Published online Apr 28, 2015. doi: 10.3748/wjg.v21.i16.4933
Peer-review started: September 30, 2014
First decision: October 29, 2014
Revised: November 11, 2014
Accepted: December 14, 2014
Article in press: December 16, 2014
Published online: April 28, 2015
Processing time: 209 Days and 2.3 Hours
AIM: To clarify the utility of using des-γ-carboxy prothrombin (DCP) and α-fetoprotein (AFP) levels to predict the prognosis of hepatocellular carcinoma (HCC) in patients with hepatitis B virus (HBV) and the hepatitis C virus (HCV) infections.
METHODS: A total of 205 patients with HCC (105 patients with HBV infection 100 patients with HCV infection) who underwent primary hepatectomy between January 2004 and May 2012 were enrolled retrospectively. Preoperative AFP and DCP levels were used to create interactive dot diagrams to predict recurrence within 2 years after hepatectomy, and cutoff levels were calculated. Patients in the HBV and HCV groups were classified into three groups: a group with low AFP and DCP levels (LL group), a group in which one of the two parameters was high and the other was low (HL group), and a group with high AFP and DCP levels (HH group). Liver function parameters, the postoperative recurrence-free survival rate, and postoperative overall survival were compared between groups. The survival curves were compared by log-rank test using the Kaplan-Meier method. Multivariate analysis using a Cox forward stepwise logistic regression model was conducted for a prognosis.
RESULTS: The preoperative AFP cutoff levels for recurrence within 2 years after hepatectomy in the HBV and HCV groups were 529.8 ng/mL and 60 mAU/mL, respectively; for preoperative DCP levels, the cutoff levels were 21.0 ng/mL in the HBV group and 67 mAU/mL in the HCV group. The HBV group was significantly different from the other groups in terms of vascular invasion, major hepatectomy, volume of intraoperative blood loss, and surgical duration. Significant differences were found between the LL group, the HL group, and the HH group in terms of both mean disease-free survival time (MDFST) and mean overall survival time (MOST): 64.81 ± 7.47 vs 36.63 ± 7.62 vs 18.98 ± 6.17 mo (P = 0.001) and 85.30 ± 6.55 vs 59.44 ± 7.87 vs 46.57 ± 11.20 mo (P = 0.018). In contrast, the HCV group exhibited a significant difference in tumor size, vascular invasion, volume of intraoperative blood loss, and surgical duration; however, no significant difference was observed between the three groups in liver function parameters except for albumin levels. In the LL group, the HL group, and the HH group, the MDFST was 50.09 ± 5.90, 31.01 ± 7.21, and 14.81 ± 3.08 mo (log-rank test, P < 0.001), respectively, and the MOST was 79.45 ± 8.30, 58.82 ± 7.56, and 32.87 ± 6.31 mo (log-rank test, P < 0.001), respectively.
CONCLUSION: In the HBV group, the prognosis was poor when either AFP or DCP levels were high. In the HCV group, the prognosis was good when either or both levels were low; however, the prognosis was poor when both levels were high. High levels of both AFP and DCP were an independent risk factor associated with tumor recurrence in the HBV and HCV groups. The relationship between tumor marker levels and prognosis was characteristic to the type of viral hepatitis.
Core tip: There is no consensus regarding using cutoff levels of tumor markers to predict survival and recurrence after hepatectomy for hepatocellular carcinoma. Furthermore, the prognostic characteristics of these tumor markers according to hepatitis type remain unclear. The α-fetoprotein (AFP) cutoff level for recurrence within 2 years after surgery was 21.0 ng/mL in the hepatitis C virus (HCV) group compared with 529.8 ng/mL in the hepatitis B virus (HBV) group. Furthermore, patients in the HBV group with high levels of either AFP or des-γ-carboxy prothrombin (DCP) had poor prognoses, as did those patients with high levels of both tumor markers. In contrast, only those patients in the HCV group who had high levels of both AFP and DCP had poor prognoses. We believe that to predict prognosis, preoperative levels of tumor markers should be distinguished and assessed according to the type of viral hepatitis.
- Citation: Meguro M, Mizuguchi T, Nishidate T, Okita K, Ishii M, Ota S, Ueki T, Akizuki E, Hirata K. Prognostic roles of preoperative α-fetoprotein and des-γ-carboxy prothrombin in hepatocellular carcinoma patients. World J Gastroenterol 2015; 21(16): 4933-4945
- URL: https://www.wjgnet.com/1007-9327/full/v21/i16/4933.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i16.4933
Chronic hepatitis caused by viral hepatitis often progresses to cirrhosis and hepatocellular carcinoma (HCC)[1]. In Asia, HCC is mainly caused by infection with the hepatitis B virus (HBV), whereas in western countries, it is characteristically caused by infection with the hepatitis C virus (HCV)[2]. The oncogenic mechanisms differ between the two virus types[3,4], and these mechanisms should be taken into consideration when evaluating prognosis and establishing treatment regimens.
Various staging systems have been developed to predict the survival for HCC patients, such as the tumor-node-metastasis[5], Okuda et al[6], the Cancer of the Liver Italian Program (CLIP)[7], Japan Integrated Staging (JIS)[8], and the Barcelona Clinic Liver Cancer[9] staging systems. These systems classify tumors according to tumor size, tumor number, vascular invasion, and metastatic regions (regardless of whether they are intrahepatic or extrahepatic metastases). Each of these parameters is closely associated with the overall prognosis of HCC patients[5-9].
Liver function parameters are also important prognostic factors for HCC. In fact, the Okuda[6], CLIP[7], and JIS[8] staging systems consider both tumor extension and liver function parameters in tumor classification. Accordingly, it has been reported that tumor-related factors and liver function parameters are also both closely associated with the prognosis of patients with HCC[6-10].
In addition to these factors that impact tumorigenesis and liver function, α-fetoprotein (AFP) and des-γ-carboxy prothrombin (DCP) levels are tumor markers and known prognostic factors for HCC[11,12]. Discovered by Abelev et al[13] in 1963, AFP is a glycoprotein with an albumin-like structure produced by liver cells and in the yolk sac during the fetal stage with a half-life of 4-6 d and a molecular weight of 65 kDa. AFP production in the liver is increased in hepatocellular carcinoma as well as in chronic hepatitis and cirrhosis[14]; therefore, AFP is considered to have low specificity for the diagnosis of cancer. In contrast, prothrombin is formed after the γ-carboxylation of vitamin K-dependent propeptides, and DCP is produced as a result of an acquired posttranslational defect in the vitamin K-dependent carboxylase system. According to a 1984 report by Liebman et al[15], DCP has a molecular weight of 72 kDa and a half-life of 40-72 h. DCP production does not increase in chronic hepatitis or cirrhosis, and DCP is considered to have high specificity for the diagnosis of cancer. However, DCP has no prognostic value in cases with vitamin K deficiency or vitamin K function inhibition.
Although the associations between these tumor markers and postoperative prognosis in HCC patients have been reported[16-18], there is no consensus regarding the cutoff levels of these markers to predict survival and recurrence after hepatectomy. Furthermore, the prognostic characteristics of these tumor markers according to hepatitis type remain unclear. Therefore, the aim of the present study was both to compare preoperative tumor marker levels and prognosis after hepatectomy and to clarify the characteristics of these tumor marker levels according to hepatitis type. The prognostic findings associated with these tumor marker levels could inform the development of new selection criteria for living donor liver transplantation candidates with HCC, especially beyond the Milan criteria[19].
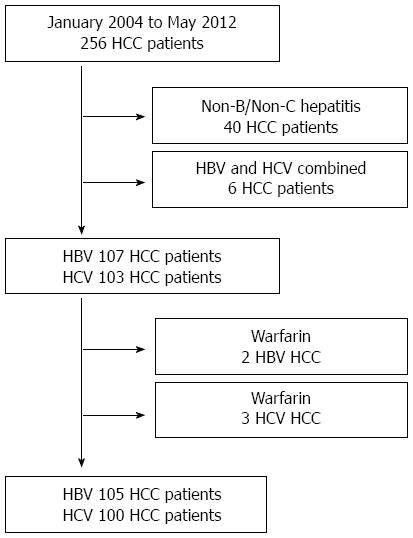
We retrospectively reviewed the medical records of 256 consecutive HCC patients who underwent primary hepatectomy at the Sapporo Medical University Hospital (Sapporo, Japan) from January 2004 to May 2012. Using the Child-Pugh classification system[20], the volume of resectable liver was determined, including the following items: prothrombin time (PT), serum total bilirubin (TBIL) levels, and serum albumin (ALB) levels, all of which are measured on both preoperative function tests and the indocyanine green retention rate at 15 min (ICGR15)[21] as measured by three-dimensional computed tomography. AFP and DCP levels were measured immediately before surgery. The present study excluded 40 patients with non-B/non-C hepatitis and six patients with concurrent HBV and HCV infections. In addition, two patients in the HBV group and three in the HCV group receiving warfarin were also excluded. The final sample included 105 patients with HBV infection and 100 with HCV infection (Figure 1).
The operation type was classified as follows: partial hepatic resection including tumor enucleation (Hr0); subsegmentectomy (HrS); monosegmentectomy (Hr1); bisegmentectomy, including right hepatectomy, left hepatectomy, and central bisegmentectomy (Hr2); and trisegmentectomy (Hr3). Hr0, HrS, and Hr1 were defined as minor hepatectomy, whereas Hr2 and Hr3 resections were considered major hepatectomy. Red cell concentrate transfusion was administered at the discretion of the anesthetist on the basis of intraoperative factors, such as systemic hemodynamic factors, hemoglobin levels, and blood lactate levels on blood gas analysis. Following hepatectomy, the number of tumors, tumor size, presence or absence of vascular invasion, and condition of underlying liver tissue (normal liver, chronic hepatitis, and liver cirrhosis) were determined.
All patients were followed up every 3 mo until the end of March 2013, until their last visit to our hospital, or until death. The study design conformed to the ethical guidelines of the Declaration of Helsinki, and informed consent was obtained from each subject before registration.
The surgery included total and pure laparoscopic procedures and laparoscopically assisted approaches. We used either five or six ports (5-12 mm in diameter) depending on the tumor location, and the first periumbilical port for the laparoscopic camera was inserted using the open technique. If the volume of blood loss exceeded 300 mL from any of these ports, the Pringle maneuver[22] was performed for hepatectomy. The procedures were performed under carbon dioxide pneumoperitoneum, and intra-abdominal pressure was maintained at < 12 mmHg based on electronic readings. We used a variable view angle, high-definition endoscopic camera. Intraoperative ultrasonography (BK Medical, Herlev, Denmark) was performed routinely to examine the location and diameter of the hepatic tumor as well as the positional relationship of the tumor with the main hepatic vessels. Parenchymal transection and hemostasis were performed with a laparoscopic Cavitron ultrasonic surgical aspirator (CUSA; Valleylab, Boulder, CO, United States), a harmonic scalpel (UltraCision; Ethicon Endo-Surgery, Inc., Blue Ash, OH, United States), saline-associated monopolar electrocautery, and a thermofusion device (BiClamp; ERBE, Marietta, GA, United States). The resected specimen was placed in a plastic bag and extracted through a slightly enlarged periumbilical port site or additional minilaparotomy. For open laparotomy, right subcostal, upper middle, or inverted L-shaped or T-shaped incisions were made depending on tumor location. Intraoperative ultrasonography (Hitachi-Aloka Medical, Ltd., Tokyo, Japan) was performed routinely. Parenchymal transection and hemostasis were performed primarily using a CUSA, saline-associated monopolar electrocautery, and an absorbable fibrin sealant patch (Tachosil, Baxter Healthcare Corporation, Irvine, CA, United States) as necessary.
Data are presented as medians (25th-75th percentile range) for skewed distributions and as mean ± SD for normal distributions. The Pearson χ2 analysis or Fisher exact test was used to compare categorical variables, whereas the Kruskal-Wallis, Mann-Whitney U test, or analysis of variance was used for comparisons of continuous variables. Recurrence-free survival or overall survival rates were estimated using the Kaplan-Meier method and compared using the log-rank test. A P value < 0.05 was considered statistically significant. Variables with statistical significance (P < 0.05) in the univariate analysis were subjected to multivariate analysis using a Cox forward stepwise logistic regression model. Statistical analysis was performed using StatView® software (version 5.0; SAS Institute Inc., Cary, NC, United States) and SPSS version 21.0 for Windows (IBM-SPSS Inc., Chicago, IL, United States). Interactive dot diagrams were created using MedCalc® software (version 10.2.0.0; Mariakerke, Ostend, Belgium).
The clinical characteristics of all patients in the HBV group (n = 105) are shown in Table 1. The median age was 62 years, and there were more males than females. Major hepatectomy was performed in 16 patients (15.2%), and anatomical resection was performed in 55 patients (52.4%). Preoperative liver function was good in most patients, with a median ICGR15 level of 9.6%. The median preoperative levels of AFP and DCP were 24.2 ng/mL and 38 mAU/mL, respectively. The median volume of intraoperative blood loss was 400 mL and median surgical duration was 332 min. The mean tumor size was 4.27 ± 3.21 cm; 39 patients (37.1%) had multiple tumors, and vascular invasion was observed in 34 patients (32.4%). Sixty patients (61.0%) presented with either normal liver function or chronic hepatitis. Fifty patients (47.6%) developed recurrence within 2 years after surgery.
| Variables | All patients (n = 105) | LL group (n = 55) | HL group (n = 34) | HH group (n = 16) | P value |
| Age (yr) | 62 (57-68) | 62 (59-66) | 64 (56-70) | 63 (56-70) | 0.962 |
| Sex, male/female | 90/15 | 48/7 | 29/5 | 13/3 | 0.829 |
| Body mass index (kg/m2) | 23.7 ± 3.1 | 24.0 ± 2.6 | 23.5 ± 3.3 | 23.1 ± 4.3 | 0.691 |
| Open/pure lap/lap-assisted | 83/8/14 | 43/6/6 | 29/1/4 | 11/1/4 | 0.390 |
| Hr | |||||
| 0/S/1/2/3 | 50/25/14/14/2 | 34/13/6/2/0 | 14/10/4/4/2 | 2/2/4/8/0 | < 0.001 |
| Preoperative laboratory values | |||||
| Aspartate transaminase (U/L) | 31 (22-46) | 31 (22-41) | 30 (25-48) | 43 (24-62) | 0.153 |
| Alanine transaminase (U/L) | 29 (20-39) | 32 (17-42) | 27 (21-42) | 27 (19-52) | 0.978 |
| Platelets (× 104/μL) | 16.6 ± 13.3 | 16.4 ± 12.7 | 16.2 ± 16.2 | 18.0 ± 8.6 | 0.168 |
| Serum ALB (g/dL) | 3.95 ± 0.46 | 3.99 ± 0.46 | 3.87 ± 0.43 | 3.98 ± 0.53 | 0.334 |
| PT (%) | 93.3 ± 12.2 | 95.5 ± 11.1 | 90.3 ± 14.1 | 92.2 ± 10.6 | 0.062 |
| Serum TBIL (mg/dL) | 0.79 ± 0.44 | 0.80 ± 0.45 | 0.85 ± 0.49 | 0.65 ± 0.24 | 0.681 |
| ICGR15 | 9.6 (5.7-14.9) | 9.4 (5.9-14.0) | 12.5 (5.7-22.5) | 8.5 (5.0-14.6) | 0.334 |
| AFP (ng/mL) | 24.2 (3.8-165.0) | 5.2 (2.4-31.1) | 45.0 (5.1-246.5) | 1950 (753.5-2827.0) | < 0.001 |
| DCP (mAU/mL) | 38 (20-364) | 22 (15-28) | 399 (90-1,667) | 4460 (223-34373) | < 0.001 |
| Intraoperative data | |||||
| Blood loss (mL) | 400 (130-660) | 360 (110-560) | 435 (100-1,040) | 880 (130-1330) | 0.042 |
| Surgical duration (min) | 332 (243-411) | 313 (236-370) | 361 (243-450) | 432 (270-604) | 0.020 |
| Blood transfusion | |||||
| RCC: yes/no (%) | 21/84 (20.0%) | 4/51 (7.2%) | 10/24 (29.4%) | 7/9 (43.8%) | 0.001 |
| Pathological results | |||||
| Tumor size (cm) | 4.27 ± 3.21 | 3.26 ± 2.56 | 4.97 ± 2.71 | 6.24 ± 4.77 | < 0.001 |
| Multiple tumors: yes/no (%) | 39/66 (37.1%) | 19/36 (34.5%) | 15/19 (44.1%) | 5/11 (31.3%) | 0.575 |
| Vascular invasion: yes/no (%) | 34/71 (32.4%) | 10/45 (18.2%) | 14/20 (41.1%) | 10/6 (62.5%) | 0.002 |
| Remnant liver: NL/CH/LC | 13/47/41 | 6/23/26 | 3/17/14 | 4/7/5 | 0.464 |
| Histological tumor differentiation | |||||
| Well/moderately/poorly | 14/63/28 | 11/35/9 | 3/18/13 | 0/10/6 | 0.048 |
| Recurrence within 2 yr after liver resection | |||||
| Yes/no (%) | 50/55 (47.6%) |
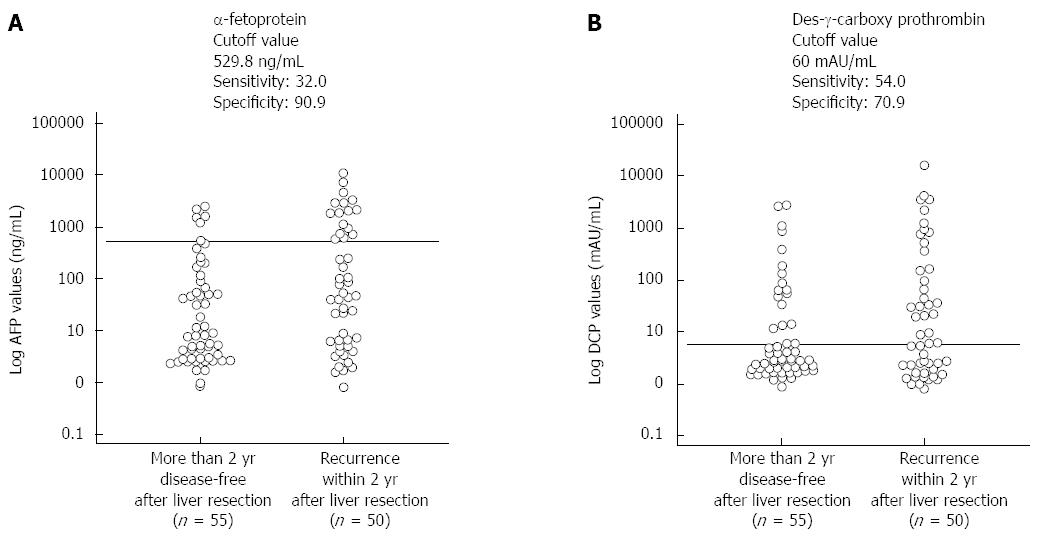
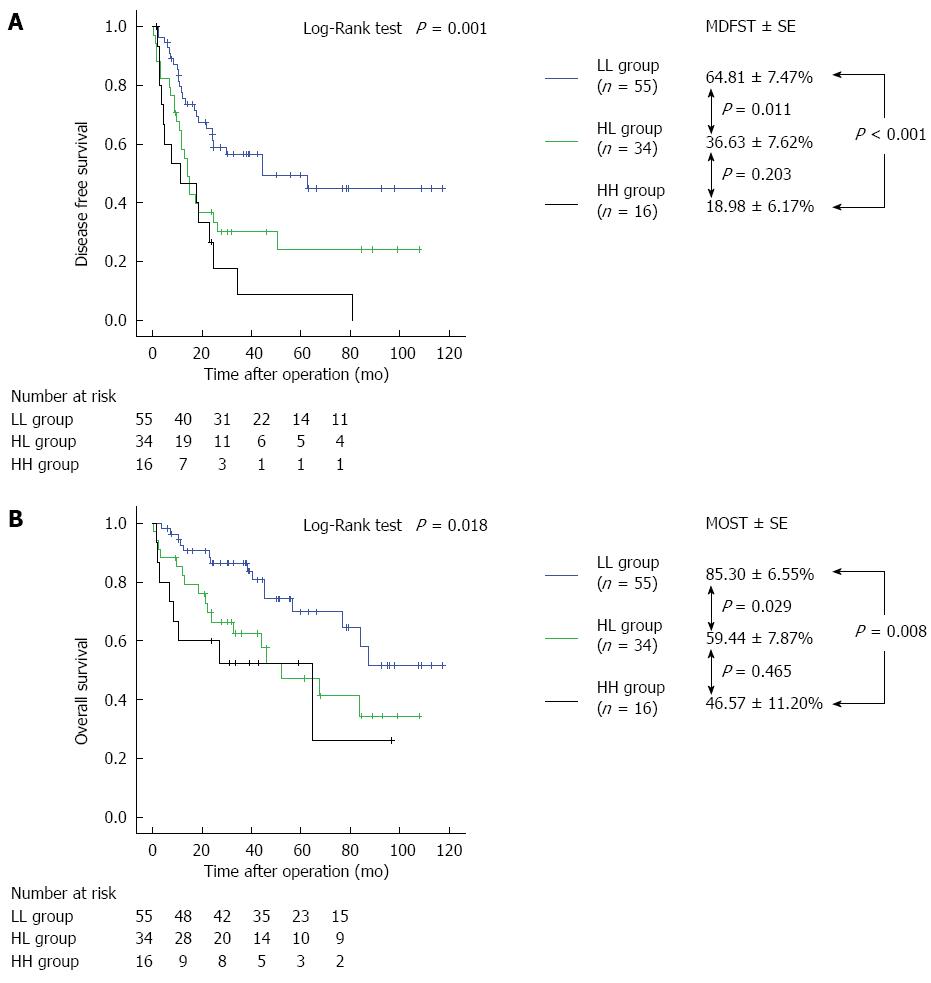
The preoperative AFP and DCP cutoff levels for recurrence within 2 years of hepatectomy calculated using an interactive dot diagram were 529.8 ng/mL (sensitivity, 32.0%; specificity, 90.0%) and 60 mAU/mL (sensitivity, 54.0%; specificity, 70.9%), respectively (Figure 2). Table 1 depicts the characteristics of patients in three groups when these cutoff levels were used as the reference levels: a group with low AFP and DCP levels (LL group, n = 55), a group in which one of the two parameters was high and the other was low [high and low (HL) group, n = 34], and a group with high AFP and DCP levels (HH group, n = 16). The rates of anatomical resection in the LL, HL, and HH groups were significantly different (38.2%, 58.8%, and 87.5%; P = 0.002). There were no significant differences observed between groups in any preoperative liver function parameter, including aspartate transaminase levels, alanine transaminase levels, platelet count, serum ALB, serum TBIL, PT, and ICGR15 levels. However, there were significant differences in AFP levels (P < 0.001), DCP levels (P < 0.001), volume of intraoperative blood loss (P = 0.042), surgical duration (P = 0.020), rate of intraoperative transfusion (P = 0.001), tumor size (P < 0.001), vascular invasion (P = 0.002), and histological tumor differentiation (P = 0.048). The mean disease-free survival time (MDFST) in the LL, HL, and HH groups was 64.81 ± 7.47, 36.63 ± 7.62, and 18.98 ± 6.17 mo, respectively (log-rank test, P = 0.001); the mean overall survival time (MOST) in the LL, HL, and HH groups was 85.30 ± 6.55, 59.44 ± 7.87, and 46.57 ± 11.20 mo, respectively (log-rank test, P = 0.018). Significant differences were observed between all groups on both measures (Figure 3).
Our univariate and multivariate analyses to determine the risk factors associated with tumor recurrence after hepatectomy in the patients with HBV infection (n = 105) are shown in Table 2. The multivariate analysis revealed that high levels of TBIL (P = 0.004), HH group (P = 0.031), large tumor size (P = 0.003), and the presence of vascular invasion (P = 0.002) were associated with significantly higher incidences of tumor recurrence after liver resection.
| Variables | Univariate analysis | Multivariate analysis | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Age | 0.977 | 0.952-1.003 | 0.086 | |||
| Sex, male | 1.077 | 0.512-2.264 | 0.843 | |||
| Preoperative laboratory values | ||||||
| Aspartate transaminase | 1.008 | 1.003-1.013 | 0.0011 | 1.001 | 0.993-1.010 | 0.778 |
| Alanine transaminase | 0.999 | 0.989-1.009 | 0.811 | |||
| Platelets | 1.001 | 0.979-1.024 | 0.929 | |||
| Serum ALB | 0.648 | 0.367-1.142 | 0.134 | |||
| PT | 0.974 | 0.953-0.996 | 0.0211 | 0.976 | 0.946-1.007 | 0.124 |
| Serum TBIL | 2.305 | 1.346-3.947 | 0.0021 | 4.068 | 1.586-10.436 | 0.0041 |
| ICGR15 | 1.033 | 1.008-1.059 | 0.0111 | 0.967 | 0.919-1.018 | 0.967 |
| Tumor markers | ||||||
| HH group | 3.235 | 1.673-6.257 | < 0.0011 | 2.464 | 1.086-5.587 | 0.0311 |
| HL group | 2.042 | 1.164-3.583 | 0.0131 | 1.083 | 0.497-2.360 | 0.841 |
| Intraoperative data | ||||||
| Blood loss | 1.001 | 1.000-1.001 | 0.0021 | 1.000 | 1.000-1.001 | 0.416 |
| Surgical duration | 1.002 | 1.000-1.004 | 0.0031 | 1.000 | 0.997-1.003 | 0.868 |
| Pathologic results | ||||||
| Tumor size | 1.148 | 1.068-1.234 | < 0.0011 | 1.182 | 1.059-1.318 | 0.0031 |
| Multiple tumors | 1.363 | 1.152-1.612 | < 0.0011 | 1.336 | 0.955-1.869 | 0.091 |
| Vascular invasion | 2.088 | 1.261-3.458 | 0.0041 | 2.624 | 1.408-4.892 | 0.0021 |
Our univariate and multivariate analyses to determine the risk factors associated with poor overall survival after hepatectomy in patients with HBV infection (n = 105) are shown in Table 3. The multivariate analysis revealed that the presence of vascular invasion (P = 0.008) was associated with significantly higher incidences of poor overall survival after liver resection.
| Variables | Univariate analysis | Multivariate analysis | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Age | 0.996 | 0.964-1.028 | 0.797 | |||
| Sex, male | 1.440 | 0.511-4.056 | 0.490 | |||
| Preoperative laboratory values | ||||||
| Aspartate transaminase | 1.008 | 1.002-1.014 | 0.0121 | 1.004 | 0.992-1.016 | 0.518 |
| Alanine transaminase | 0.992 | 0.976-1.007 | 0.296 | |||
| Platelets | 1.015 | 0.994-1.035 | 0.158 | |||
| Serum ALB | 0.469 | 0.234-0.939 | 0.033 | |||
| PT | 0.975 | 0.950-1.000 | 0.051 | |||
| Serum TBIL | 2.249 | 1.125-4.495 | 0.0221 | 1.584 | 0.447-5.613 | 0.476 |
| ICGR15 | 1.056 | 1.025-1.087 | < 0.0011 | 1.040 | 0.976-1.108 | 0.229 |
| Tumor markers | ||||||
| HH group | 2.985 | 1.259-7.075 | 0.0131 | 2.274 | 0.698-7.403 | 0.173 |
| HL group | 2.112 | 1.054-4.232 | 0.0351 | 1.025 | 0.390-2.693 | 0.960 |
| Intraoperative data | ||||||
| Blood loss | 1.001 | 1.001-1.001 | < 0.0011 | 1.000 | 1.000-1.001 | 0.391 |
| Surgical duration | 1.004 | 1.001-1.008 | 0.0051 | 1.002 | 0.996-1.007 | 0.534 |
| Pathologic results | ||||||
| Tumor size | 1.239 | 1.133-1.356 | < 0.0011 | 1.127 | 0.969-1.310 | 0.122 |
| Multiple tumors | 1.385 | 1.121-1.711 | 0.0021 | 1.200 | 0.813-1.772 | 0.358 |
| Vascular invasion | 2.491 | 1.335-4.647 | 0.0041 | 3.173 | 1.352-7.447 | 0.0081 |
The clinical characteristics of all patients in the HCV group (n = 100) are shown in Table 4. The median age was 71 years, and there were more males than females. Major hepatectomy of type Hr2 or higher was performed in five patients (5.0%), and anatomical resection combining subsegmentectomy of the liver, segmentectomy of the liver, and hepatic lobe resection was performed in 43 patients (43.0%). Many patients had poor preoperative liver function, and 58 patients (58.0%) had liver cirrhosis with a median ICGR15 level of 14.2%. The median preoperative levels of AFP and DCP were 18.6 ng/mL and 45 mAU/mL, respectively. The median volume of intraoperative blood loss was 330 mL, and the median surgical duration was 304 min. The mean tumor size was 3.51 ± 2.48 cm, and multiple tumors were observed in 36 patients (36.0%). Furthermore, vascular invasion was observed in 30 patients (30.0%), and 51 patients (51.0%) developed recurrence within 2 years after hepatectomy.
| Variables | All patients (n = 100) | LL group (n = 34) | HL group (n = 39) | HH group (n = 27) | P value |
| Age (yr) | 71 (62-77) | 72 (64-77) | 70 (62-75) | 70 (65-77) | 0.766 |
| Sex, male/female | 82/18 | 22/12 | 29/10 | 16/11 | 0.413 |
| Body mass index | 23.6 ± 3.6 | 23.6 ± 2.9 | 23.8 ± 3.8 | 23.4 ± 4.1 | 0.737 |
| Open/Pure Lap/Lap-assisted | 77/12/11 | 26/3/5 | 27/8/4 | 24/1/2 | 0.218 |
| Hr | |||||
| 0/S/1/2/3 | 57/27/11/2/3 | 23/8/3/0/0 | 21/10/5/1/2 | 13/9/3/1/1 | 0.784 |
| Preoperative laboratory values | |||||
| Aspartate transaminase (U/L) | 47 (30-64) | 41 (23-56) | 48 (35-67) | 53 (47-71) | 0.101 |
| Alanine transaminase (U/L) | 38 (26-57) | 33 (21-53) | 41 (28-57) | 42 (29-62) | 0.496 |
| Platelets (× 104/μL) | 13.5 ± 6.8 | 13.9 ± 4.7 | 13.9 ± 9.2 | 12.5 ± 4.4 | 0.804 |
| Serum ALB (g/dL) | 3.76 ± 0.39 | 3.94 ± 0.40 | 3.67 ± 0.35 | 3.67 ± 0.38 | 0.0061 |
| PT (%) | 89.8 ± 13.4 | 92.5 ± 11.4 | 87.8 ± 15.0 | 89.2 ± 13.5 | 0.254 |
| Serum TBIL (mg/dL) | 0.78 ± 0.34 | 0.74 ± 0.32 | 0.79 ± 0.33 | 0.81 ± 0.39 | 0.777 |
| ICGR15 | 14.2 (8.4-18.5) | 12.1 (8.1-15.4) | 14.7 (8.4-21.5) | 15.6 (13.0-24.6) | 0.163 |
| AFP (ng/mL) | 18.6 (5.6-134.0) | 5.3 (3.8-11.0) | 21.1 (6.9-99.2) | 299.7 (68.1-1046.0) | < 0.0011 |
| DCP (mAU/mL) | 45 (21-244) | 22 (15-35) | 37 (23-134) | 429 (124-1902) | < 0.0011 |
| Intraoperative data | |||||
| Blood loss (mL) | 330 (20-650) | 263 (70-500) | 230 (20-500) | 535 (300-1270) | 0.0061 |
| Surgical duration (min) | 304 (230-377) | 303 (175-377) | 296 (230-352) | 345 (282-475) | 0.0401 |
| Blood transfusion | |||||
| RCC: yes/no (%) | 12/88 (12.0%) | 3/31 (8.8%) | 2/37 (5.1%) | 7/20 (25.9%) | 0.0301 |
| Pathologic results | |||||
| Tumor size (cm) | 3.51 ± 2.48 | 2.84 ± 1.23 | 3.26 ± 2.31 | 4.72 ± 3.39 | 0.0081 |
| Multiple tumor: yes/no (%) | 36/64 (36.0%) | 8/26 (23.5%) | 17/22 (43.6%) | 11/16 (40.7%) | 0.171 |
| Vascular invasion: yes/no (%) | 30/70 (30.0%) | 5/29 (14.7%) | 12/27 (30.8%) | 13/14 (48.1%) | 0.0181 |
| Remnant liver: NL/CH/LC | 5/37/58 | 2/18/14 | 2027/10/2 | 2017/9/1 | 0.161 |
| Histological tumor differentiation | |||||
| Well/Moderately/Poorly | 17/63/20 | 7/23/4 | 8/24/7 | 2/16/9 | 0.213 |
| Recurrence within 2 yr after hepatectomy | |||||
| Yes/no (%) | 51/49 (51.0%) |
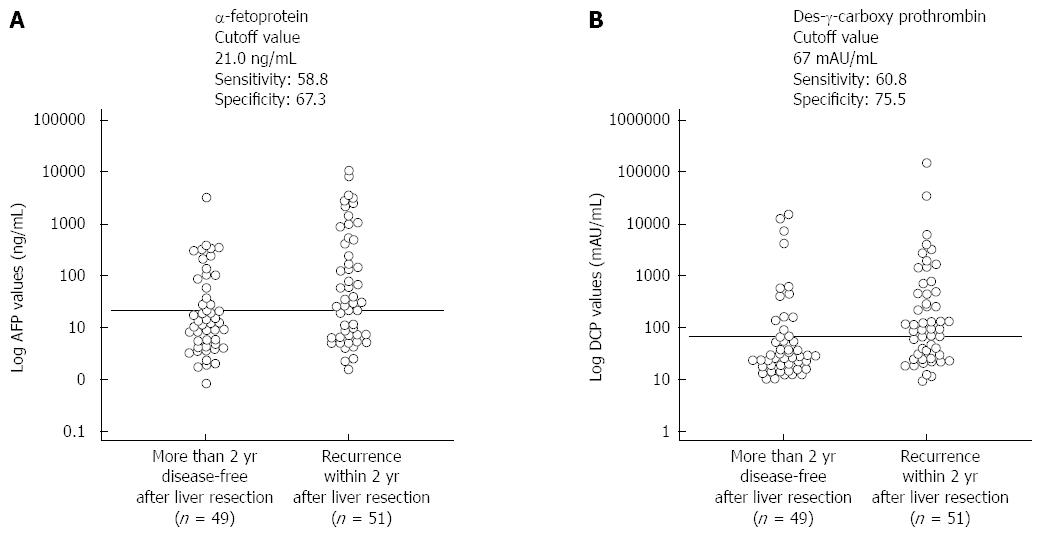
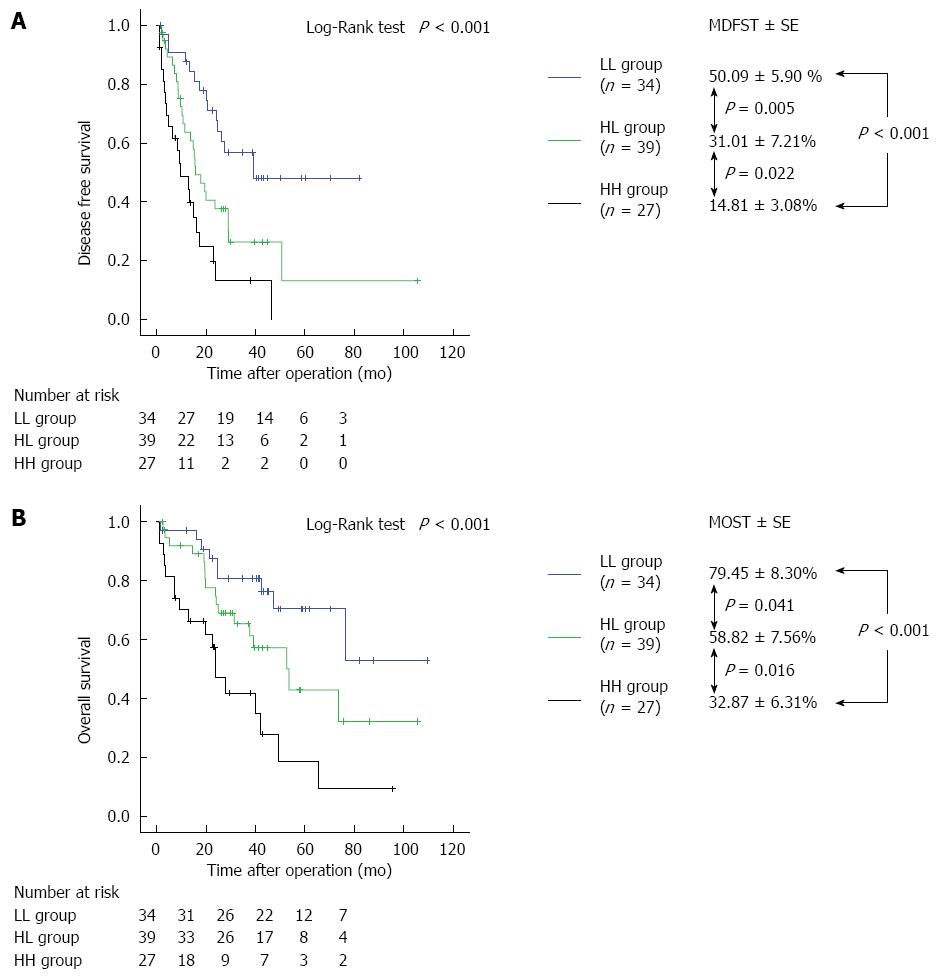
The preoperative cutoff levels for recurrence within 2 years after hepatectomy, as calculated using an interactive dot diagram, were 21.0 ng/mL for AFP (sensitivity, 58.8%; specificity, 67.3%) and 67 mAU/mL for DCP (sensitivity, 60.8%; specificity, 75.5%) (Figure 4). Table 4 summarizes the characteristics of HCC patients with HCV infection in the LL group (n = 34), HL group (n = 39), and HH group (n = 27) with these cutoff levels as baselines. There were no significant differences observed in pathological background (P = 0.161), type of operation (P = 0.784), or histological differentiation (P = 0.213). There were significant differences in preoperative ALB levels (P = 0.006); however, no significant difference was observed for other laboratory variables. There were also significant differences between groups in AFP levels (P < 0.001), DCP levels (P < 0.001), volume of intraoperative blood loss (P = 0.006), surgical duration (P = 0.040), intraoperative transfusion rate (P = 0.030), tumor size (P = 0.008), and vascular invasion (P = 0.018). MDFST and MOST for the LL, HL, and HH groups were 50.09 ± 5.90, 31.01 ± 7.21, and 14.81 ± 3.08 mo (log-rank test, P < 0.001), respectively, and 79.45 ± 8.30, 58.82 ± 7.56, and 32.87 ± 6.31 mo (log-rank test, P < 0.001), respectively, with a significant difference observed between all groups (Figure 5).
Our univariate and multivariate analyses to determine the risk factors associated with tumor recurrence after hepatectomy in the patients with HCV infection (n = 100) are shown in Table 5. The multivariate analysis revealed that high levels of ICGR15 (P < 0.001), HH group (P < 0.001), HL group (P = 0.032), long surgical duration (P = 0.031), large tumor size (P = 0.012), and the presence of multiple tumors (P = 0.018) were associated with significantly higher incidences of tumor recurrence after liver resection.
| Variables | Univariate analysis | Multivariate analysis | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Age | 1.001 | 0.972-1.031 | 0.950 | |||
| Sex, male | 1.455 | 0.823-2.570 | 0.197 | |||
| Preoperative laboratory values | ||||||
| Aspartate transaminase | 1.005 | 0.999-1.011 | 0.134 | |||
| Alanine transaminase | 1.003 | 0.966-1.010 | 0.412 | |||
| Platelets | 1.015 | 0.983-1.049 | 0.363 | |||
| Serum ALB | 0.569 | 0.297-1.092 | 0.090 | |||
| PT | 0.999 | 0.980-1.019 | 0.943 | |||
| Serum TBIL | 1.281 | 0.610-2.692 | 0.513 | |||
| ICGR15 | 1.049 | 1.017-1.081 | 0.0021 | 1.069 | 1.032-1.108 | < 0.0011 |
| Tumor markers | ||||||
| HH group | 4.427 | 2.238-8.760 | < 0.0011 | 5.098 | 2.165-12.005 | < 0.0011 |
| HL group | 2.210 | 1.166-4.189 | 0.0151 | 2.325 | 1.077-5.018 | 0.0321 |
| Intraoperative data | ||||||
| Blood loss | 1.001 | 1.000-1.001 | < 0.0011 | 1.000 | 0.999-1.000 | 0.292 |
| Surgical duration | 1.003 | 1.002-1.004 | < 0.0011 | 1.002 | 0.998-1.006 | 0.129 |
| Pathologic results | ||||||
| Tumor size | 1.290 | 1.178-1.413 | < 0.0011 | 1.160 | 1.033-1.302 | 0.0121 |
| Multiple tumors | 1.264 | 1.073-1.487 | 0.0051 | 1.264 | 1.041-1.534 | 0.0181 |
| Vascular invasion | 2.454 | 1.479-4.071 | 0.0011 | 1.597 | 0.890-2.867 | 0.117 |
Our univariate and multivariate analyses to determine the risk factors associated with poor overall survival after hepatectomy in patients with HCV infection (n = 100) are shown in Table 6. The multivariate analysis revealed that high levels of ICGR15 (P = 0.047), HH group (P = 0.009), and the presence of multiple tumors (P = 0.028) were associated with significantly higher incidences of poor overall survival after liver resection.
| Variables | Univariate analysis | Multivariate analysis | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Age | 1.025 | 0.987-1.065 | 0.195 | |||
| Sex, male | 1.328 | 0.668-2.642 | 0.418 | |||
| Preoperative laboratory values | ||||||
| Aspartate transaminase | 1.006 | 0.999-1.013 | 0.106 | |||
| Alanine transaminase | 1.001 | 0.992-1.009 | 0.849 | |||
| Platelets | 1.036 | 0.995-1.077 | 0.084 | |||
| Serum ALB | 0.246 | 0.114-0.531 | < 0.0011 | 0.611 | 0.219-1.706 | 0.347 |
| PT | 0.994 | 0.971-1.018 | 0.643 | |||
| Serum TBIL | 1.206 | 0.523-2.780 | 0.660 | |||
| ICGR15 | 1.054 | 1.021-1.087 | 0.0011 | 1.042 | 1.001-1.084 | 0.0471 |
| Tumor markers | ||||||
| HH group | 4.562 | 2.031-10.248 | < 0.0011 | 4.018 | 1.424-11.337 | 0.0091 |
| HL group | 2.049 | 0.911-4.609 | 0.083 | 2.209 | 0.829-5.885 | 0.113 |
| Intraoperative data | ||||||
| Blood loss | 1.001 | 1.000-1.001 | < 0.0011 | 1.000 | 0.999-1.001 | 0.727 |
| Surgical duration | 1.003 | 1.002-1.004 | < 0.0011 | 1.002 | 0.998-1.005 | 0.414 |
| Pathologic results | ||||||
| Tumor size | 1.285 | 1.162-1.421 | < 0.0011 | 1.081 | 0.946-1.235 | 0.255 |
| Multiple tumors | 1.204 | 1.021-1.420 | 0.0271 | 1.257 | 1.025-1.542 | 0.0281 |
| Vascular invasion | 2.223 | 1.221-4.045 | 0.0091 | 1.089 | 0.490-2.421 | 0.835 |
We investigated the clinical correlations between prognosis in HCC patients who underwent initial hepatectomy and levels of the tumor markers AFP and DCP. There was a significant difference between the HBV and HCV groups in the AFP and DCP cutoff levels to predict recurrence within 2 years after hepatectomy. In the HBV group, high AFP or DCP levels were sufficient to predict poor prognosis. In contrast, a low level of either or both tumor markers was correlated with a good prognosis in the HCV group. However, when levels of both markers were high, prognosis was poor in both the HBV and HCV groups. Furthermore, HH group membership was an independent risk factor associated with tumor recurrence in both the HBV and HCV group.
It has been reported that among HCC patients who acquired HBV infection at a relatively young age (approximately 60 years), many do not have liver cirrhosis and have normal liver function[23-25]. In the HBV group in the present study (Table 1), the median patient age was 62 years, the median ICGR15 was 9.6%, and liver function parameters were good, with median ALB levels, PTs, and TBIL levels of 3.95 ± 0.46 g/dL, 93.3% ± 12.2%, and 0.79 ± 0.44 mg/dL, respectively. The literature finds that few HCC patients with HBV infection present with liver cirrhosis[23-25]. Similarly, in this study, relatively few patients in the HBV group had liver cirrhosis [41 patients (39.0%)], and the platelet count, which is decreased in cirrhosis, was maintained at 16.6 × 104/μL (Table 1). In the present study, the subjects in the HBV group were comparable to subjects in other reports to date; thus, we believe that this sample was not biased.
A study evaluating the relationship between prognosis and levels of AFP and DCP in 1447 HCC patients used AFP and DCP cutoff levels randomly set at 400 ng/mL and 100 mAU/mL, respectively. The patients with high levels of both AFP and DCP had poor prognoses, which is similar to the findings of our study. These cutoff levels were similar to the levels in our HBV group. In addition, of the 1447 patients in the abovementioned report, 1048 had HBV-induced HCC[26]. The cutoff levels commonly used to diagnose HCC are 20 ng/mL to 200 ng/mL for AFP and 40 mAU/mL to 100 mAU/mL for DCP[27-30]. The cutoff levels to predict cancer prognosis are almost twice the levels used for cancer diagnosis. Therefore, once cancer develops, treatment should be administered before the tumor marker levels increase to levels indicative of poor prognosis.
MDFST and MOST in the HBV group were significantly different from the other groups in all analyses; however, there was no significant difference in MDFST and MOST between the HH and HL groups (P = 0.203 and P = 0.465, respectively) (Figure 3). The HH group had a relatively large median tumor size of 6.24 ± 4.77 cm, and there was a high frequency of patients with vascular invasion (10/16 patients, 62.5%). Tumor size was smaller in the HL group than in the HH group, and there was less vascular invasion. However, the prevalence of poorly differentiated HCC on histological examination in the HL group was comparable to that in the HH group. Moreover, the liver function parameters, including the ICGR15 level, ALB level, PT, and TBIL level, were worse in the HL group than in the HH group. Although the underlying mechanisms of poor liver function in the HL group remain unclear, poor liver function may explain the similar long-term prognoses found in the HH and HL groups. It is assumed that when the HBV group had high levels of either AFP or DCP, liver function parameters strongly affected prognosis.
Studies suggest that liver function decreases and liver cirrhosis progresses with age in many HCC patients with HCV infection[31,32]. In the present study, the median age in the HCV group was 71 years, and many patients had decreased liver function parameters (Table 4). Furthermore, the majority of patients (58/100, 58.0%) had liver cirrhosis (Table 4), and the characteristics of subjects in the HCV group were similar to the characteristics of subjects in other studies; thus, we believe that there was no selection bias.
In the HCV group, the AFP and DCP cutoff levels for recurrence within 2 years after hepatectomy were 21.0 ng/mL and 67 mAU/mL, respectively (Figure 4). The specificity of AFP levels to predict recurrence within 2 years after hepatectomy was relatively low (67.3%), which is similar to the results of other studies[33]. Furthermore, the DCP cutoff level was approximately the same as that for the HBV group. The lack of association between DCP cutoff level and the underlying virus may be because unlike the AFP level, the DCP level is typically not elevated in chronic hepatitis or liver cirrhosis[14]; thus, it is not affected by liver function parameters, but rather fluctuates specifically in response to tumor marker levels. The AFP cutoff level varied between the HCV and HBV groups. However, the lens culinaris agglutinin-reactive fraction of AFP (AFP-L3) has been shown to have a high specificity for a cancer diagnosis[34]. We believe that the AFP-L3 cutoff level would have been similar in the HCV and HBV groups.
MDFST and MOST in the HCV group varied by subgroup (Figure 5). This difference demonstrates that high levels of either AFP or DCP were clinically significant[31]. The analysis of the clinical characteristics (Table 4) revealed no significant differences in liver function parameters; however, tumor-related factors, including tumor size and incidence of vascular invasion, were different between the groups. This difference suggests that tumor-related characteristics strongly affected prognosis with HCV infection; therefore, we believe that prognosis can be predicted by AFP and DCP levels together with tumor-related factors.
In our study, various clinical factors were associated with tumor recurrence and overall survival; therefore, we conducted a multivariate analysis for prognosis using a Cox forward stepwise logistic regression model. In the HBV group, the independent prognostic factors associated with tumor recurrence were tumor size, vascular invasion, and TBIL (Table 2). Significant differences in tumor size and vascular invasion were found between the three groups (Table 1). Tumor recurrence prognosis among the three groups was affected by these two factors, and a significant difference in disease-free survival was found (Figure 3A). However, membership in the HH group was an independent prognostic factor associated with tumor recurrence. Therefore, high levels of both AFP and DCP may be a prognostic predictor of tumor recurrence in the HBV group. The only independent prognostic factor associated with poor overall survival was vascular invasion (Table 3), which differed significantly between the three groups (Table 1). Therefore, a significant difference in overall survival rates was found between groups (Figure 3B). The identification of AFP and DCP levels was not always useful for predicting the prognosis associated with poor overall survival in the HBV group because neither HH group membership nor HL group membership was an independent prognostic factor associated with poor overall survival (Table 3). In the HCV group, the independent prognostic factors associated with tumor recurrence were ICGR15, surgical duration, tumor size, vascular invasion, and multiple tumors (Table 5). Surgical duration and tumor size differed significantly between the three groups (Table 4). Tumor recurrence prognosis in all three groups was affected by these two factors, yielding a significant difference between groups in disease-free survival (Figure 5A). However, membership in the HH group and membership in the HL group were independent prognostic factors associated with tumor recurrence (Table 5), suggesting that high levels of AFP and/or DCP are prognostic predictors of tumor recurrence in the HCV group. The independent prognostic factors associated with poor overall survival were ICGR15 and multiple tumors (Table 6), neither of which differed significantly between the three groups (Table 4). A prognosis of poor overall survival in all three groups was not affected by ICGR15 or multiple tumors. Membership in the HH group was an independent prognostic factor associated with poor overall survival (Table 6), indicating that high levels of both AFP and DCP are a prognostic predictor of poor overall survival in the HCV group.
In conclusion, among HCC patients treated in our department, the AFP cutoff level for recurrence within 2 years after surgery was 21.0 ng/mL in the HCV group and 529.8 ng/mL in the HBV group. Furthermore, patients in the HBV group with high levels of either AFP or DCP had poor prognoses, as did patients with high levels of both AFP and DCP. In contrast, poor prognoses were found in patients in the HCV group only when both levels were high. High AFP and DCP levels were an independent risk factor for tumor recurrence in both the HBV and HCV groups. We believe that to predict prognosis, preoperative levels of tumor markers should be distinguished and assessed according to the type of viral hepatitis.
The authors thank Crimson Interactive Pvt. Ltd. for their assistance in manuscript translation and editing.
There is no consensus regarding preoperative α-fetoprotein (AFP) and des-γ-carboxy prothrombin (DCP) cutoff levels to predict survival and recurrence after hepatectomy. Furthermore, the prognostic characteristics of these tumor markers according to hepatitis type remain unclear. Therefore, the aim of the present study was to compare these preoperative tumor marker levels and prognosis after hepatectomy as well as to clarify the characteristics of these tumor marker levels according to hepatitis type.
Chronic hepatitis caused by viral hepatitis often progresses to cirrhosis and hepatocellular carcinoma. The oncogenic mechanisms differ between hepatitis B and C viral infections, and these mechanisms should be taken into consideration when evaluating prognosis and establishing treatment regimens. In this study, the authors demonstrate that the relationship between preoperative tumor marker levels and prognosis varied by the type of viral hepatitis.
Although associations between preoperative tumor markers and postoperative hepatocellular carcinoma prognosis have been reported, this is the first study to report that the AFP cutoff level for recurrence within 2 years after surgery was 21.0 ng/mL in hepatitis C virus (HCV) patients compared with a higher level of 529.8 ng/mL in hepatitis B virus (HBV) patients. Furthermore, patients in the HBV group with high levels of either AFP or DCP had poor prognoses, as did those patients with high levels of both tumor markers. In contrast, in patients in the HCV group, poor prognoses were found only when levels of both AFP and DCP were high.
In the HBV group, prognosis was poor when either AFP or DCP levels were high. In the HCV group, prognosis was good when either or both levels were low; however, prognosis was poor when both levels were high.
AFP is a glycoprotein produced by liver cells and in the yolk sac during the fetal stage with an albumin-like structure, a half-life of 4-6 d and a molecular weight of 65 kDa. AFP production in the liver is increased in hepatocellular cancer as well as in chronic hepatitis and cirrhosis; therefore, AFP is considered to have low specificity for the diagnosis of cancer. Prothrombin is formed after the γ-carboxylation of vitamin K-dependent propeptides, and DCP is produced as a result of an acquired posttranslational defect in the vitamin K-dependent carboxylase system. DCP has a molecular weight of 72 kDa and a half-life of 40-72 h. DCP production does not increase in chronic hepatitis or cirrhosis, and it is considered to have high specificity for the diagnosis of cancer. However, DCP has no prognostic value in cases with vitamin K deficiency or vitamin K function inhibition.
Nice paper. Many patients included but a regression model is needed to confirm data. If they would do these statistical analysis, paper is interesting enough to be published.
Biostatistics: The statistical methods of this study were mainly reviewed by prof. Koichi Hirata from Sapporo Medical University School of Medicine.
P- Reviewer: Cerwenka HR, Festi D, Ramia JM S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN
| 1. | Ince N, Wands JR. The increasing incidence of hepatocellular carcinoma. N Engl J Med. 1999;340:798-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 109] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 2. | Kim MN, Kim BK, Han KH. Hepatocellular carcinoma in patients with chronic hepatitis C virus infection in the Asia-Pacific region. J Gastroenterol. 2013;48:681-688. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 3. | Liu L, Dong Z, Liang J, Cao C, Sun J, Ding Y, Wu D. As an independent prognostic factor, FAT10 promotes hepatitis B virus-related hepatocellular carcinoma progression via Akt/GSK3β pathway. Oncogene. 2014;33:909-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 4. | Mas VR, Maluf DG, Archer KJ, Yanek K, Kong X, Kulik L, Freise CE, Olthoff KM, Ghobrial RM, McIver P. Genes involved in viral carcinogenesis and tumor initiation in hepatitis C virus-induced hepatocellular carcinoma. Mol Med. 2009;15:85-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 239] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 5. | Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5537] [Cited by in RCA: 6457] [Article Influence: 430.5] [Reference Citation Analysis (0)] |
| 6. | Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, Hasegawa H, Nakajima Y, Ohnishi K. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56:918-928. [PubMed] |
| 7. | A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998;28:751-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 977] [Cited by in RCA: 963] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 8. | Kudo M, Chung H, Osaki Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score). J Gastroenterol. 2003;38:207-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 501] [Cited by in RCA: 537] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 9. | Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2645] [Cited by in RCA: 2871] [Article Influence: 110.4] [Reference Citation Analysis (1)] |
| 10. | Mizuguchi T, Kawamoto M, Meguro M, Nakamura Y, Harada K, Kukita K, Hirata K. Prognostic impact of preoperative the branched-chain amino acid to the tyrosine ratio in hepatocellular carcinoma patients after initial hepatectomy. J Gastrointest Surg. 2011;15:1433-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Toyoda H, Kumada T, Tada T, Niinomi T, Ito T, Kaneoka Y, Maeda A. Prognostic significance of a combination of pre- and post-treatment tumor markers for hepatocellular carcinoma curatively treated with hepatectomy. J Hepatol. 2012;57:1251-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Tandon P, Garcia-Tsao G. Prognostic indicators in hepatocellular carcinoma: a systematic review of 72 studies. Liver Int. 2009;29:502-510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 228] [Cited by in RCA: 274] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 13. | Abelev GI, Perova SD, Khramkova NI, Postnikova ZA, Irlin IS. Production of embryonal alpha-globulin by transplantable mouse hepatomas. Transplantation. 1963;1:174-180. [PubMed] |
| 14. | Giannini EG, Sammito G, Farinati F, Ciccarese F, Pecorelli A, Rapaccini GL, Di Marco M, Caturelli E, Zoli M, Borzio F. Determinants of alpha-fetoprotein levels in patients with hepatocellular carcinoma: implications for its clinical use. Cancer. 2014;120:2150-2157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Liebman HA, Furie BC, Tong MJ, Blanchard RA, Lo KJ, Lee SD, Coleman MS, Furie B. Des-gamma-carboxy (abnormal) prothrombin as a serum marker of primary hepatocellular carcinoma. N Engl J Med. 1984;310:1427-1431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 420] [Article Influence: 10.2] [Reference Citation Analysis (16)] |
| 16. | Shimada M, Takenaka K, Fujiwara Y, Gion T, Kajiyama K, Maeda T, Shirabe K, Sugimachi K. Des-gamma-carboxy prothrombin and alpha-fetoprotein positive status as a new prognostic indicator after hepatic resection for hepatocellular carcinoma. Cancer. 1996;78:2094-2100. [PubMed] |
| 17. | Huo TI, Huang YH, Lui WY, Wu JC, Lee PC, Chang FY, Lee SD. Selective prognostic impact of serum alpha-fetoprotein level in patients with hepatocellular carcinoma: analysis of 543 patients in a single center. Oncol Rep. 2004;11:543-550. [PubMed] |
| 18. | Kim HS, Park JW, Jang JS, Kim HJ, Shin WG, Kim KH, Lee JH, Kim HY, Jang MK. Prognostic values of alpha-fetoprotein and protein induced by vitamin K absence or antagonist-II in hepatitis B virus-related hepatocellular carcinoma: a prospective study. J Clin Gastroenterol. 2009;43:482-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Shirabe K, Taketomi A, Morita K, Soejima Y, Uchiyama H, Kayashima H, Ninomiya M, Toshima T, Maehara Y. Comparative evaluation of expanded criteria for patients with hepatocellular carcinoma beyond the Milan criteria undergoing living-related donor liver transplantation. Clin Transplant. 2011;25:E491-E498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. [PubMed] |
| 21. | Imamura H, Sano K, Sugawara Y, Kokudo N, Makuuchi M. Assessment of hepatic reserve for indication of hepatic resection: decision tree incorporating indocyanine green test. J Hepatobiliary Pancreat Surg. 2005;12:16-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 253] [Article Influence: 12.7] [Reference Citation Analysis (1)] |
| 22. | Pringle JH. V. Notes on the Arrest of Hepatic Hemorrhage Due to Trauma. Ann Surg. 1908;48:541-549. [PubMed] |
| 23. | Chon YE, Choi GH, Lee MH, Kim SU, Kim do Y, Ahn SH, Kim KS, Choi JS, Han KH, Chon CY. Combined measurement of preoperative α-fetoprotein and des-γ-carboxy prothrombin predicts recurrence after curative resection in patients with hepatitis-B-related hepatocellular carcinoma. Int J Cancer. 2012;131:2332-2341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Hung HH, Su CW, Lai CR, Chau GY, Chan CC, Huang YH, Huo TI, Lee PC, Kao WY, Lee SD. Fibrosis and AST to platelet ratio index predict post-operative prognosis for solitary small hepatitis B-related hepatocellular carcinoma. Hepatol Int. 2010;4:691-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 25. | Ishikawa T. Clinical features of hepatitis B virus-related hepatocellular carcinoma. World J Gastroenterol. 2010;16:2463-2467. [PubMed] |
| 26. | Kang SH, Kim do Y, Jeon SM, Ahn SH, Park JY, Kim SU, Kim JK, Lee KS, Chon CY, Han KH. Clinical characteristics and prognosis of hepatocellular carcinoma with different sets of serum AFP and PIVKA-II levels. Eur J Gastroenterol Hepatol. 2012;24:849-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 27. | Fujiyama S, Izuno K, Yamasaki K, Sato T, Taketa K. Determination of optimum cutoff levels of plasma des-gamma-carboxy prothrombin and serum alpha-fetoprotein for the diagnosis of hepatocellular carcinoma using receiver operating characteristic curves. Tumour Biol. 1992;13:316-323. [PubMed] |
| 28. | Lok AS, Sterling RK, Everhart JE, Wright EC, Hoefs JC, Di Bisceglie AM, Morgan TR, Kim HY, Lee WM, Bonkovsky HL. Des-gamma-carboxy prothrombin and alpha-fetoprotein as biomarkers for the early detection of hepatocellular carcinoma. Gastroenterology. 2010;138:493-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 450] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 29. | Tateishi R, Yoshida H, Matsuyama Y, Mine N, Kondo Y, Omata M. Diagnostic accuracy of tumor markers for hepatocellular carcinoma: a systematic review. Hepatol Int. 2008;2:17-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 137] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 30. | Choi JY, Jung SW, Kim HY, Kim M, Kim Y, Kim DG, Oh EJ. Diagnostic value of AFP-L3 and PIVKA-II in hepatocellular carcinoma according to total-AFP. World J Gastroenterol. 2013;19:339-346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 69] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (1)] |
| 31. | Kumada T, Toyoda H, Kiriyama S, Tanikawa M, Hisanaga Y, Kanamori A, Tada T, Tanaka J, Yoshizawa H. Predictive value of tumor markers for hepatocarcinogenesis in patients with hepatitis C virus. J Gastroenterol. 2011;46:536-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 32. | Nagaoki Y, Aikata H, Miyaki D, Murakami E, Hashimoto Y, Katamura Y, Azakami T, Kawaoka T, Takaki S, Hiramatsu A. Clinical features and prognosis in patients with hepatocellular carcinoma that developed after hepatitis C virus eradication with interferon therapy. J Gastroenterol. 2011;46:799-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 33. | Tyson GL, Duan Z, Kramer JR, Davila JA, Richardson PA, El-Serag HB. Level of α-fetoprotein predicts mortality among patients with hepatitis C-related hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2011;9:989-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 34. | Saito Y, Shimada M, Utsunomiya T, Morine Y, Imura S, Ikemoto T, Mori H, Hanaoka J, Yamada S, Asanoma M. Prediction of recurrence of hepatocellular carcinoma after curative hepatectomy using preoperative Lens culinaris agglutinin-reactive fraction of alpha-fetoprotein. Hepatol Res. 2012;42:887-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |