrum JT, Nielsen OH, Riis LB, Pittet V, Mueller C, Rogler G, Olsen J. Transcriptional analysis of left-sided colitis, pancolitis, and ulcerative colitis-associated dysplasia. Inflamm Bowel Dis. 2014;20:2340-2352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 43. | Soetikno R, Subramanian V, Kaltenbach T, Rouse RV, Sanduleanu S, Suzuki N, Tanaka S, McQuaid K. The detection of nonpolypoid (flat and depressed) colorectal neoplasms in patients with inflammatory bowel disease. Gastroenterology. 2013;144:1349-1352, 1352.e1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 44. | Ignjatovic A, East JE, Subramanian V, Suzuki N, Guenther T, Palmer N, Bassett P, Ragunath K, Saunders BP. Narrow band imaging for detection of dysplasia in colitis: a randomized controlled trial. Am J Gastroenterol. 2012;107:885-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 125] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 45. | van den Broek FJ, Fockens P, van Eeden S, Stokkers PC, Ponsioen CY, Reitsma JB, Dekker E. Narrow-band imaging versus high-definition endoscopy for the diagnosis of neoplasia in ulcerative colitis. Endoscopy. 2011;43:108-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 131] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 46. | Omata F, Ohde S, Deshpande GA, Kobayashi D, Masuda K, Fukui T. Image-enhanced, chromo, and cap-assisted colonoscopy for improving adenoma/neoplasia detection rate: a systematic review and meta-analysis. Scand J Gastroenterol. 2014;49:222-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 47. | Isomoto H, Uehara R, Hayashi T, Shiota J, Matsushima K, Chen CC, Takeshima F, Nakayama T, Nakao K. Magnifying Endoscopic Findings Can Predict Clinical Outcome during Long-Term Follow-Up of More Than 12 Months in Patients with Ulcerative Colitis. Gastroenterol Res Pract. 2013;2013:671576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 48. | Efthymiou M, Allen PB, Taylor AC, Desmond PV, Jayasakera C, De Cruz P, Kamm MA. Chromoendoscopy versus narrow band imaging for colonic surveillance in inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:2132-2138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 49. | Blackstone MO, Riddell RH, Rogers BH, Levin B. Dysplasia-associated lesion or mass (DALM) detected by colonoscopy in long-standing ulcerative colitis: an indication for colectomy. Gastroenterology. 1981;80:366-374. [PubMed] |
| 50. | Wanders LK, Dekker E, Pullens B, Bassett P, Travis SP, East JE. Cancer risk after resection of polypoid dysplasia in patients with longstanding ulcerative colitis: a meta-analysis. Clin Gastroenterol Hepatol. 2014;12:756-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 133] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 51. | Rutter MD, Saunders BP, Wilkinson KH, Rumbles S, Schofield G, Kamm MA, Williams CB, Price AB, Talbot IC, Forbes A. Cancer surveillance in longstanding ulcerative colitis: endoscopic appearances help predict cancer risk. Gut. 2004;53:1813-1816. [PubMed] |
| 52. | Rutter MD, Saunders BP, Wilkinson KH, Kamm MA, Williams CB, Forbes A. Most dysplasia in ulcerative colitis is visible at colonoscopy. Gastrointest Endosc. 2004;60:334-339. [PubMed] |
| 53. | Soetikno R, Sanduleanu S, Kaltenbach T. An atlas of the nonpolypoid colorectal neoplasms in inflammatory bowel disease. Gastrointest Endosc Clin N Am. 2014;24:483-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 54. | Kisiel JB, Loftus EV, Harmsen WS, Zinsmeister AR, Sandborn WJ. Outcome of sporadic adenomas and adenoma-like dysplasia in patients with ulcerative colitis undergoing polypectomy. Inflamm Bowel Dis. 2012;18:226-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 55. | Pekow JR, Hetzel JT, Rothe JA, Hanauer SB, Turner JR, Hart J, Noffsinger A, Huo D, Rubin DT. Outcome after surveillance of low-grade and indefinite dysplasia in patients with ulcerative colitis. Inflamm Bowel Dis. 2010;16:1352-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 56. | Rubin DT, Rothe JA, Hetzel JT, Cohen RD, Hanauer SB. Are dysplasia and colorectal cancer endoscopically visible in patients with ulcerative colitis? Gastrointest Endosc. 2007;65:998-1004. [PubMed] |
| 57. | Rubin DT, Turner JR. Surveillance of dysplasia in inflammatory bowel disease: The gastroenterologist-pathologist partnership. Clin Gastroenterol Hepatol. 2006;4:1309-1313. [PubMed] |
| 58. | Chawla A, Judge TA, Lichtenstein GR. Evaluation of polypoid lesions in inflammatory bowel disease. Gastrointest Endosc Clin N Am. 2002;12:525-534, ix. [PubMed] |
| 59. | Sanchez-Yague A, Kaltenbach T, Raju G, Soetikno R. Advanced endoscopic resection of colorectal lesions. Gastroenterol Clin North Am. 2013;42:459-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 60. | Soetikno R, Kaltenbach T. Dynamic submucosal injection technique. Gastrointest Endosc Clin N Am. 2010;20:497-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 61. | Hogan RB, Hogan RB. Epinephrine volume reduction of giant colon polyps facilitates endoscopic assessment and removal. Gastrointest Endosc. 2007;66:1018-1022. [PubMed] |
| 62. | Lee SH, Chung IK, Kim SJ, Kim JO, Ko BM, Kim WH, Kim HS, Park DI, Kim HJ, Byeon JS. Comparison of postpolypectomy bleeding between epinephrine and saline submucosal injection for large colon polyps by conventional polypectomy: a prospective randomized, multicenter study. World J Gastroenterol. 2007;13:2973-2977. [PubMed] |
| 63. | Kishihara T, Chino A, Uragami N, Yoshizawa N, Imai M, Ogawa T, Igarashi M. Usefulness of sodium hyaluronate solution in colorectal endoscopic mucosal resection. Dig Endosc. 2012;24:348-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 64. | Al-Taie OH, Bauer Y, Dietrich CG, Fischbach W. Efficacy of submucosal injection of different solutions inclusive blood components on mucosa elevation for endoscopic resection. Clin Exp Gastroenterol. 2012;5:43-48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 65. | Moss A, Bourke MJ, Metz AJ. A randomized, double-blind trial of succinylated gelatin submucosal injection for endoscopic resection of large sessile polyps of the colon. Am J Gastroenterol. 2010;105:2375-2382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 66. | Facciorusso A, Di Maso M, Antonino M, Del Prete V, Panella C, Barone M, Muscatiello N. Polidocanol injection decreases the bleeding rate after colon polypectomy: a propensity score analysis. Gastrointestinal Endoscopy. 2014;In press. |
| 67. | Din S, Ball AJ, Riley SA, Kitsanta P, Johal S. Cold snare polypectomy: Does snare type influence outcomes? Dig Endosc. 2015;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 68. | Tribonias G, Komeda Y, Voudoukis E, Bassioukas S, Viazis N, Manola ME, Giannikaki E, Papalois A, Paraskeva K, Karamanolis D. Cold snare polypectomy with pull technique of flat colonic polyps up to 12 mm: a porcine model. Ann Gastroenterol. 2015;28:141-143. [PubMed] |
| 69. | Beppu K, Osada T, Sakamoto N, Shibuya T, Matsumoto K, Nagahara A, Terai T, Ogihara T, Watanabe S. Optimal timing for resuming antithrombotic agents and risk factors for delayed bleeding after endoscopic resection of colorectal tumors. Gastroenterol Res Pract. 2014;2014:825179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 70. | Kume K, Watanabe T, Yoshikawa I, Harada M. Endoscopic measurement of polyp size using a novel calibrated hood. Gastroenterol Res Pract. 2014;2014:714294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 71. | Zlatanic J, Waye JD, Kim PS, Baiocco PJ, Gleim GW. Large sessile colonic adenomas: use of argon plasma coagulator to supplement piecemeal snare polypectomy. Gastrointest Endosc. 1999;49:731-735. [PubMed] |
| 72. | Liaquat H, Rohn E, Rex DK. Prophylactic clip closure reduced the risk of delayed postpolypectomy hemorrhage: experience in 277 clipped large sessile or flat colorectal lesions and 247 control lesions. Gastrointest Endosc. 2013;77:401-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 206] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 73. | Facciorusso A, Antonino M, Di Maso M, Muscatiello N. Endoscopic submucosal dissection vs endoscopic mucosal resection for early gastric cancer: A meta-analysis. World J Gastrointest Endosc. 2014;6:555-563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 110] [Cited by in RCA: 119] [Article Influence: 10.8] [Reference Citation Analysis (2)] |
| 74. | Kaltenbach T, Soetikno R. Endoscopic resection of large colon polyps. Gastrointest Endosc Clin N Am. 2013;23:137-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 75. | Tanaka S, Kashida H, Saito Y, Yahagi N, Yamano H, Saito S, Hisabe T, Yao T, Watanabe M, Yoshida M. JGES guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc. 2015;27:417-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 437] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 76. | Asayama N, Oka S, Tanaka S, Hayashi N, Arihiro K, Chayama K. Endoscopic submucosal dissection as total excisional biopsy for clinical T1 colorectal carcinoma. Digestion. 2015;91:64-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 77. | Fujiya M, Tanaka K, Dokoshi T, Tominaga M, Ueno N, Inaba Y, Ito T, Moriichi K, Kohgo Y. Efficacy and adverse events of EMR and endoscopic submucosal dissection for the treatment of colon neoplasms: a meta-analysis of studies comparing EMR and endoscopic submucosal dissection. Gastrointest Endosc. 2015;81:583-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 269] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 78. | Hong MJ, Kim JH, Lee SY, Sung IK, Park HS, Shim CS. Prevalence and clinical features of coagulation syndrome after endoscopic submucosal dissection for colorectal neoplasms. Dig Dis Sci. 2015;60:211-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 79. | Horiuchi A, Tanaka N. Improving quality measures in colonoscopy and its therapeutic intervention. World J Gastroenterol. 2014;20:13027-13034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 80. | Anderloni A, Jovani M, Hassan C, Repici A. Advances, problems, and complications of polypectomy. Clin Exp Gastroenterol. 2014;7:285-296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 81. | Othman MO, Wallace MB. Endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) in 2011, a Western perspective. Clin Res Hepatol Gastroenterol. 2011;35:288-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 82. | Okamoto K, Kitamura S, Muguruma N, Takaoka T, Fujino Y, Kawahara Y, Okahisa T, Takayama T. Mucosectom2-short blade for safe and efficient endoscopic submucosal dissection of colorectal tumors. Endoscopy. 2013;45:928-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 83. | Kyzer S, Bégin LR, Gordon PH, Mitmaker B. The care of patients with colorectal polyps that contain invasive adenocarcinoma. Endoscopic polypectomy or colectomy? Cancer. 1992;70:2044-2050. [PubMed] |
| 84. | Minamoto T, Mai M, Ogino T, Sawaguchi K, Ohta T, Fujimoto T, Takahashi Y. Early invasive colorectal carcinomas metastatic to the lymph node with attention to their nonpolypoid development. Am J Gastroenterol. 1993;88:1035-1039. [PubMed] |
| 85. | Mou S, Soetikno R, Shimoda T, Rouse R, Kaltenbach T. Pathologic predictive factors for lymph node metastasis in submucosal invasive (T1) colorectal cancer: a systematic review and meta-analysis. Surg Endosc. 2013;27:2692-2703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 86. | Rex DK, Kahi CJ, Levin B, Smith RA, Bond JH, Brooks D, Burt RW, Byers T, Fletcher RH, Hyman N. Guidelines for colonoscopy surveillance after cancer resection: a consensus update by the American Cancer Society and US Multi-Society Task Force on Colorectal Cancer. CA Cancer J Clin. 2006;56:160-167; quiz 185-186. [PubMed] |
| 87. | Winawer SJ. Long-term follow-up after removal of colorectal adenomas provides evidence for risk stratification of patients at colonoscopic polypectomy. Evid Based Med. 2015;20:29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 88. | Wu XR, Liang J, Church JM. Management of sessile malignant polyps: is colonoscopic polypectomy enough? Surg Endosc. 2014;Epub ahead of print. [PubMed] |
| 89. | Seo JY, Chun J, Lee C, Hong KS, Im JP, Kim SG, Jung HC, Kim JS. Novel risk stratification for recurrence after endoscopic resection of advanced colorectal adenoma. Gastrointest Endosc. 2015;81:655-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 90. | Winawer SJ, Zauber AG, Fletcher RH, Stillman JS, O’brien MJ, Levin B, Smith RA, Lieberman DA, Burt RW, Levin TR. Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. CA Cancer J Clin. 2006;56:143-159; quiz 184-185. [PubMed] |
| 91. | Matsuda T, Kawano H, Chiu HM. Screening colonoscopy: What is the most reliable modality for the detection and characterization of colorectal lesions? Dig Endosc. 2015;27 Suppl 1:25-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 92. | Saito Y, Yamada M, So E, Abe S, Sakamoto T, Nakajima T, Otake Y, Ono A, Matsuda T. Colorectal endoscopic submucosal dissection: Technical advantages compared to endoscopic mucosal resection and minimally invasive surgery. Dig Endosc. 2014;26 Suppl 1:52-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 93. | Lee S, Moon CM, Kim YJ, Cho JH, Kim HM, Han KJ, Cho HG, Lee SW, Oh HE, Song JS. Diagnostic accuracy of narrow band imaging for predicting colon polyp histology can be affected by polyp characteristics. Hepatogastroenterology. 2013;60:1028-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 94. | Masci E, Mangiavillano B, Crosta C, Fiori G, Trovato C, Viaggi P, Zambelli A, Buffoli F, Staiano T, Manfredi G. Interobserver agreement among endoscopists on evaluation of polypoid colorectal lesions visualized with the Pentax i-Scan technique. Dig Liver Dis. 2013;45:207-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 95. | Iacucci M, Hassan C, Fort Gasia M, Urbanski S, Gui X, Eustace G, Kaplan G, Eksteen B, Panaccione R. Serrated adenoma prevalence in inflammatory bowel disease surveillance colonoscopy, and characteristics revealed by chromoendoscopy and virtual chromoendoscopy. Can J Gastroenterol Hepatol. 2014;28:589-594. [PubMed] |
| 96. | Li M, Ali SM, Umm-a-OmarahGilani S, Liu J, Li YQ, Zuo XL. Kudo’s pit pattern classification for colorectal neoplasms: a meta-analysis. World J Gastroenterol. 2014;20:12649-12656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 61] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 97. | Miyamoto H, Ikematsu H, Fujii S, Osera S, Odagaki T, Oono Y, Yano T, Ochiai A, Sasaki Y, Kaneko K. Clinicopathological differences of laterally spreading tumors arising in the colon and rectum. Int J Colorectal Dis. 2014;29:1069-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 98. | Kim JS, Lee BI, Choi H, Kang BK, Kim JI, Lee HM, Im EJ, Kim BW, Kim SW, Choi MG. Brief education on microvasculature and pit pattern for trainees significantly improves estimation of the invasion depth of colorectal tumors. Gastroenterol Res Pract. 2014;2014:245396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 99. | Sakamoto T, Matsuda T, Nakajima T, Saito Y, Fujii T. Impact of clinical experience on type V pit pattern analysis using magnifying chromoendoscopy in early colorectal cancer: a cross-sectional interpretation test. BMC Gastroenterol. 2014;14:100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |