Published online Apr 28, 2015. doi: 10.3748/wjg.v21.i16.4883
Peer-review started: September 19, 2014
First decision: October 29, 2014
Revised: December 17, 2014
Accepted: January 16, 2015
Article in press: January 16, 2015
Published online: April 28, 2015
Processing time: 224 Days and 4.5 Hours
AIM: To investigate the change in intestinal dendritic cell (DC) number in fulminant hepatic failure (FHF).
METHODS: An animal model of FHF was created. Intestinal CD11b/c was detected by immunohistochemistry and Western blot. Quantitative real-time polymerase chain reaction (PCR) was used to detect intestinal integrin-α mRNA expression. Intestinal CD83, CD86, CD74, CD3 and AKT were detected by immunohistochemistry, Western blot and PCR. Phosphorylated-AKT (p-AKT) was detected by immunohistochemistry and Western blot.
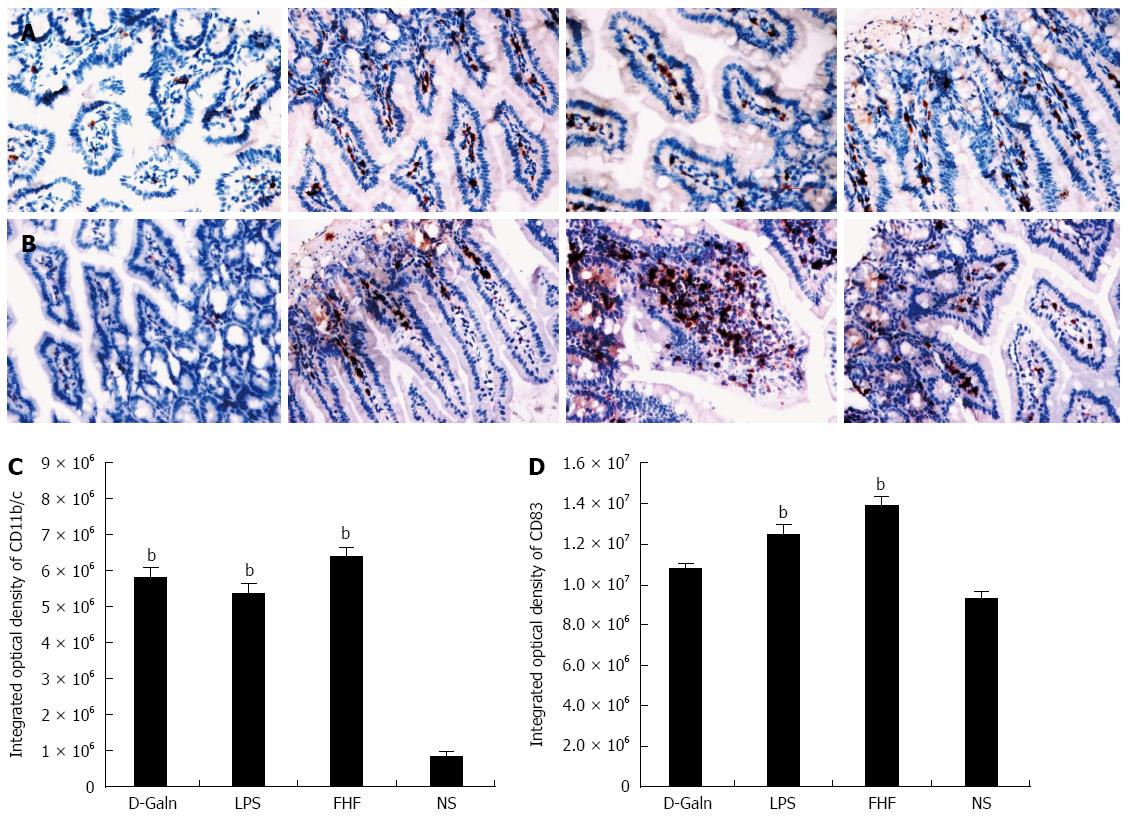
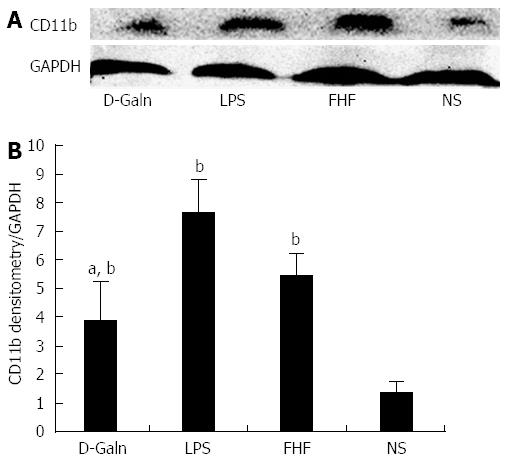
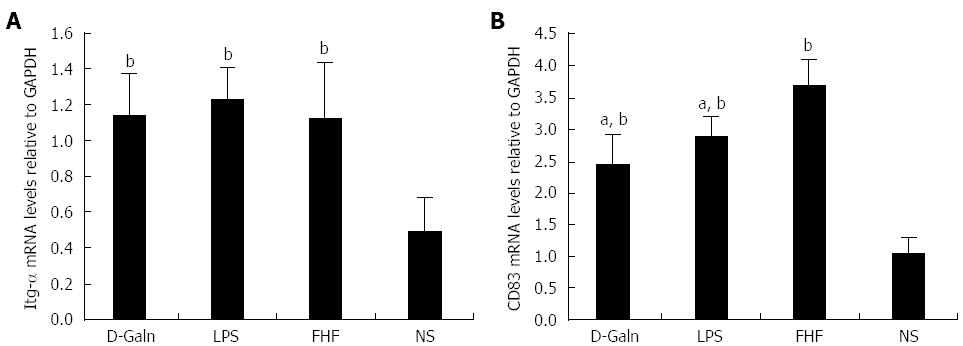
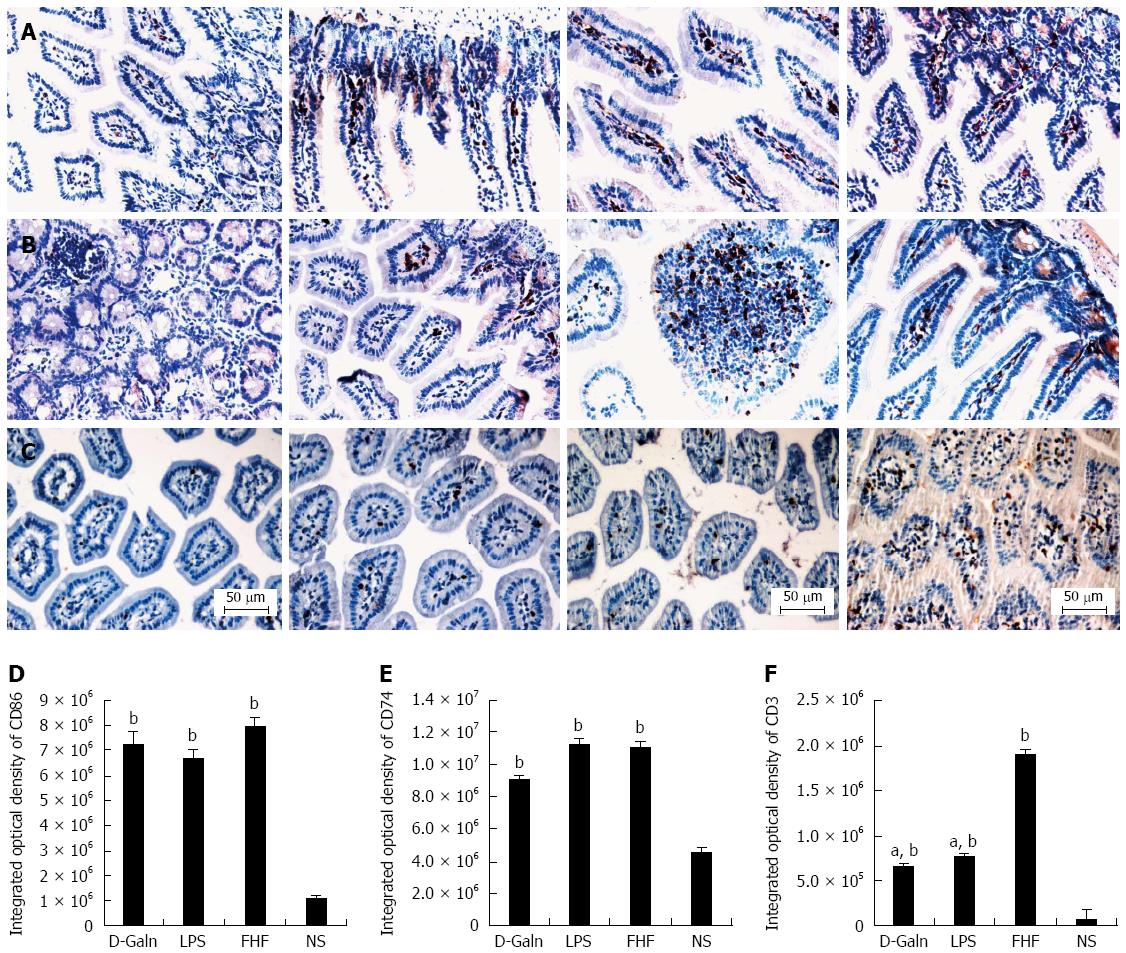
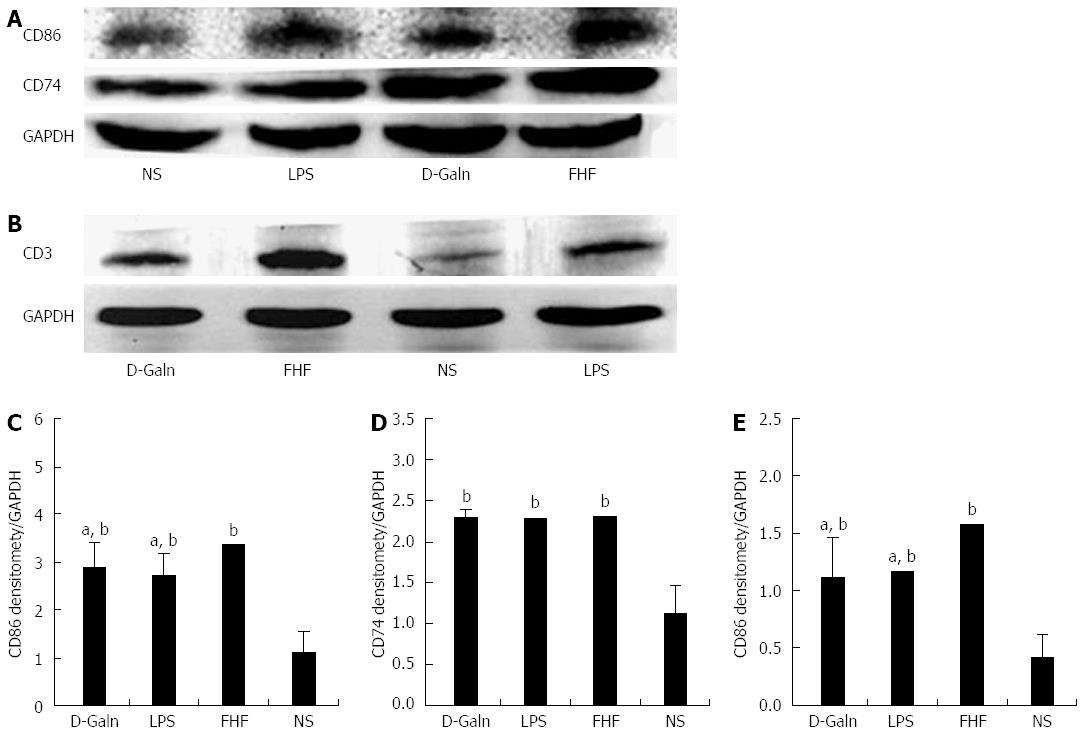
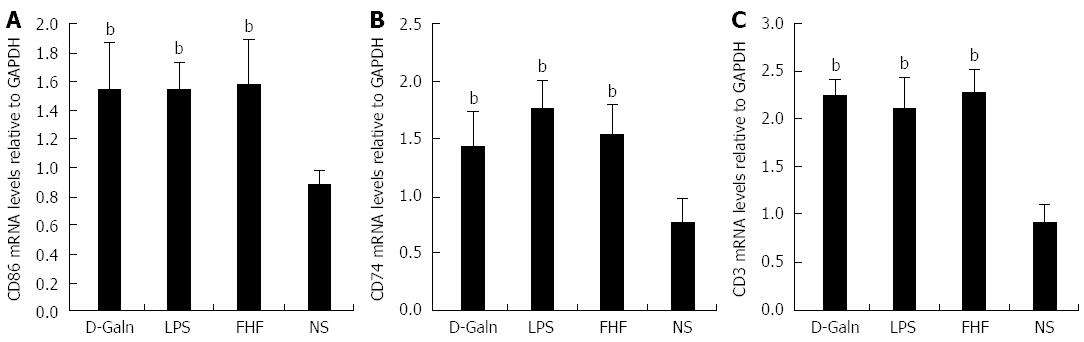
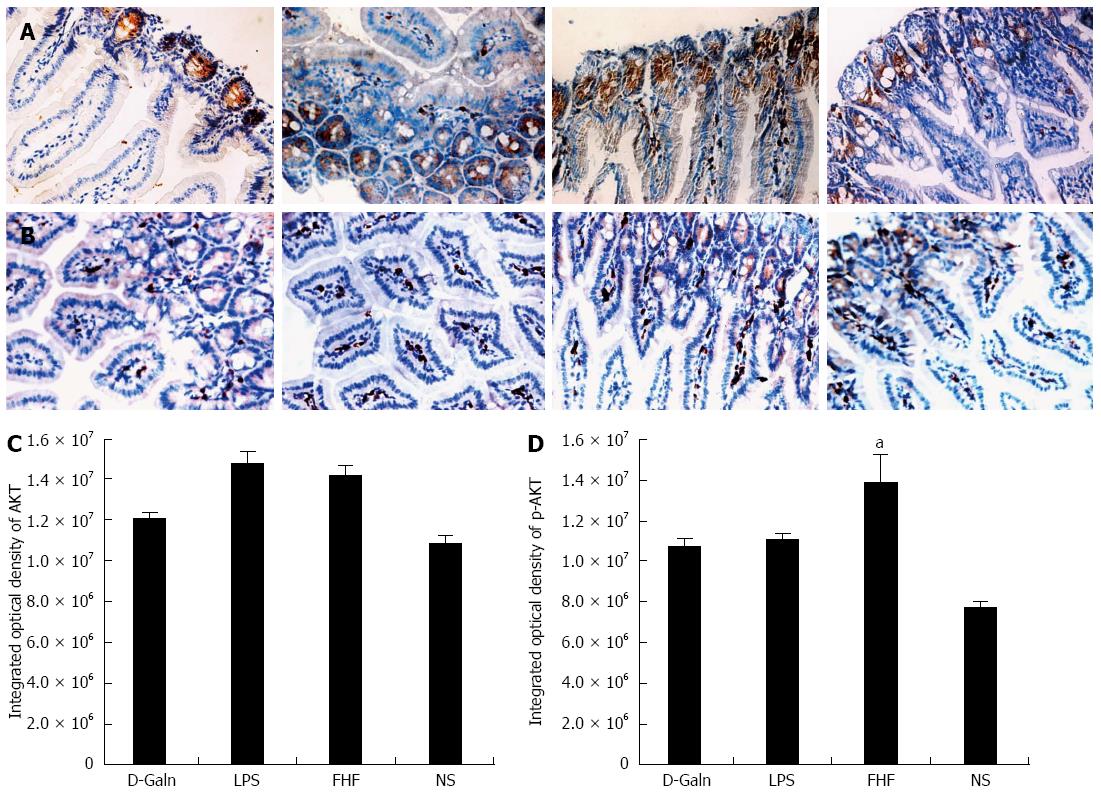
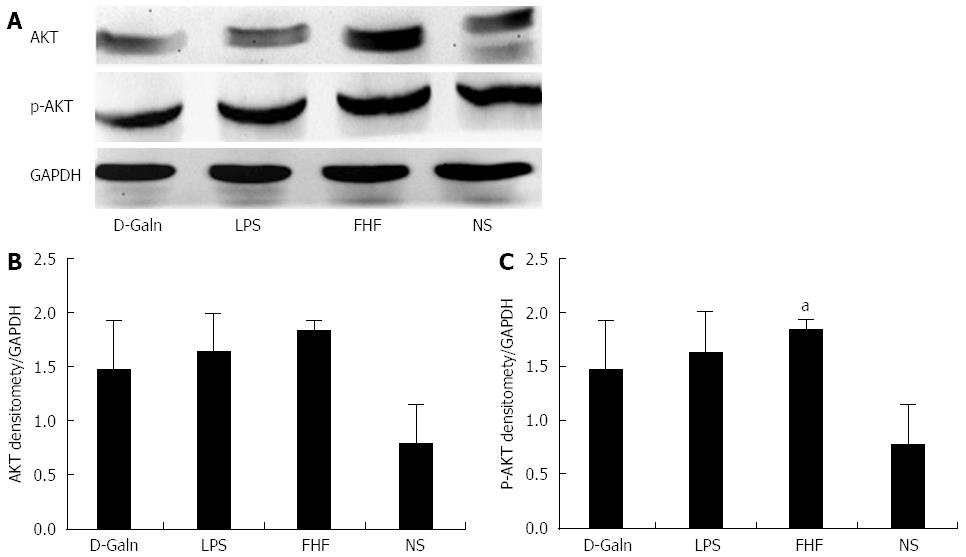
RESULTS: In the FHF group [D-galactosamine (D-Galn) + lipopolysaccharide (LPS) group], the mice began to die after 6 h; conversely, in the D-Galn and LPS groups, the activity of mice was poor, but there were no deaths. Immunohistochemistry results showed that in FHF, the expression of CD11b/c (7988400 ± 385941 vs 1102400 ± 132273, P < 0.05), CD83 (13875000 ± 467493 vs 9257600 ± 400364, P < 0.05), CD86 (7988400 ± 385941 vs 1102400 ± 13227, P < 0.05) and CD74 (11056000 ± 431427 vs 4633400 ± 267903, P < 0.05) was significantly increased compared with the normal saline (NS) group. Compared with the NS group, the protein expression of CD11b/c (5.4817 ± 0.77 vs 1.4073 ± 0.37, P < 0.05) and CD86 (4.2673 ± 0.69 vs 1.1379 ± 0.42, P < 0.05) was significantly increased. Itg-α (1.1224 ± 0.3 vs 0.4907 ± 0.19, P < 0.05), CD83 (3.6986 ± 0.40 vs 1.0762 ± 0.22, P < 0.05) and CD86 (1.5801 ± 0.32 vs 0.8846 ± 0.10, P < 0.05) mRNA expression was increased significantly in the FHF group. At the protein level, expression of CD74 in the FHF group (2.3513 ± 0.52) was significantly increased compared with the NS group (1.1298 ± 0.33), whereas in the LPS group (2.3891 ± 0.47), the level of CD74 was the highest (P < 0.05). At the gene level, the relative expression of CD74 mRNA in the FHF group (1.5383 ± 0.26) was also significantly increased in comparison to the NS group (0.7648 ± 0.22; P < 0.05). CD3 expression was the highest in the FHF group (P < 0.05). In the FHF, LPS and D-Galn groups, the expression of AKT at the protein and mRNA levels was elevated compared with the NS group, but there was no statistical significance (P > 0.05). The p-AKT protein expression in the FHF (1.54 ± 0.06), LPS (1.56 ± 0.05) and D-Galn (1.29 ± 0.03) groups was higher than that in the NS group (1.07 ± 0.03) (P < 0.05).
CONCLUSION: In FHF, a large number of DCs mature, express CD86, and activate MHC class II molecular pathways to induce a T cell response, and the AKT pathway is activated.
Core tip: In this study, we showed that the expression of CD11b/c, CD83, CD86, CD74 and CD3 in the fulminant hepatic failure (FHF) group [D-galactosamine (D-Galn) + lipopolysaccharide (LPS) group] was significantly higher compared with the normal saline (NS) group. Additionally, the phosphorylated-AKT protein expression in the FHF, LPS and D-Galn groups was higher than that in the NS group. This result showed that a large number of dendritic cells mature, express CD86, and activate MHC class II molecular pathways to induce a T cell response in FHF. In addition, the AKT pathway in FHF was activated.
- Citation: Cao X, Liu M, Wang P, Liu DY. Intestinal dendritic cells change in number in fulminant hepatic failure. World J Gastroenterol 2015; 21(16): 4883-4893
- URL: https://www.wjgnet.com/1007-9327/full/v21/i16/4883.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i16.4883
Fulminant hepatic failure (FHF) is a clinical syndrome resulting from a rapid loss of hepatocyte function with acute morbidity. A lack of effective treatment has resulted in high mortality so far[1,2]. During the progression of FHF, bacterial overgrowth in the small intestine affects intracellular signal transduction pathways, leading to the disruption of epithelial tight junctions and an increase in the paracellular permeability to macromolecules. Furthermore, intestinal bacterial translocation (BT) through the gastrointestinal barrier into the blood is associated with decreased intestinal resistance. This mechanism may lead to primary peritonitis. Clinically, this event is diagnosed as spontaneous bacterial peritonitis (SBP). Therefore, a decrease in intestinal permeability would prevent BT, suppress intestinal endotoxemia, prevent the occurrence of SBP, and thus enhance recovery from liver injury.
In the gut, dendritic cells (DCs), which are professional antigen presenting cells (APC), play a crucial role in both innate and adaptive immunity against microbial antigens. Activated mature intestinal DCs are the main stimulators of naive T cells, ultimately shaping the adaptive mucosal immune system[3]. Additionally, the invariant natural killer T cells regulate DC numbers[4]. T cell responses are initiated with specific peptide-MHC protein complexes on the surface of APC and through signals provided by co-stimulators expressed on APC[5]. In response to BT, gut epithelial cells release chemokines that induce the recruitment of DCs to the mucosa[6]. DCs that underlie the epithelium may open tight junctions between epithelial cells, sending dendrites into the lumen to directly sample bacteria[7,8]. Previous studies have shown that both viable and killed probiotic bacteria had strain-specific effects on the phenotype of human and murine DCs[9-11]. For example, lamina propria DCs can be distinguished according to CD11c (high and low), CD11b, CD103, CX3CR1, and CD70[12] expression. LP-DCs can be divided into two different subsets: CD103+CX3CR1-DCs that induce the development of regulatory T cells and CD103-CX3CR1+DCs with features of macrophages, which promote TNF production and the development of Th1/Th17 T cells. It has been reported that bacterial translocation in rats with cirrhotic ascites was associated with an increase in the total number of intestinal CD103+DCs in the lamina propria and in mesenteric lymph nodes[13].
Preventing the occurrence of SBP is essential to avoid liver damage and reduce mortality in patients with FHF. In this study, the intestinal CD11b/c, CD83, CD86, the major histocompatibility complex II-associated invariant chain Ii (also known as CD74), the T cell marker (CD3), and AKT/ phosphorylated-AKT (p-AKT) were assessed to gain a better understanding of how the distribution of DCs and the corresponding immune response change after the onset of FHF.
All animals in this study were from the Animal Center of Shengjing Hospital of China Medical University. Adult wild-type rats were anesthetized and killed by cervical dislocation. All studies were performed in accordance with the protocol approved by the Institutional Animal Care and Use Committee of the China Medical University for Basic Research in Developmental Disabilities. All surgery was performed under anesthesia, and all efforts were made to minimize suffering.
A mouse model of FHF was established as described previously[14]. BALB/C mice housed in a specific-pathogen-free environment, weighing 18-22 g, were purchased from the Experimental Animal Center of China Medical University. All pups were randomly divided into four groups: normal saline (NS, 15 mice), lipopolysaccharide (LPS, 30 mice), D-galactosamine (D-Galn, 30 mice), and FHF (LPS + D-Galn, 100 mice). Mice were injected intraperitoneally with LPS (10 μg/kg, Sigma, United States) and/or D-Galn (800 mg/kg, Sigma, United States). Mice were sacrificed via decapitation 9 h after the injection of NS, LPS, or D-Galn.
BALB/C mice were sacrificed by decapitation. To standardize analysis, half of the small intestine was fixed in situ with 4% paraformaldehyde in phosphate-buffered saline (pH 7.4) and then embedded in paraffin. The rest of the small intestine was harvested for mRNA and protein analysis.
Paraffin-embedded sections of intestinal tissues were deparaffinized, rehydrated, and incubated with mouse anti-mouse CD11b/c (Abcam, United Kingdom), mouse anti-mouse CD86 (Santa Cruz Biotechnology, United States), rabbit anti-mouse CD3 (Sigma, United States), rabbit anti-mouse AKT (Thermo Fisher Scientificm, United States), rabbit anti-mouse CD74 (Santa Cruz Biotechnology, United States), rabbit anti-mouse p-AKT(Santa Cruz Biotechnology, United States) and goat anti-mouse CD83 (Santa Cruz Biotechnology, United States). Slides were rinsed three times with PBS between incubations, and sections were incubated with biotinylated secondary antibodies and horseradish peroxidase labeled avidin (ZSGB-BIO, China). Slides were rinsed three times with PBS after each incubation, and sections were counterstained with hematoxylin. For negative controls, the primary antibody was replaced with PBS. After scanning, the median absorbance values were determined using Image-Pro analysis software (Media Cybernetics, United States).
A BCA Protein Assay Kit (Beyotime, Shanghai, China) was used to determine the protein concentration of intestinal tissue according to the manufacturer’s instructions.
Proteins extracted from intestinal tissues were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinyl fluoride membranes. Membranes were blocked with Tris-buffer containing 5% skim milk and probed with anti-CD11b/c, -CD86, -CD74, -CD3, -AKT and -p-AKT antibodies or anti-GAPDH followed by a peroxidase-conjugated secondary antibody. They were then incubated with an enhanced chemiluminescent substrate and exposed to X-OMAT film (Perkin Elmer, American). Images were scanned, and densitometry was analyzed for protein levels normalized to GAPDH using the Image-pro plus 6.0 software (Media Cybernetics, American).
Total RNA was extracted from the intestinal tissue of FHF mice using an RNA Mini Kit from Takara (Takara Biotechnology Co., Dalian, China). The quality of extracted RNA was determined by the number and size of the bands obtained using agarose gel electrophoresis. cDNA (Takara Biotechnology Co., Dalian, China) was synthesized using 100 ng RNA. The levels of individual RNA transcripts were quantified using quantitative real-time polymerase chain reaction (PCR). The primers used were as follows: CD3ε-Forward (F): 5’-TCACGGAGATGTCGCCAGA-3’, CD3ε-Reverse (R): 5’-TTGCCATCCAGTTTGCCCTTA-3’; integrin-α (Itg-α)-F: 5’-ATGGCTCCGGTAGCATCAACA-3’, Itg-α-R: 5’-CTCGTCCGAGTACTGCATCAAAG-3’; CD74-F: 5’-AGCCAGATGCGGATGGCTA-3’, CD74-R: 5’-TCCTGGGTCATGTTGCCGTA-3’; CD86-F: 5’-ATATGACCGTTGTGTGTGTTCTGGA-3’, CD86-R: 5’-AGGGCCACAGTAACTGAAGCTGTAA-3’; CD83-F: 5’-AAGTACAGGGCAGAAGCTGTGTTG-3’, CD83-R: 5’-AAGCTTGTTCCGTACCAGGTTTAGA-3’; AKT-F: 5’-GAGGTTGCCCACACGCTTA-3’, AKT-R: 5’-GGCGTACTCCATGACAAAGCAG-3’; GAPDH-F: 5’-GGTGAAGGTCGGTGTGAACG-3’, GAPDH-R: 5’-CTCGCTCCTGGAAGATGGTG-3’.
The primers and fluorescent probes were purchased from Takara. The PCR conditions were as follows: a preliminary cycle at 95 °C for 10 s, followed by 45 cycles at 95 °C for 5 s and at 60 °C for 20 s, followed by 1 min at 60 °C and 5 s at 95 °C. We also confirmed that the efficiency of amplification for GAPDH was 100% in the exponential phase of PCR. The mRNA levels were normalized to GADPH mRNA according to the following formula: relative levels of target mRNA = 2-ΔΔCT× 100 %, where ΔΔ CT = (CTFHF, LPS, D-Galn groups - CT GAPDH) - (CTNS group - CTGAPDH). The intestinal mRNA levels of the FHF groups were compared with those of other groups.
For each experiment, at least 15 mice were tested per group. Data regarding group differences were reported as mean ± SD. Significant differences between treatment groups were determined using one-way analysis of variance with the Dunnett test.
The DC surface markers, CD11b/c (Figure 1A) and CD83 (Figure 1B), were detected using immunohistochemistry. Mature DC markers were expressed on the cytoplasmic membrane and in the cytoplasm of intestinal mucosal cells. In the FHF group, DCs were found to be distributed throughout the basal layer and in the lymphatic vessels, but they only appeared on the basal layer in the NS group (Figure 1A). In the FHF, LPS, and D-Galn groups, CD11b/c staining (Figure 1C) was significantly higher than that of the NS group. CD83 staining (Figure 1D) in the other three groups was higher than that of the NS group. To further confirm DC maturation, CD11b protein expression was measured (Figure 2A). The results demonstrated that the CD11b protein expression in the FHF, D-Galn and LPS groups was higher than that of the NS group (Figure 2B). At the genetic level, CD11b is encoded by the Itg-α, which is part of the integrin family. The Itg-α (Figure 3A) and CD83 (Figure 3B) mRNA expression changes were consistent with the changes in their protein levels; the protein levels observed in each of the three treatment groups were higher than those of the NS group. Additionally, an increase in protein expression was more pronounced in the LPS and FHF groups. These data indicated that a greater number of DCs matured in the intestinal tissues of the FHF mice.
To explore how DCs might influence the development of FHF, the co-stimulatory molecule, CD86, was tested. The staining results (Figure 4A and D) showed that CD86 expression in the FHF, LPS and D-Galn groups was significantly higher than that of the NS group. The CD86 protein (Figure 5A and C), approximately 56 kDa, was remarkably increased in the FHF group compared with the other groups. Similarly, CD86 mRNA (Figure 6A) in the three treatment groups was much higher than in the NS group. To determine whether the MHC II molecular pathway was activated, CD74 was measured. As shown in Figure 4B, CD74 was expressed both on the cell membrane and in the cytoplasm and was significantly increased (Figure 4E) in the FHF group. In all the three treatment groups, CD74 protein was expressed at a significantly higher level compared with the NS group (Figure 5A), and CD74 mRNA was significantly increased as well (Figure 6B). To determine whether there was an increased number of T cells present in intestinal tissue samples from the FHF group, we measured CD3 staining. CD3 was distributed in the intestinal mucosal tissue (Figure 4C) and in the FHF group its number was the highest (Figure 4F). The CD3 protein expression (Figure 5B and 5) in the FHF group was also much higher than in other groups. The CD3 mRNA (Figure 6C) of the three treatment groups was still far higher than that of the NS group. However, there was no difference among the three treatment groups. Therefore, we concluded that in the FHF group, the number and activity of intestinal mature DCs were significantly increased and that the T cells were also increased. Thus, these data suggested that the entire intestinal mucosal immune barrier was influenced.
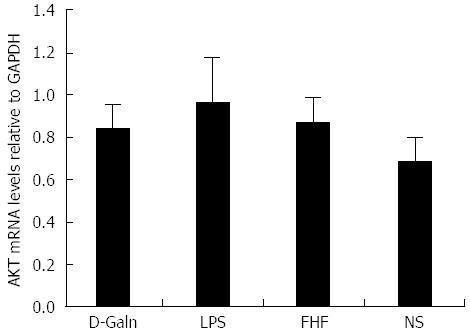
The AKT signal transduction pathway is an important mechanism by which cells avoid apoptosis because it promotes the progression of the cell cycle and thus cell proliferation and activation. The activation of AKT is usually characterized by phosphorylation. AKT is primarily expressed in the basal layer of the intestinal mucosa. The results of immunohistochemistry staining showed that in the FHF, LPS and D-Galn groups, AKT (Figure 7A and C) was not significantly changed, but p-AKT (Figure 7B and D) was higher than that of the NS group. Compared with the NS group, AKT protein (Figure 8A and B) was only non-significantly increased in the FHF, LPS and D-Galn groups. However, the expression of p-AKT protein (Figure 8A and C) showed a significant difference. In addition, the AKT mRNA (Figure 9) levels were not significantly different among these groups. We concluded that the AKT signaling pathway was activated in the FHF group.
The gut microbiome plays both physiological and pathological roles in intestinal homeostasis and the pathogenesis of inflammatory bowel diseases[15]. As effector cells of both innate and adaptive immune responses, DCs are central not only for maintaining protective immunity against pathogens but also for preventing inflammatory intestinal immune responses against the microbiota and food antigens. Similar effector functions to those involved in protective immunity against pathogens are engaged during inappropriate inflammatory responses against harmless antigens[16]. The pathological changes of other organs often affect the gut, as occurs in many hepatic diseases. In the case of FHF, SBP is a common complication caused by pathological BT. The clinical pathological causes of BT are the increase in intestinal permeability and the disruption of the intestinal mucosal barrier[17,18]. These changes indicate that DCs may be involved in FHF due to their role as professional APCs present in the gut and their ability to disrupt tight junctions to sample bacteria in the lumen.
The intestinal mucosa contains many subtypes of DCs, which are present in two functionally distinct states; i.e., immature and mature cells. The subtypes of DCs vary based on their typical tissue localization. For example, DCs isolated from intestinal tissues are endowed with unique mucosal functions that are specific to these DC subsets. It was originally thought that mature DCs induced protective immunity to infectious agents but that immature DCs induced tolerance to innocuous antigens[19]. CD83 has been implicated in the regulation of inflammation, and it is expressed on a number of cell types[20]. In this study, by using CD11b/c and CD83 as DC maturation markers, we demonstrated that mature intestinal DCs in the FHF group showed a significantly increased trend. These data were in agreement with other studies in which CD83 expression was upregulated in a chemical-induced murine colitis model[21]. Some studies showed that LPS injection can influence the expression of MCH-II and CD11b in brain sections[22]. Our results might also be associated with the concentration of LPS used in vivo[23].
DCs have roles in both the innate and adaptive immune responses. DCs are sentinels of the immune system recognizing and translating pathogenic signals into immune responses[24]. Thus, an increase of intestinal DCs suggested that the intestinal mucosa had been injured in FHF. Some studies have shown that the presence of CD83 on mDC membranes enhanced T lymphocyte proliferation[25]. For instance, CD80/CD86, expressed in DCs, plays a central role in T cell activation by delivery of co-stimulatory signals to naïve T cells, activating them to become effector T cells[26]. The report showed that the expression intensity of CD86 with LPS stimulation was significantly higher[27]. The results of the current study demonstrated that CD86 and CD3 were significantly increased in FHF tissues. These data indicated that intestinal T cell activation was increased in FHF as a result of ligation with CD80/CD86 on DCs.
CD74 is a type II integral membrane protein and acts as a chaperone for MHC II protein expression[28]. It is a key substrate that contributes to the survival of DCs[29,30]. Downregulation of CD74 has been shown to regulate DC migration in vitro and in vivo[31,32]. It was also reported that post-infectious intestinal lamina propria DCs displayed increased CD86 and MHC class II, resulting in the enhanced induction of T cell proliferation[33]. Other reports suggested that DCs displayed higher expression of MHC II and CD86 in LPS-induced liver injury[34]. In this study, CD74 was significantly increased in FHF tissue. Collectively, these results imply that in FHF, SBP would lead to the rapid maturation of DCs and the subsequent activation of T cells to produce an adaptive immune response.
AKT signaling has been reported to play a key role in regulating cell growth, survival, and metabolism in a variety of apoptotic paradigms[35]. Activated AKT, which in turn phosphorylates and inactivates components of the apoptotic machinery, participates in cell survival, proliferation and apoptosis inhibition[36,37]. Abnormal activation of the AKT pathway may lead to disease and dysfunction. Studies have found that crosslinking CD80/CD86 in human DCs activated the PI3K/AKT pathway[38]. AKT activation has also been shown to be an early sign of intestinal microenvironmental change and is closely related to the treatment of gut injury[39]. Therefore, we measured p-AKT as a first attempt to understand the role of the AKT pathway in FHF. Our results demonstrated that p-AKT was significantly increased in FHF. In addition, other researchers have shown that activation of the AKT pathway might be involved in the pathogenesis of Crohn’s disease[40]. Moreover, AKT activation might be involved in the transition from intestinal inflammation to cancer[41]. Taken together, these results suggested that the AKT pathway was activated in FHF and might participate in early intestinal mucosal injury.
DCs play an important role in intestinal mucosal health because they can induce a T cell adaptive immune response. In this study, a large number of DCs matured, expressed CD86, and activated MHC class II molecular pathways to induce a T cell response in FHF. In addition, the AKT signaling pathway was activated in FHF.
Fulminant hepatic failure (FHF) is often associated with spontaneous bacterial peritonitis (SBP), which is caused by bacterial translocation from the gut after serious liver damage and is associated with significantly increased mortality. Dendritic cells (DCs) play an important role in the generation and regulation of immune responses and oversee intestinal immune homeostasis in SBP. Studies on intestinal DCs were performed to understand the mechanisms of SBP.
DCs are sentinels of the immune system that recognize and translate pathogenic signals into immune responses. Intestinal DC numbers and function in liver diseases have become a topic of interest. This study is basic to understanding the mechanisms of SBP.
To date, many studies have been carried out primarily on bacterial translation and cytokine expression by T cells and DCs. In this study, the authors carefully studied changes in the number of intestinal DCs by employing immunohistochemistry, Western blot and polymerase chain reaction and found that a large number of DCs matured, expressed CD86, and activated the MHC II molecular pathway to induce a T cell response in FHF. Furthermore, the AKT signaling pathway was activated in FHF.
By identifying the intestinal DCs in FHF, the authors evaluated CD86, CD83 and CD74 in intestinal DCs, which could improve our understanding of the mechanisms of SBP in FHF.
Integrin-α (Itg-α): At the genetic level, CD11b is encoded by the Itg-α gene, which is part of the integrin family.
The authors performed experiments to detect CD86, CD83 and CD74 in the intestinal DCs in FHF and found that a large number of DCs matured, expressed CD86, and activated the MHC II molecular pathway to induce a T cell response in FHF. Furthermore, the authors detected AKT and phosphorylated-AKT, and the results showed that the AKT signaling pathway was activated in FHF.
P- Reviewer: Wan JY S- Editor: Yu J L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Lu H, Zhang CY, Ding W, Lu YJ, Li GQ, Zhang F, Lu L. Severe hepatic necrosis of unknown causes following ABO-incompatible liver transplantation. World J Gastroenterol. 2013;19:964-967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 2. | Lee WM. Acute liver failure. Semin Respir Crit Care Med. 2012;33:36-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 187] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 3. | Kalliomäki M, Kirjavainen P, Eerola E, Kero P, Salminen S, Isolauri E. Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. J Allergy Clin Immunol. 2001;107:129-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 894] [Cited by in RCA: 811] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 4. | Pilones KA, Aryankalayil J, Babb JS, Demaria S. Invariant natural killer T cells regulate anti-tumor immunity by controlling the population of dendritic cells in tumor and draining lymph nodes. J Immunother Cancer. 2014;2:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Lintermans LL, Stegeman CA, Heeringa P, Abdulahad WH. T cells in vascular inflammatory diseases. Front Immunol. 2014;5:504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 6. | Wiest R, Lawson M, Geuking M. Pathological bacterial translocation in liver cirrhosis. J Hepatol. 2014;60:197-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 562] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 7. | Rescigno M, Rotta G, Valzasina B, Ricciardi-Castagnoli P. Dendritic cells shuttle microbes across gut epithelial monolayers. Immunobiology. 2001;204:572-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 193] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 8. | Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl JP, Ricciardi-Castagnoli P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1870] [Cited by in RCA: 1822] [Article Influence: 75.9] [Reference Citation Analysis (0)] |
| 9. | Christensen HR, Frøkiaer H, Pestka JJ. Lactobacilli differentially modulate expression of cytokines and maturation surface markers in murine dendritic cells. J Immunol. 2002;168:171-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 649] [Cited by in RCA: 622] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 10. | Hart AL, Lammers K, Brigidi P, Vitali B, Rizzello F, Gionchetti P, Campieri M, Kamm MA, Knight SC, Stagg AJ. Modulation of human dendritic cell phenotype and function by probiotic bacteria. Gut. 2004;53:1602-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 382] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 11. | Drakes M, Blanchard T, Czinn S. Bacterial probiotic modulation of dendritic cells. Infect Immun. 2004;72:3299-3309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 149] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 12. | Rescigno M. Intestinal dendritic cells. Adv Immunol. 2010;107:109-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 13. | Muñoz L, José Borrero M, Ubeda M, Lario M, Díaz D, Francés R, Monserrat J, Pastor O, Aguado-Fraile E, Such J. Interaction between intestinal dendritic cells and bacteria translocated from the gut in rats with cirrhosis. Hepatology. 2012;56:1861-1869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Dong-Yan L, Weiguo J, Pei L. Reduction of the amount of intestinal secretory IgA in fulminant hepatic failure. Braz J Med Biol Res. 2011;44:477-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Xue X, Cao AT, Cao X, Yao S, Carlsen ED, Soong L, Liu CG, Liu X, Liu Z, Duck LW. Downregulation of microRNA-107 in intestinal CD11c(+) myeloid cells in response to microbiota and proinflammatory cytokines increases IL-23p19 expression. Eur J Immunol. 2014;44:673-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Mann ER, Li X. Intestinal antigen-presenting cells in mucosal immune homeostasis: crosstalk between dendritic cells, macrophages and B-cells. World J Gastroenterol. 2014;20:9653-9664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 76] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (1)] |
| 17. | Al-Sadi R, Ye D, Said HM, Ma TY. IL-1beta-induced increase in intestinal epithelial tight junction permeability is mediated by MEKK-1 activation of canonical NF-kappaB pathway. Am J Pathol. 2010;177:2310-2322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 169] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 18. | Miele L, Valenza V, La Torre G, Montalto M, Cammarota G, Ricci R, Mascianà R, Forgione A, Gabrieli ML, Perotti G. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877-1887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1133] [Cited by in RCA: 1101] [Article Influence: 68.8] [Reference Citation Analysis (1)] |
| 19. | Rescigno M, Di Sabatino A. Dendritic cells in intestinal homeostasis and disease. J Clin Invest. 2009;119:2441-2450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 244] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 20. | Bates JM, Flanagan K, Mo L, Ota N, Ding J, Ho S, Liu S, Roose-Girma M, Warming S, Diehl L. Dendritic cell CD83 homotypic interactions regulate inflammation and promote mucosal homeostasis. Mucosal Immunol. 2015;8:414-428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Eckhardt J, Kreiser S, Döbbeler M, Nicolette C, DeBenedette MA, Tcherepanova IY, Ostalecki C, Pommer AJ, Becker C, Günther C. Soluble CD83 ameliorates experimental colitis in mice. Mucosal Immunol. 2014;7:1006-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Deng X, Li M, Ai W, He L, Lu D, Patrylo PR, Cai H, Luo X, Li Z, Yan X. Lipolysaccharide-Induced Neuroinflammation Is Associated with Alzheimer-Like Amyloidogenic Axonal Pathology and Dendritic Degeneration in Rats. Adv Alzheimer Dis. 2014;3:78-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 23. | Koido S, Ohkusa T, Kan S, Takakura K, Saito K, Komita H, Ito Z, Kobayashi H, Takami S, Uchiyama K. Production of corticotropin-releasing factor and urocortin from human monocyte-derived dendritic cells is stimulated by commensal bacteria in intestine. World J Gastroenterol. 2014;20:14420-14429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Cabezón R, Benítez-Ribas D. Therapeutic potential of tolerogenic dendritic cells in IBD: from animal models to clinical application. Clin Dev Immunol. 2013;2013:789814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Pinho MP, Migliori IK, Flatow EA, Barbuto JA. Dendritic cell membrane CD83 enhances immune responses by boosting intracellular calcium release in T lymphocytes. J Leukoc Biol. 2014;Epub ahead of print. [PubMed] |
| 26. | MacDonald TT, Monteleone I, Fantini MC, Monteleone G. Regulation of homeostasis and inflammation in the intestine. Gastroenterology. 2011;140:1768-1775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 206] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 27. | Jiang H, Zhang Y, Yin X, Hu H, Hu X, Fei Y, Tu Y, Zhang Y. Construction and evaluation of rats’ tolerogenic dendritic cells (DC) induced by NF-κB Decoy method. Afr Health Sci. 2014;14:626-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Maharshak N, Cohen S, Lantner F, Hart G, Leng L, Bucala R, Shachar I. CD74 is a survival receptor on colon epithelial cells. World J Gastroenterol. 2010;16:3258-3266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Munn DH, Sharma MD, Mellor AL. Ligation of B7-1/B7-2 by human CD4+ T cells triggers indoleamine 2,3-dioxygenase activity in dendritic cells. J Immunol. 2004;172:4100-4110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 357] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 30. | Beisner DR, Langerak P, Parker AE, Dahlberg C, Otero FJ, Sutton SE, Poirot L, Barnes W, Young MA, Niessen S. The intramembrane protease Sppl2a is required for B cell and DC development and survival via cleavage of the invariant chain. J Exp Med. 2013;210:23-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 31. | Powis SJ. CLIP-region mediated interaction of Invariant chain with MHC class I molecules. FEBS Lett. 2006;580:3112-3116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Basha G, Omilusik K, Chavez-Steenbock A, Reinicke AT, Lack N, Choi KB, Jefferies WA. A CD74-dependent MHC class I endolysosomal cross-presentation pathway. Nat Immunol. 2012;13:237-245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 33. | Long Y, Wang W, Wang H, Hao L, Qian W, Hou X. Characteristics of intestinal lamina propria dendritic cells in a mouse model of postinfectious irritable bowel syndrome. J Gastroenterol Hepatol. 2012;27:935-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 34. | Zhang Y, Cai W, Huang Q, Gu Y, Shi Y, Huang J, Zhao F, Liu Q, Wei X, Jin M. Mesenchymal stem cells alleviate bacteria-induced liver injury in mice by inducing regulatory dendritic cells. Hepatology. 2014;59:671-682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 35. | Yang L, Wang R, Gao Y, Xu X, Fu K, Wang S, Li Y, Peng R. The protective role of interleukin-11 against neutron radiation injury in mouse intestines via MEK/ERK and PI3K/Akt dependent pathways. Dig Dis Sci. 2014;59:1406-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 36. | Gao Z, Liu F, Yin P, Wan C, He S, Liu X, Zhao H, Liu T, Xu J, Guo S. Inhibition of heat-induced apoptosis in rat small intestine and IEC-6 cells through the AKT signaling pathway. BMC Vet Res. 2013;9:241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 37. | Zhang Y, Yao X, Jiang C, Yue J, Guan J, Cheng H, Hajirashid M, Wang Y, Fan L. Expression of PI3K, PTEN and Akt in small intestinal adenocarcinoma detected by quantum dots-based immunofluorescence technology. Cancer Biomark. 2013;13:299-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 38. | Koorella C, Nair JR, Murray ME, Carlson LM, Watkins SK, Lee KP. Novel regulation of CD80/CD86-induced phosphatidylinositol 3-kinase signaling by NOTCH1 protein in interleukin-6 and indoleamine 2,3-dioxygenase production by dendritic cells. J Biol Chem. 2014;289:7747-7762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 39. | Niederlechner S, Baird C, Wischmeyer PE. P38MAP kinase, but not phosphoinositol-3 kinase, signal downstream of glutamine-mediated fibronectin-integrin signaling after intestinal injury. Nutr J. 2013;12:88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 40. | Long SH, He Y, Chen MH, Cao K, Chen YJ, Chen BL, Mao R, Zhang SH, Zhu ZH, Zeng ZR. Activation of PI3K/Akt/mTOR signaling pathway triggered by PTEN downregulation in the pathogenesis of Crohn’s disease. J Dig Dis. 2013;14:662-669. [PubMed] |
| 41. | Josse C, Bouznad N, Geurts P, Irrthum A, Huynh-Thu VA, Servais L, Hego A, Delvenne P, Bours V, Oury C. Identification of a microRNA landscape targeting the PI3K/Akt signaling pathway in inflammation-induced colorectal carcinogenesis. Am J Physiol Gastrointest Liver Physiol. 2014;306:G229-G243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |