Published online Apr 28, 2015. doi: 10.3748/wjg.v21.i16.4852
Peer-review started: September 24, 2014
First decision: October 29, 2014
Revised: December 7, 2014
Accepted: January 30, 2015
Article in press: January 30, 2015
Published online: April 28, 2015
Processing time: 217 Days and 6.1 Hours
AIM: To determine the molecular mechanisms of Shugan decoction (SGD) in the regulation of colonic motility and visceral hyperalgesia (VHL) in irritable bowel syndrome (IBS).
METHODS: The chemical compounds contained in SGD were measured by high-performance liquid chromatography. A rat model of IBS was induced by chronic water avoidance stress (WAS). The number of fecal pellets was counted after WAS and the pain pressure threshold was measured by colorectal distension. Morphological changes in colonic mucosa were detected by hematoxylin-eosin staining. The contents of tumor necrosis factor (TNF)-α in colonic tissue and calcitonin-gene-related peptide (CGRP) in serum were measured by ELISA. The protein expression of serotonin [5-hydroxytryptamide (5-HT)], serotonin transporter (SERT), chromogranin A (CgA) and CGRP in colon tissue was measured by immunohistochemistry.
RESULTS: SGD inhibited colonic motility dysfunction and VHL in rats with IBS. Blockers of transient receptor potential (TRP) vanilloid 1 (TRPV1) (Ruthenium Red) and TRP ankyrin-1 (TRPA1) (HC-030031) and activator of protease-activated receptor (PAR)4 increased the pain pressure threshold, whereas activators of PAR2 and TRPV4 decreased the pain pressure threshold in rats with IBS. The effect of SGD on pain pressure threshold in these rats was abolished by activators of TRPV1 (capsaicin), TRPV4 (RN1747), TRPA1 (Polygodial) and PAR2 (AC55541). In addition, CGRP levels in serum and colonic tissue were both increased in these rats. TNF-α level in colonic tissue was also significantly upregulated. However, the levels of 5-HT, SERT and CgA in colonic tissue were decreased. All these pathological changes in rats with IBS were attenuated by SGD.
CONCLUSION: SGD alleviated VHL and attenuated colon motility in IBS, partly by regulating TRPV1, TRPV4, TRPA1, PAR2, 5-HT, CgA and SERT, and reducing CGRP and TNF-α level.
Core tip: The present study demonstrated that Shugan decoction alleviated visceral hyperalgesia and attenuated colon motility in a rat model of irritable bowel syndrome, partly by regulating the expression of transient receptor potential (TRP) vanilloid 1, TRP vanilloid 4, TRP ankyrin-1 and protease-activated receptor 2, serotonin, chromogranin A and serotonin transporter, and reducing the levels of calcitonin-gene-related peptide and tumor necrosis factor-α.
- Citation: Shi HL, Liu CH, Ding LL, Zheng Y, Fei XY, Lu L, Zhou XM, Yuan JY, Xie JQ. Alterations in serotonin, transient receptor potential channels and protease-activated receptors in rats with irritable bowel syndrome attenuated by Shugan decoction. World J Gastroenterol 2015; 21(16): 4852-4863
- URL: https://www.wjgnet.com/1007-9327/full/v21/i16/4852.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i16.4852
Irritable bowel syndrome (IBS) is a functional gastrointestinal disorder characterized by altered bowel evacuation, bloating and visceral pain, without anatomical or biochemical abnormalities[1,2]. The pathological mechanism of IBS is not well understood. In recent years, laxatives or antidiarrheal agents, with or without spasmolytics, as well as serotonergic agents [e.g., tegaserod, a serotonin 5-hydroxytryptamine (5-HT4) agonist, and alosetron, a 5-HT3 antagonist], have achieved some therapeutic effects, but have not obtained satisfactory results due to their adverse effects[3]. Thus, to date, there are no standard therapeutic agents to treat IBS. Acupuncture and traditional Chinese medicine (TCM) have attracted increased attention due to their potential in the treatment of IBS. The efficacy of acupuncture on visceral hypersensitivity and related motility dysfunction in IBS has been demonstrated[4]. In China, there are many clinical formulas of TCM used in the treatment of IBS[2,5], for example, TongXieYaoFang (TXYF), is a classic TCM prescription[2] consisting of white attractylodes rhizome (Baizhu), white peony root (Baishao), dried old orange peel (Chenpi) and Ledebouriella root (Fangfeng). Shugan decoction (SGD) is another formula used to treat patients with diarrhea-predominant IBS, which is composed of TXYF and Radix bupleuri (Chaihu), and can alleviate visceral pain and improve bowel habits[6,7]. SGD shows an excellent therapeutic effect on patients who lack coordination between the liver and the spleen (TCM syndrome type)[6]. However, the functional mechanism of SGD is poorly understood.
Visceral hypersensitivity and gastrointestinal dysmotility are two primary characteristics of IBS. Transient receptor potential vanilloid type 1 (TRPV1) and TRP ankyrin-1 (TRPA1) have been shown to play an important role in visceral hypersensitivity, and are correlated with severity of abdominal pain in patients with IBS and inflammatory bowel disease (IBD), as well as IBS-like symptoms in rats with water avoidance stress (WAS)[8-12]. Alterations in protease-activated receptor (PAR)2 and PAR4 have also been indicated in visceral pain in IBS patients and stressed mice[13-16]. Serotonin (5-HT) in both the central and peripheral nervous systems, is involved in visceral hypersensitivity and gut motility disorders which can be attenuated by acupuncture and TCM[2,4,5,17]. Although their mechanisms have not been fully elucidated, regulation of the 5-HT signaling pathway has been shown to play an important role in relieving the symptoms of IBS. In recent studies, it was demonstrated that many types of endocrine cells are affected in IBS patients[18-20]. Lower densities of cells positive for 5-HT and peptide YY are found in the colon of patients with IBS, compared with healthy individuals. In addition, the density of chromogranin A (CgA) is reduced in the colon of patients with IBS[18-20].
A suitable animal model is important for elucidating the pathophysiological process of IBS. Previous studies have shown that repeated exposure to WAS induces sustained visceral hypersensitivity and gastrointestinal motility disorders in rats[21]. Therefore, WAS-exposed rats could reflect the typical characteristics of IBS. In the present study, the efficacy of SGD and its underlying mechanisms of action on visceral hypersensitivity and colonic dysmotility were evaluated using WAS in a rat model of IBS.
White attractylodes rhizome (Baizhu), white peony root (Baishao), dried old orange peel (Chenpi), ledebouriella root (Fangfeng), and Radix bupleuri (Chaihu) were purchased as crude herbs from the Yanghetang Pharmacy (Shanghai, China). Rabbit anti-CgA polyclonal antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, United States); the SABC kit was purchased from Boston Biochem (Wuhan, China). Methanol was obtained from Merck (Darmstadt, Germany). Triple deionized water was from Millipore (Bedford, MA, United States) and was prepared for all aqueous solutions. Ruthenium Red, capsaicin, HC-030031, polygodial, AY-NH2, RN1747 and AC55541 were obtained from Tocris Bioscience (MI, United Kingdom). Saikosaponin d, penoniflorin, poncirin, synephrine, atractylenolide III, isofraxidin, hesperidin and neohesperidin were purchased from Shanghai Research and Development Centre for Standardization of Chinese Medicines (Shanghai, China). Calcitonin-gene-related peptide (CGRP) ELISA kit was purchased from Cusabio Biotech (Wuhan, China). Tumor necrosis factor (TNF)-α ELISA kit was obtained from ExCell (Shanghai, China). Rabbit anti-5-HT antibody was obtained from ShangHai Xiangsheng Biotechnology Co. Ltd. (Shanghai, China).
SGD is comprised of five types of herbs with different mass ratios, which are listed in Table 1, and the total mass used was 31.5 g (common dose for adult humans). TXYF contained four types of herbs with a total mass of 25.5 g dry herbs, which are also listed in Table 1. The dry powder of an aqueous extract of SGD or TXYF was prepared by the Herbal Chemistry Laboratory at Shanghai University of Traditional Chinese Medicine. SGD and TXYF extracts were prepared as follows. Briefly, 315 g crude SGD and 255 g crude TXYF were powdered and soaked in 8 volumes of water for 12 h, boiled for 2 h, filtered, and the liquid collected. The procedure was then repeated. The liquid from the two procedures was mixed together and evaporated under vacuum until the aqueous phase was extracted completely. After drying in a vacuum drying oven, 125 g SGD powder and 100 g TXYF powder were obtained separately.
| Chinese name | Latin name | Common name | Drug (dry, g) | Formula names | |
| Baizhu | Rhizoma Atractylodis Macrocephalae | White attractylodes rhizome | 9.0 | TongXieYaoFang | Shugan decoction |
| Baishao | Radix Paeoniae Alba | white peony root | 6.0 | ||
| Chenpi | Pericarpium Citri Reticulatae | dried old orange peel | 6.0 | ||
| Fangfeng | Radix Saposhnikoviae | Ledebouriella root | 4.5 | ||
| Chaihu | Radix bupleuri | Radix bupleuri | 6.0 | ||
The samples were determined using the Agilent 1100 chromatographic system, consisting of a solvent degasser, a quaternary gradient pump, a diode array detector, and a data station with analytical software (Chemstation 8.03; Agilent, SF, CA, United States). The separation was performed on a Kromasil C18 analytical column (250 mm × 4.6 mm, 5 μm) and maintained at 25 °C. The mobile phase was a mixture of methanol/0.1% phosphoric acid aqueous solution (85:15, v:v) with a flow rate of 1 mL/min. The injection volume was 10 μL and the detection wavelength was set at 210 nm. The standard solutions of saikosaponin d, penoniflorin, poncirin and atractylenolide III were prepared with methanol.
Male Sprague-Dawley rats (150-200 g body weight) were purchased from SLAC Laboratory Animal Co. Ltd. (Shanghai, China), and fed in the Experimental Animal Center of Shanghai University of Traditional Chinese Medicine. All animals were maintained at 22 °C with a 12-h light/dark cycle and had free access to rodent chow and water. All animal experiments were performed according to the protocols approved by the Animal Care and Use Committee of Shanghai University of Traditional Chinese Medicine. The rats were randomly divided into the control and WAS groups. After 3 d, the WAS rats were randomly divided into five subgroups: (1) IBS model group; (2) SGD-H group (1.97 g/kg); (3) SGD-M group (0.98 g/kg); (4) SGD-L group (0.49 g/kg); and (5) TXYF group (0.68 g/kg). SGD or TXYF extract was dissolved in distilled water and rats were treated by intragastric administration 1 h before WAS on day 4 and maintained for 7 d. The rats in the control and model groups were treated with distilled water.
WAS was induced as described previously[21], with minor modifications. The rats were placed on an island-like plastic platform (10 cm × 8 cm × 8 cm), which was fixed in the center of a plexiglass pool (45 cm × 25 cm × 25 cm) filled with fresh tap water (25 °C) up to 7 cm (1 cm below the top of the platform), for 1 h once daily and repeated for 10 consecutive days. Stress procedures were performed between 09:00 and 11:30 am to minimize diurnal variations in response.
Fecal pellet output was used to estimate distal colonic motility as a validated index[22]. Fecal pellets found in the pool were counted at the end of 1 h WAS. Control animals were left in the home cage for 1 h to count the fecal pellets.
Colorectal distension (CRD) induces contraction of the abdominal and hind limb muscles, and is termed abdominal withdrawal reflex (AWR), which has been validated as a quantitative method to determine visceral sensitivity in rats[10,23]. Behavioral responses to CRD were assessed by measuring the pressure threshold of CRD, that is, the minimum pressure that induced the first AWR. Immediately after the end of 1 h WAS on the 10th day, the rats were lightly anesthetized with ether while a deflated latex balloon (5 mm diameter at full inflation) was inserted intra-anally into the descending colon and rectum with its end 1 cm proximal to the anal sphincter. The balloon catheter (2 mm diameter) was fixed at the root of the tail. The rats were then placed into a small Lucite cubicle (20 cm × 8 cm × 8 cm) and allowed to recover from anesthesia for 30 min before testing. To measure the pressure threshold of CRD, the colorectal balloon was progressively inflated with an increment of 5 mmHg until pain behavior was observed, namely, at the first sign of obvious contraction in the abdominal wall. The measurement was repeated three times with at least 5-min intervals for recovery. During the measurement, the investigators were blinded to the treatment groups.
Rats were deeply anesthetized with sodium pentobarbital, and the colonic tissues were excised and fixed in 10% formaldehyde solution. After being paraffin embedded and sectioned, the paraffin slides were deparaffinized in xylene I, II and III for 15, 10 and 10 min, respectively, and dehydrated in 100%, 95%, 85% and 75% ethanol for 5 min, respectively. The sections were stained with hematoxylin-eosin (HE), dehydrated in 95%, 85% and 75% ethanol, cleared in xylene, and finally mounted with Permount Mounting Medium (Shanghai, China). Morphological changes in the colonic mucosa were observed and photographed under a light microscope (200 × objective) equipped with a Nikon color digital camera system.
Blood samples were centrifuged at 3000 rpm, at 4 °C for 10 min. Serum was collected and immediately frozen in liquid nitrogen and stored at -80 °C for further study. The frozen colonic tissues were homogenized and lysed in tissue lysis buffer, and centrifuged at 12000 g, at 4 °C for 10 min. The supernatant was collected. The level of calcitonin-gene-related peptide (CGRP) in serum was measured by ELISA (Cusabio Biotech), and the level of TNF-α in colonic tissue was determined by ELISA (ExCell), according to the manufacturer’s instructions.
The sections were deparaffinized in xylene and an ethanol gradient. The endogenous peroxidase was quenched in methanol: 30% H2O2 (9:1) for 10 min, and microwaved for 10 min in citrate buffer (10 mmol/L, pH 6.0) for antigen retrieval. Sections were blocked in 5% bovine serum albumin at 37 °C for 1 h, followed by incubation with goat anti-rabbit polyclonal CgA antibody (Santa Cruz Biotechnology) at 1:30 dilution, or rabbit anti-5-HT polyclonal antibody (Xiangsheng, Shanghai, China) at 1:100 dilution overnight at 4 °C, then followed by incubation with secondary antibody-peroxidase conjugate at 37 °C for 1 h. PBS was used for washing after each step. Subsequent visualization was performed using diaminobenzidine as a chromogen (Dako, Denmark).
Positive expression was displayed by brown color, and the cell nuclei were stained blue by hematoxylin. Positive expression of CgA and 5-HT in the colon was observed and photographed under a light microscope at × 200 magnification. Five randomly selected × 200 magnification fields were evaluated using a BH2 microscope (Olympus, Tokyo, Japan) equipped with a Nikon 4500 digital camera. The area and optical density (OD) of CgA- and 5-HT-positive cells in each field were counted and processed by the computer-aided automatic image analysis system (Qiu Wei, Shanghai, China). The immunohistochemical index was calculated as the average integral OD: [(positive area × OD)/ total area].
Each value represents mean ± SD. Differences between two groups were analyzed using the Student’s t test, and statistical comparisons among more than two groups were subjected to One-way analysis of variance (ANOVA) followed by Dunnett’s test using GraphPad Prism 5.0. P < 0.05 was considered statistically significant.
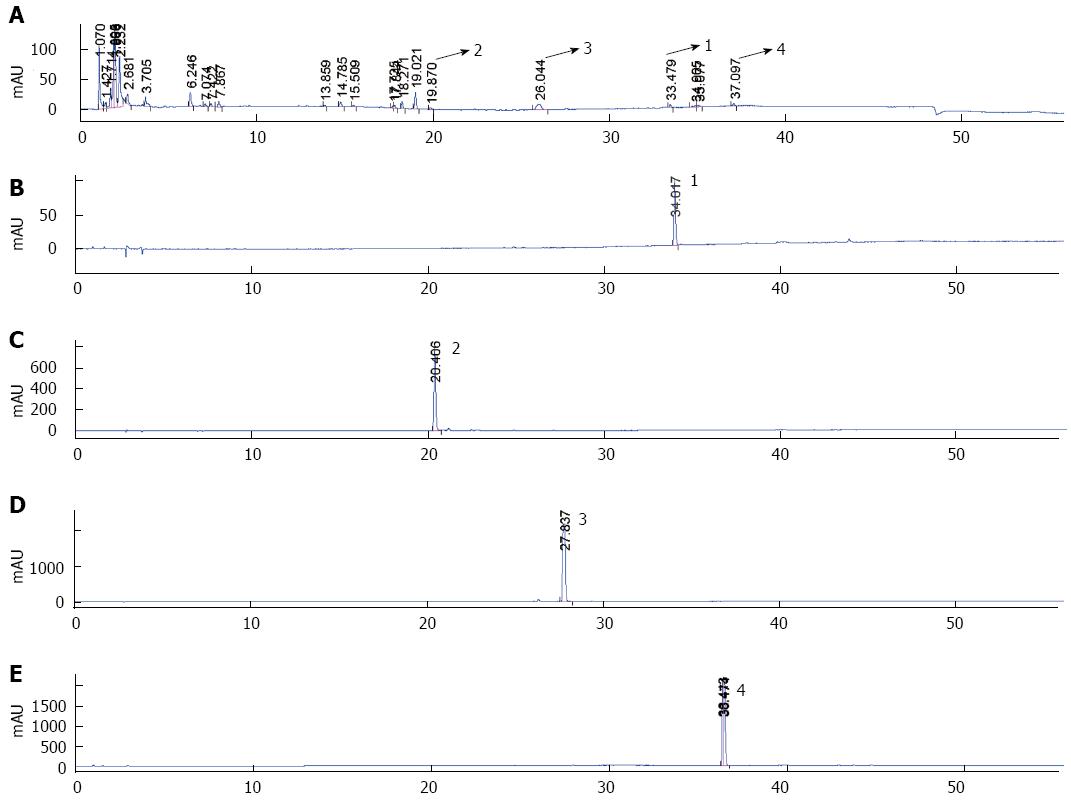
The ingredients in the water extract of SGD were determined by high-performance liquid chromatography (HPLC) analysis. Although many chemical compounds were detected by HPLC-MS/MS analysis (data not shown), we could not obtain all the standard chemical compounds shown in the HPLC-MS/MS analysis, thus, we were only able to confirm the following chemical ingredients in the water extract of SGD: saikosaponin d, penoniflorin, poncirin, and atractylenolide III (Figure 1). The other chemical ingredients should be analyzed further.
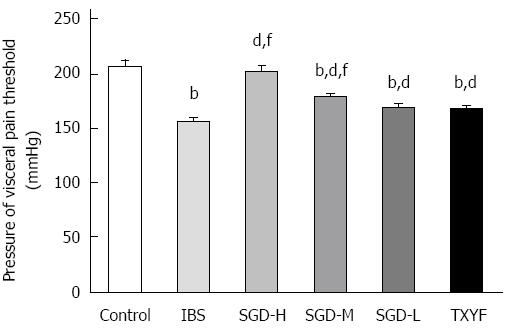
At the end of 10 d of WAS stimulation, the AWR pressure threshold of CRD was measured. The AWR pressure threshold of rats in the IBS model group was significantly lower than that of rats in the control group (P < 0.01), which demonstrated that visceral hyperalgesia (VHL) was successfully induced by WAS (Figure 2). The AWR pressure threshold of SGD-treated rats was significantly higher than that of the IBS model rats (P < 0.01). TXYF was able to reverse the AWR pressure threshold in model rats (P < 0.01). However, SGD-H and SGD-M had a greater effect on AWR pressure threshold than TXYF (P < 0.01, P < 0.01, respectively).
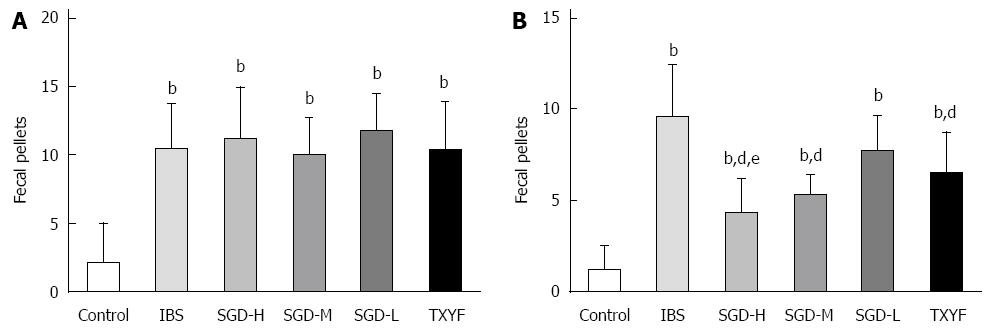
As shown in Figure 3, compared with control rats, the number of fecal pellets produced by model rats was significantly increased (P < 0.01), then attenuated by the water extract of SGD (P < 0.01). TXYF also decreased the number of fecal pellets produced by model rats (P < 0.01). However, SGD-H had a better effect on fecal pellet output than TXYF (P < 0.05).
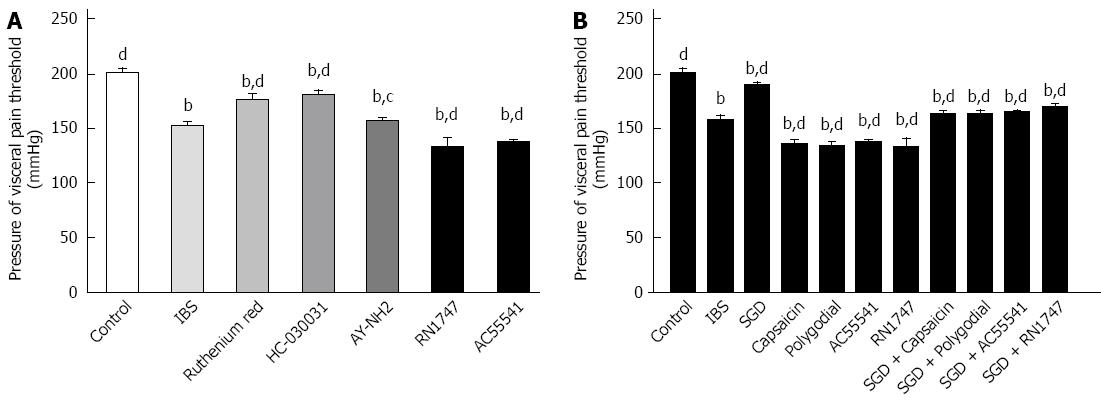
Blockers of TRPV1 (Ruthenium Red) and TRPA1 (HC-030031), or the PAR4 agonist (AY-NH2) increased the AWR pressure threshold in IBS model rats (P < 0.01, P < 0.01, P < 0.05, respectively) (Figure 4A). In contrast, agonists of PAR2 (AC55541), TRPV1 (capsaicin), TRPV4 (RN1747) and TRPA1 (polygodial) decreased AWR pressure threshold in model rats (P < 0.01, P < 0.01, P < 0.01, P < 0.01, respectively) (Figure 4A and B). The effect of SGD on AWR pressure threshold was weakened or abolished by agonists of PAR2 (AC55541), TRPV1 (capsaicin), TRPV4 (RN1747) and TRPA1 (polygodial) (P < 0.01, P < 0.01, P < 0.01, P < 0.01, respectively).
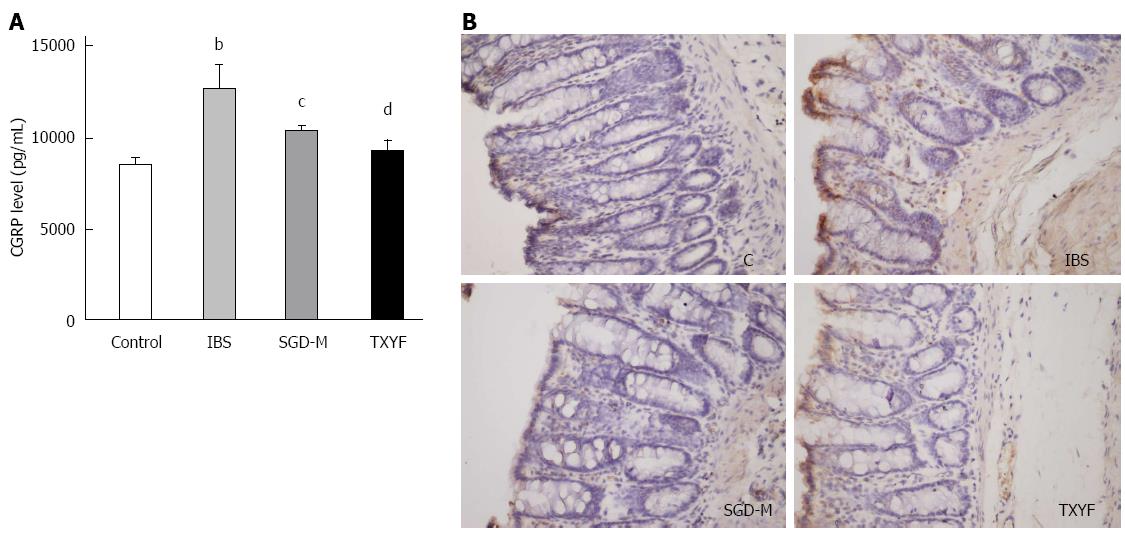
ELISA and immunohistochemistry indicated that CGRP in serum (P < 0.01) or in colonic tissue of WAS rats was elevated, compared with control rats. The water extract of SGD and TXYF reduced CGRP expression in serum (P < 0.05, P < 0.01, respectively) (Figure 5A) and colonic tissue (Figure 5B) of WAS rats.
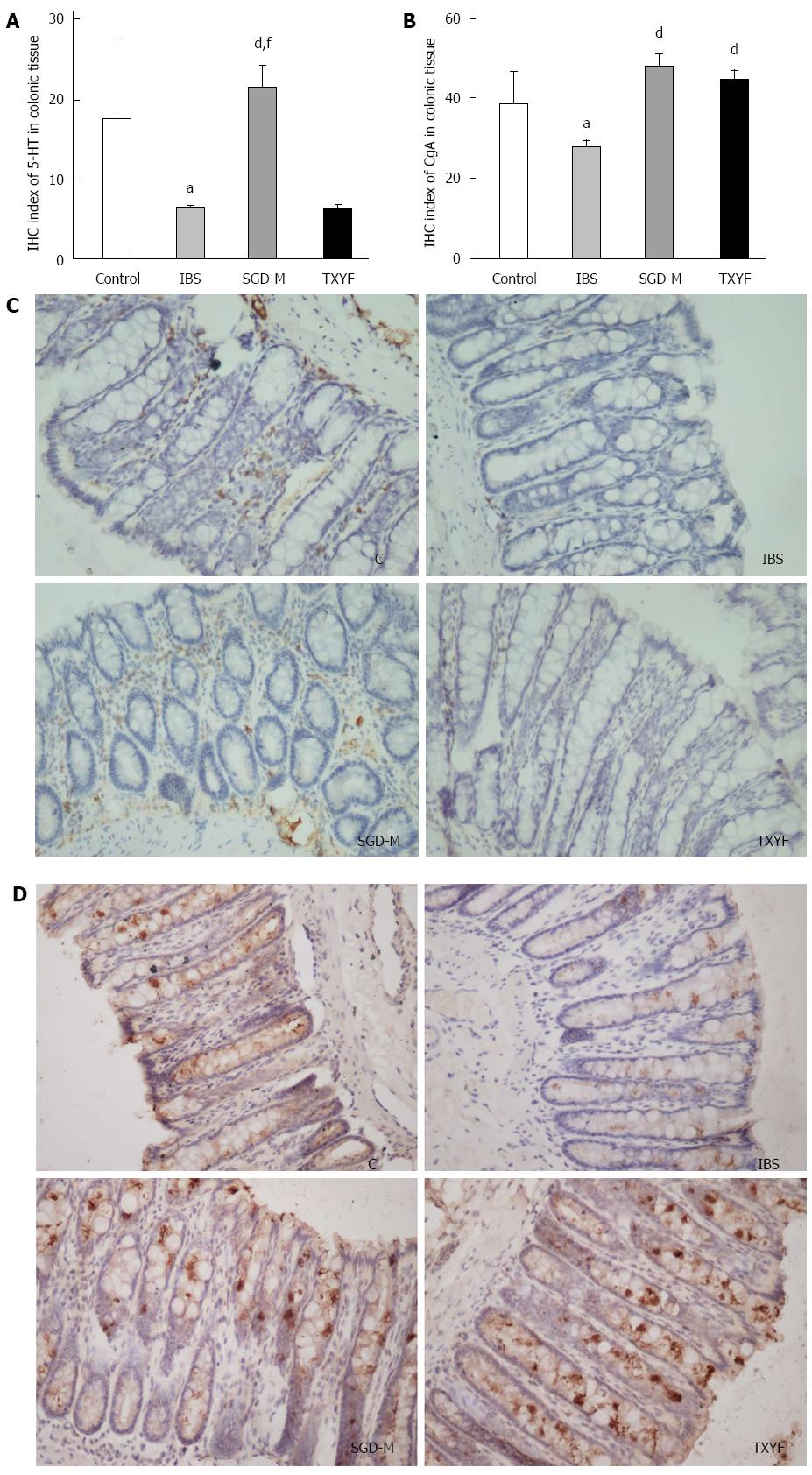
To determine whether the expression of 5-HT and CgA in the colonic tissue of rats was affected by WAS, the expression of 5-HT and CgA was detected by immunohistochemistry. As shown in Figure 6, after repeated exposure to WAS, the expression of 5-HT or CgA in colonic mucosa of IBS model rats was lower than that in the colonic mucosa of control rats (P < 0.05, P < 0.05, respectively). Treatment with the water extract of SGD increased expression of 5-HT and CgA in the colonic mucosa of IBS model rats (P < 0.01, P < 0.01, respectively). TXYF increased the expression of CgA (P < 0.01), but not 5-HT (P > 0.05) in colonic mucosa.
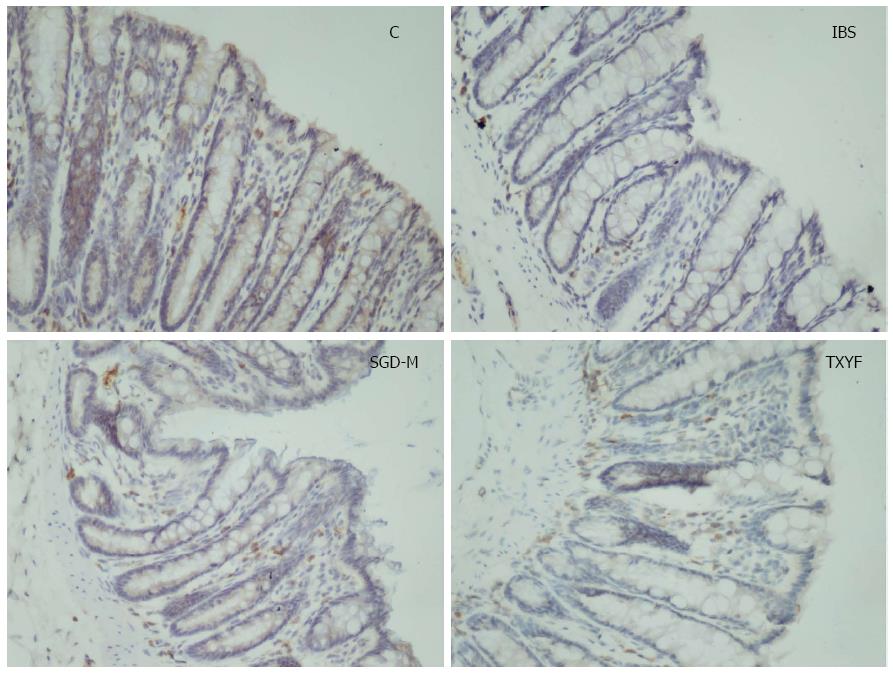
Figure 7 shows that the protein expression of serotonin transporter (SERT) was significantly decreased in colonic tissues of IBS model rats, compared with control rats. After treatment with the water extract of SGD or TXYF, SERT expression in colonic tissues was increased.
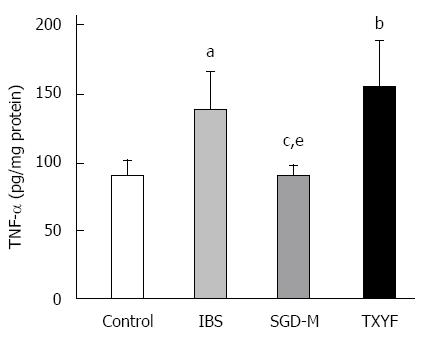
Although HE staining showed no inflammation in the colonic tissues of rats with chronic WAS (data not shown), Figure 8 shows that the level of TNF-α in colonic tissue of IBS model rats was higher than that in control rats (P < 0.05). The water extract of SGD, but not TXYF significantly reduced the TNF-α level in colonic tissue of WAS rats (P < 0.05, P > 0.05, respectively).
In this study, we found that the water extract of SGD attenuated VHL by inhibiting the activation of TRPV1, TRPV4, TRPA1 and PAR2 and regulating 5-HT, SERT and CgA expression, as well as TNF-α and CGRP levels, which may contribute to the improvement in VHL and abnormal colonic motility in WAS rats.
Currently, the detailed pathological mechanism of IBS is not clear. Due to the difficulty in removing colonic tissue samples from IBS patients, animal models of IBS are pivotal in clarifying the pathogenesis of IBS. To date, repeated WAS and neonatal maternal separation have been used to create animal models of IBS to study the pathological process or to evaluate the efficacy of western medicines or TCM[21,23]. In previous studies, rats with repeated exposure to WAS developed persistent VHL, exhibited anxiety-like behaviors, and increased fecal pellet output[21,23]. In our study, the AWR pressure threshold in response to CRD in model rats was lower than that in control rats, which indicated that the WAS rats suffered from VHL. The number of fecal pellets excreted after 1 h exposure to WAS in model rats was increased compared with control rats, which showed that abnormal colonic motility was present in the model rats. The water extract of SGD significantly improved this dysfunction (visceral hypersensitivity and abnormal colonic motility).
Abnormal pain perception or visceral hypersensitivity is considered to be an important pathological basis underlying the symptoms of some IBS patients[11]. The mechanism of action of SGD on VHL was also investigated in the present study. TRPV1 is widely expressed in the colonic afferent dorsal root ganglia (DRG) neurons and throughout the gastrointestinal tract. TRPV1 is a ligand-gated ion channel that modulates the sensation of pain and thermal hyperalgesia as well as colonic hypersensitivity[24,25]. TRPA1 is coexpressed with TRPV1 in primary sensory neurons[26], and has attracted the attention of researchers investigating gut sensation. TRPA1 deletion markedly reduces colitis-induced mechanical hyperalgesia in mice[27]. Moreover, activation of TRPA1 is proposed to contribute to the somatic hyperalgesia triggered by tissue damage and inflammation[28]. In WAS rats, protein expression of TRPV1 and TRPA1 in the colonic afferent DRG is significantly upregulated[10]. In our previous studies, increased expression of TRPV1 was also found in the DRG and colonic tissue of WAS rats with IBS-like symptoms[23,29]. Some studies have shown that increased TRPV1 expression is also present in the colonic tissue of IBS patients[11,30]. However, evidence of the involvement of TRPV1 in visceral perception in IBS and in WAS rats with IBS-like symptoms is still lacking. In our previous studies, SGD decreased the elevated expression of TRPV1, substance P and CGRP in the DRG and colonic tissue[23,29]. However, whether TRPV1 is involved in the therapeutic effect of SGD on VHL of WAS-induced IBS-like symptoms has not been clarified. In a neonatal maternal separation IBS murine model, expression of the TRPV4 gene was significantly increased[31]. However, the role of TRPV4 in visceral perception in IBS is not well understood. In the present study, blockers of TRPV1 and TRPA1 and an activator of TRPV4 showed that TRPV1, TRPV4 and TRPA1 are involved in WAS-induced VHL. PARs play an important role in the modulation of visceral pain[32]. PAR2 is highly expressed in the gastrointestinal tract and implicated in the genesis of hyperalgesia in a number of models of colitis[32]. Activation of PAR2 in the lumen affects visceral pain[33]. In rats, the intracolonic infusion of PAR2 agonists (SLIGRL and trypsin) induces delayed hypersensitivity to colonic distension[34]. In contrast, PAR4 may mediate antinociceptive activity. PAR4 activation significantly increases the nociceptive threshold in response to noxious stimuli[35]. Thus, blockade of PAR2 at the periphery and/or inhibition of colonic luminal protease activity may be new targets for treating gut hypersensitivity and IBS. In the present study, activators of PAR2 and PAR4 showed that activation of PAR2, but not PAR4 was involved in WAS-induced VHL. However, PAR4 activation increased AWR pressure threshold. Moreover, activators of TRPV1, TRPV4, TRPA1 and PAR2 also demonstrated that SGD attenuated VHL in WAS rats by inhibiting TRPV1, TRPV4, TRPA1 and PAR2. Whether there is crosstalk between TRPV1, TRPV4, TRPA1 and PAR2 warrants further study.
5-HT is an important neurotransmitter which is involved in mediating psychological stress, VHL and gastrointestinal dysmotility by influencing the sympathetic, parasympathetic, and enteric nervous systems[18,36]. In healthy individuals, 5-HT is released mainly from enterochromaffin (EC) cells in the gastrointestinal tract and is inactivated via reuptake by SERT from the mucosa into nerve fibers[37]. Altered SERT expression and function in IBS could result in abdominal hypersensitivity and abnormal colonic motility[5,18]. Previous studies indicated that mucosal 5-HT and SERT were both decreased in ulcerative colitis, diarrhea-predominant IBS (IBS-D), and constipation-predominant IBS (IBS-C)[18]. However, Kerckhoffs et al[38] reported that IBS-C patients have increased mucosal 5-HT concentration and EC cell numbers, whereas IBS-D patients have reduced mucosal 5-HT concentration. Other studies have also shown that SERT expression was increased in the colon together with an increased concentration of 5-HT in neonatal maternally separated rats[5]. Thus, altered SERT expression in different states may be associated with different processes of IBS development, which should be investigated in further studies. In the present study, compared with control rats, expression of SERT and 5-HT was significantly decreased in the colonic mucosa of WAS rats. The water extract of SGD upregulated the expression of SERT and 5-HT in the colon. The above results indicated that the water extract of SGD regulated the expression of 5-HT and SERT to attenuate IBS-like symptoms. These results also suggested that the decreased level of 5-HT in the colon possibly resulted from reduced biosynthesis of 5-HT, but not a change in 5-HT reuptake by SERT.
Endocrine cells are also important in the pathological process of IBS[20]. Recent research demonstrated that colonic endocrine cells are altered in patients with IBS[18-20]. Increased numbers of mast cells in the terminal ileum are observed in patients with IBS[39]. It is reported that there are low densities of 5-HT-immunoreactive (EC) cells, peptide-YY-immunoreactive cells and CgA-immunoreactive (enteroendocrine) cells in the colon of patients with IBS[20]. In the present study, low expression of CgA and 5-HT was seen in the colonic mucosa of WAS rats, which indicated low densities of CgA-positive and 5-HT-positive endocrine cells. These changes were improved by the water extract of SGD.
Although HE staining results did not show inflammation in colonic tissue in model rats, the level of TNF-α in colonic tissue was significantly increased, but not serum TNF-α levels, and was decreased by the water extract of SGD. Foley et al[40] proved that TNF-α decreased SERT function and expression in Caco 2 cells. In the present study, the change in SERT and TNF-α was similar. However, whether there was a correlation between the expression of SERT and the level of TNF-α in this model requires clarification. In other research, TNF-α stimulated the expression and secretion of CGRP from rat trigeminal ganglion neurons[41]. In the present study, serum and colonic tissue CGRP levels were both increased in model rats after repeated exposure to WAS, accompanied by an increase in the level of TNF-α in colonic tissue. Following treatment with the water extract of SGD, the alterations in TNF-α and CGRP levels in model rats was attenuated.
In conclusion, the water extract of SGD enhanced the decreased AWR pressure threshold in response to CRD and attenuated abnormal colonic transit mainly by inhibiting TRPV1, TRPV4, TRPA1 and PAR2, but also by regulating 5-HT, SERT and CgA, as well as possibly reversing the imbalance in colonic 5-HT-positive and CgA-positive endocrine cells, thus decreasing the level of CGRP and TNF-α.
We thank Dr. Ying Dong for help in statistical analysis.
Irritable bowel syndrome (IBS) is a functional gastrointestinal disorder and not an organic disease. It is characterized by altered bowel evacuation, bloating and visceral pain, without anatomical or biochemical abnormalities. Currently, there are no standard drugs to treat IBS. Traditional Chinese medicine (TCM) has shown promising effects on IBS, however, the mechanism of action requires clarification. In this investigation, the efficacy of Shugan decoction (SGD) and its underlying mechanisms in IBS were evaluated in IBS-like rats with visceral hypersensitivity and colonic dysmotility.
The pathological mechanism of IBS is not well understood. Visceral hypersensitivity and gastrointestinal dysmotility are two pivotal characteristics of IBS. Transient receptor potential vanilloid type 1 (TRPV1), TRP ankyrin-1 (TRPA1), protease-activated receptor (PAR)2 and PAR4, as well as serotonin [5-hydroxytryptamine (5-HT)] in the central and peripheral nervous systems play important roles in abdominal pain severity and gut motility disorders in IBS and inflammatory bowel disease. In the present study, SGD alleviated visceral hyperalgesia (VHL) and attenuated colon motility in model rats, partly by regulating TRPV1, TRPV4, TRPA1, PAR2, 5-HT, chromogranin A (CgA), serotonin transporter (SERT) and reducing calcitonin-gene-related peptide (CGRP) and tumor necrosis factor (TNF)-α level.
TRPV1, TRPA1, PAR2, PAR4, 5-HT and 5-HT-positive endocrine cells are involved in abdominal pain severity and gut motility disorders in IBS patients. The effect of TCM on the above molecules, as well as its functional mechanism, is poorly understood. In the present investigation, the water extract of SGD attenuated the decrease in pain pressure threshold in response to colorectal distension and attenuated abnormal colonic transit, mainly by inhibiting TRPV1, TRPV4, TRPA1 and PAR2, and regulating 5-HT, SERT and CgA, as well as possibly reversing the imbalance in colonic 5-HT-positive and CgA-positive endocrine cells, decreasing the level of CGRP and TNF-α.
Increased stress in daily life has led to an increase in IBS, and the development of therapeutic agents is urgently needed. TCM has some beneficial effects on IBS, however, its use in IBS patients is hindered by our lack of understanding of its mechanism of action. In the present investigation, SGD relieved VHL and attenuated abnormal colonic transit, mainly by inhibiting TRPV1, TRPV4, TRPA1 and PAR2, regulating 5-HT, SERT and CgA, as well as possibly reversing the imbalance of colonic 5-HT-positive and CgA-positive endocrine cells, decreasing the level of CGRP and TNF-α. Thus, the present study shows the beneficial therapeutic effect and functional mechanism of TCM such as SGD in IBS patients.
IBS is a functional gastrointestinal disorder, and is characterized by altered bowel evacuation, bloating and visceral pain, without anatomical or biochemical abnormalities. TongXieYaoFang (TXYF) is a classic TCM prescription and consists of white attractylodes rhizome (Baizhu), white peony root (Baishao), dried old orange peel (Chenpi) and Ledebouriella root (Fangfeng). SGD is another formula used to treat patients with diarrhea-predominant IBS, which is composed of TXYF and Radix bupleuri (Chaihu), and can alleviate visceral pain and improve the bowel habits of patients with a lack of coordination between the liver and the spleen (TCM syndrome type). TRPV1 is widely expressed in the colonic afferent dorsal root ganglia neurons and throughout the gastrointestinal tract. TRPV1 is a ligand-gated ion channel that modulates pain sensation and thermal hyperalgesia as well as colonic hypersensitivity. TRPA1 is coexpressed with TRPV1 in primary sensory neurons. PAR2 is highly expressed in the gastrointestinal tract and implicated in the genesis of hyperalgesia in a number of models of colitis. 5-HT is an important neurotransmitter, which alters visceral perception and motor dysfunction in IBS by influencing the sympathetic, parasympathetic, and enteric nervous systems.
The authors described Shugan-decoction attenuates the alteration of 5-HT, TRP channels and PAR receptors of rats exposed to water avoidance stress. The article is informative and well-presented.
Institutional Review Board statement: This study has been reviewed and approved by the Institutional Review Board of Shanghai University of Traditional Chinese Medicine.
Institutional Animal Care and Use Committee statement: All animal experiments were performed according to the protocols approved by the University Animal Care and Use Committee of Shanghai University of Traditional Chinese Medicine (IACUC protocol number: 2014022).
P- Reviewer: Chiba T, Pehl C S- Editor: Ma YJ L- Editor: Webster JR E- Editor: Ma S
| 1. | Thompson WG. A world view of IBS. In Camilleri M, Spriller R, eds. Irritable bowel syndrome: diagnosis and treatment. Philadelphia and London: Saunders 2002; 17-26. |
| 2. | Hu XG, Xu D, Zhao Y, Yang XB, Meng J, Shen H, Guo J. The alleviating pain effect of aqueous extract from tong-xie-yao-fang, on experimental visceral hypersensitivity and its mechanism. Biol Pharm Bull. 2009;32:1075-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Camilleri M. LX-1031, a tryptophan 5-hydroxylase inhibitor, and its potential in chronic diarrhea associated with increased serotonin. Neurogastroenterol Motil. 2011;23:193-200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | Wu JC, Ziea ET, Lao L, Lam EF, Chan CS, Liang AY, Chu SL, Yew DT, Berman BM, Sung JJ. Effect of electroacupuncture on visceral hyperalgesia, serotonin and fos expression in an animal model of irritable bowel syndrome. J Neurogastroenterol Motil. 2010;16:306-314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Bian ZX, Zhang M, Han QB, Xu HX, Sung JJ. Analgesic effects of JCM-16021 on neonatal maternal separation-induced visceral pain in rats. World J Gastroenterol. 2010;16:837-845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 6. | Xie JQ, Zheng Y, Fei XY, Pan XX, Yuan JY, Xue J. Clinical study on “Shugan decoction” in treating diarrhea-predominant irritable bowel syndrome of live-spleen disharmony. Shanghai Zhongyiyao Daxue Xuebao. 2004;18:11-13. |
| 7. | Pan XX, Xie JQ. Clinical observation of Shugan decoction in treatment of irritable bowel syndrome. Shanghai Zhongyiyao Daxue Xuebao. 2006;20:48-50. |
| 8. | Akbar A, Yiangou Y, Facer P, Walters JR, Anand P, Ghosh S. Increased capsaicin receptor TRPV1-expressing sensory fibres in irritable bowel syndrome and their correlation with abdominal pain. Gut. 2008;57:923-929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 359] [Cited by in RCA: 391] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 9. | Akbar A, Yiangou Y, Facer P, Brydon WG, Walters JR, Anand P, Ghosh S. Expression of the TRPV1 receptor differs in quiescent inflammatory bowel disease with or without abdominal pain. Gut. 2010;59:767-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 172] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 10. | Yu YB, Yang J, Zuo XL, Gao LJ, Wang P, Li YQ. Transient receptor potential vanilloid-1 (TRPV1) and ankyrin-1 (TRPA1) participate in visceral hyperalgesia in chronic water avoidance stress rat model. Neurochem Res. 2010;35:797-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | van Wanrooij SJ, Wouters MM, Van Oudenhove L, Vanbrabant W, Mondelaers S, Kollmann P, Kreutz F, Schemann M, Boeckxstaens GE. Sensitivity testing in irritable bowel syndrome with rectal capsaicin stimulations: role of TRPV1 upregulation and sensitization in visceral hypersensitivity? Am J Gastroenterol. 2014;109:99-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 12. | Kojima R, Nozawa K, Doihara H, Keto Y, Kaku H, Yokoyama T, Itou H. Effects of novel TRPA1 receptor agonist ASP7663 in models of drug-induced constipation and visceral pain. Eur J Pharmacol. 2014;723:288-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Ibeakanma C, Ochoa-Cortes F, Miranda-Morales M, McDonald T, Spreadbury I, Cenac N, Cattaruzza F, Hurlbut D, Vanner S, Bunnett N. Brain-gut interactions increase peripheral nociceptive signaling in mice with postinfectious irritable bowel syndrome. Gastroenterology. 2011;141:2098-2108.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 14. | Zhao JH, Dong L, Shi HT, Wang ZY, Shi HY, Ding H. The expression of protease-activated receptor 2 and 4 in the colon of irritable bowel syndrome patients. Dig Dis Sci. 2012;57:58-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Valdez-Morales EE, Overington J, Guerrero-Alba R, Ochoa-Cortes F, Ibeakanma CO, Spreadbury I, Bunnett NW, Beyak M, Vanner SJ. Sensitization of peripheral sensory nerves by mediators from colonic biopsies of diarrhea-predominant irritable bowel syndrome patients: a role for PAR2. Am J Gastroenterol. 2013;108:1634-1643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 16. | Cenac N. Protease-activated receptors as therapeutic targets in visceral pain. Curr Neuropharmacol. 2013;11:598-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Yang JM, Xian YF, Ip PS, Wu JC, Lao L, Fong HH, Sung JJ, Berman B, Yeung JH, Che CT. Schisandra chinensis reverses visceral hypersensitivity in a neonatal-maternal separated rat model. Phytomedicine. 2012;19:402-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Coates MD, Mahoney CR, Linden DR, Sampson JE, Chen J, Blaszyk H, Crowell MD, Sharkey KA, Gershon MD, Mawe GM. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126:1657-1664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 554] [Cited by in RCA: 570] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 19. | El-Salhy M, Lomholt-Beck B, Hausken T. Chromogranin A as a possible tool in the diagnosis of irritable bowel syndrome. Scand J Gastroenterol. 2010;45:1435-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | El-Salhy M, Gundersen D, Ostgaard H, Lomholt-Beck B, Hatlebakk JG, Hausken T. Low densities of serotonin and peptide YY cells in the colon of patients with irritable bowel syndrome. Dig Dis Sci. 2012;57:873-878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Bradesi S, Schwetz I, Ennes HS, Lamy CM, Ohning G, Fanselow M, Pothoulakis C, McRoberts JA, Mayer EA. Repeated exposure to water avoidance stress in rats: a new model for sustained visceral hyperalgesia. Am J Physiol Gastrointest Liver Physiol. 2005;289:G42-G53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 228] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 22. | Hong S, Fan J, Kemmerer ES, Evans S, Li Y, Wiley JW. Reciprocal changes in vanilloid (TRPV1) and endocannabinoid (CB1) receptors contribute to visceral hyperalgesia in the water avoidance stressed rat. Gut. 2009;58:202-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 103] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 23. | Shang JJ, Yuan JY, Xu H, Tang RZ, Dong YB, Xie JQ. Shugan-decoction relieves visceral hyperalgesia and reduces TRPV1 and SP colon expression. World J Gastroenterol. 2013;19:8071-8077. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2577] [Cited by in RCA: 2691] [Article Influence: 107.6] [Reference Citation Analysis (0)] |
| 25. | Neri M. Irritable bowel syndrome, inflammatory bowel disease and TRPV1: how to disentangle the bundle. Eur J Pain. 2013;17:1263-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Kobayashi K, Fukuoka T, Obata K, Yamanaka H, Dai Y, Tokunaga A, Noguchi K. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J Comp Neurol. 2005;493:596-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 601] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 27. | Cattaruzza F, Spreadbury I, Miranda-Morales M, Grady EF, Vanner S, Bunnett NW. Transient receptor potential ankyrin-1 has a major role in mediating visceral pain in mice. Am J Physiol Gastrointest Liver Physiol. 2010;298:G81-G91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 28. | Obata K, Katsura H, Mizushima T, Yamanaka H, Kobayashi K, Dai Y, Fukuoka T, Tokunaga A, Tominaga M, Noguchi K. TRPA1 induced in sensory neurons contributes to cold hyperalgesia after inflammation and nerve injury. J Clin Invest. 2005;115:2393-2401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 498] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 29. | Yuan JY, Shang JJ, Xie JQ, Zheng Y, Fei XY, Shi HL, Sun QL. Effect of Shuganyin on visceral hyperalgesia in the repeated water avoidance stressed rats. Zhongyiyao Xuekan. 2012;30:2190-2192. |
| 30. | Chan CL, Facer P, Davis JB, Smith GD, Egerton J, Bountra C, Williams NS, Anand P. Sensory fibres expressing capsaicin receptor TRPV1 in patients with rectal hypersensitivity and faecal urgency. Lancet. 2003;361:385-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 232] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 31. | Distrutti E, Cipriani S, Mencarelli A, Renga B, Fiorucci S. Probiotics VSL#3 protect against development of visceral pain in murine model of irritable bowel syndrome. PLoS One. 2013;8:e63893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 32. | Karanjia R, Spreadbury I, Bautista-Cruz F, Tsang ME, Vanner S. Activation of protease-activated receptor-4 inhibits the intrinsic excitability of colonic dorsal root ganglia neurons. Neurogastroenterol Motil. 2009;21:1218-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Bueno L. Protease activated receptor 2: a new target for IBS treatment. Eur Rev Med Pharmacol Sci. 2008;12 Suppl 1:95-102. [PubMed] |
| 34. | Coelho AM, Vergnolle N, Guiard B, Fioramonti J, Bueno L. Proteinases and proteinase-activated receptor 2: a possible role to promote visceral hyperalgesia in rats. Gastroenterology. 2002;122:1035-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 162] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 35. | Asfaha S, Cenac N, Houle S, Altier C, Papez MD, Nguyen C, Steinhoff M, Chapman K, Zamponi GW, Vergnolle N. Protease-activated receptor-4: a novel mechanism of inflammatory pain modulation. Br J Pharmacol. 2007;150:176-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 103] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 36. | Faure C, Patey N, Gauthier C, Brooks EM, Mawe GM. Serotonin signaling is altered in irritable bowel syndrome with diarrhea but not in functional dyspepsia in pediatric age patients. Gastroenterology. 2010;139:249-258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 116] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 37. | Wade PR, Chen J, Jaffe B, Kassem IS, Blakely RD, Gershon MD. Localization and function of a 5-HT transporter in crypt epithelia of the gastrointestinal tract. J Neurosci. 1996;16:2352-2364. [PubMed] |
| 38. | Kerckhoffs AP, ter Linde JJ, Akkermans LM, Samsom M. SERT and TPH-1 mRNA expression are reduced in irritable bowel syndrome patients regardless of visceral sensitivity state in large intestine. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1053-G1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 39. | Wang SH, Dong L, Luo JY, Gong J, Li L, Lu XL, Han SP. Decreased expression of serotonin in the jejunum and increased numbers of mast cells in the terminal ileum in patients with irritable bowel syndrome. World J Gastroenterol. 2007;13:6041-6047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in CrossRef: 30] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 40. | Foley KF, Pantano C, Ciolino A, Mawe GM. IFN-gamma and TNF-alpha decrease serotonin transporter function and expression in Caco2 cells. Am J Physiol Gastrointest Liver Physiol. 2007;292:G779-G784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 41. | Bowen EJ, Schmidt TW, Firm CS, Russo AF, Durham PL. Tumor necrosis factor-alpha stimulation of calcitonin gene-related peptide expression and secretion from rat trigeminal ganglion neurons. J Neurochem. 2006;96:65-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 106] [Article Influence: 5.3] [Reference Citation Analysis (0)] |