Published online Apr 28, 2015. doi: 10.3748/wjg.v21.i16.4809
Peer-review started: October 27, 2014
First decision: December 11, 2014
Revised: January 14, 2015
Accepted: March 12, 2015
Article in press: March 12, 2015
Published online: April 28, 2015
Processing time: 187 Days and 7.3 Hours
Endoscopic ultrasound (EUS) is one of the most important modalities for the diagnosis of digestive tract diseases. EUS has been evolving ever since it was introduced. New techniques such as elastography and contrast enhancement have emerged, increasing the accuracy, sensitivity and specificity of EUS for the diagnosis of digestive tract diseases including pancreatic masses and lymphadenopathy. EUS-elastography evaluates tissue elasticity and therefore, can be used to differentiate various lesions. Contrast-enhanced EUS can distinguish benign from malignant pancreatic lesions and lymphadenopathy using the intravenous injection of contrast agents. This review discusses the principles and types of these new techniques, as well as their clinical applications and limitations.
Core tip: This article primarily focuses on emerging techniques such as elastography and contrast-enhanced endoscopic ultrasound. Principles, types and clinical applications are discussed. These emerging techniques have high accuracy, sensitivity and specificity in differential diagnosis between benign and malignant lesions.
- Citation: Meng FS, Zhang ZH, Ji F. New endoscopic ultrasound techniques for digestive tract diseases: A comprehensive review. World J Gastroenterol 2015; 21(16): 4809-4816
- URL: https://www.wjgnet.com/1007-9327/full/v21/i16/4809.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i16.4809
Endoscopic ultrasound (EUS) has continuously evolved since its initial introduction. With the development of accessories and technologies, EUS-guided fine-needle aspiration (FNA) has emerged as the gold standard for the diagnosis of gastrointestinal lesions. However, EUS-FNA is technically demanding and is associated with a low (but not negligible) risk of complications. EUS-elastography and contrast-enhanced EUS have emerged as non-invasive techniques in diagnosis of digestive disorders. Recently, 3-D EUS technology and EUS-guided interventions such as biliary and pancreatic fluid collection drainage and fine-needle injections have been introduced and are rapidly gaining in popularity. EUS-guided interventions will be discussed elsewhere.
Recently, many studies have demonstrated that elastography and contrast-enhanced EUS have high accuracy, sensitivity and specificity in discriminating between benign and malignant lesions (Table 1).
| Ref. | No. of cases | Target lesions | Techniques | Accuracy | Specificity | Sensitivity |
| König et al[13] | 151 | Prostatic lesions | RTE | 84.10% | N/A | N/A |
| Kanamori et al[48] | 46 | LNs lesions | CE | 82.10% | 77.30% | 88.20% |
| Alam et al[12] | 85 | LNs lesions | RTE | 84% | 59% | 98% |
| Kamoi et al[54] | 107 | Prostatic | RTE | 76% | 81% | 68% |
| Lesions | ||||||
| Ohno et al[44] | 87 | IPMNs | CE | 75.90% | 92.90% | 60% |
| Giovannini et al[33] | 222 | LNs and PLs | RTE | N/A | 82.5% (LN) | 91.8% (LN) |
| 80.0% (PL) | 92.3% (PL) | |||||
| Săftoiu et al[22] | 54 | Pancreatic masses | CE and RTE | 83.30% | 95.20% | 75.80% |
| Napoleon et al[42] | 35 | Pancreatic masses | CE | 86% | 88% | 89% |
| Xia et al[49] | 43 | Intra-abdominal lesions | CE | 97.60% | 100% | 96.30% |
| Săftoiu et al[6] | 258 | Pancreatic masses | RTE | 85.40% | 66% | 93.40% |
| Xu et al[7] | 368 | LNs lesions | RTE | N/A | 91% | 85% |
| Sakamoto et al[51] | 76 | GISTs | CH | 83% | 63% | 100% |
| Kapoor et al[55] | 50 | Prostatic lesions | RTE | N/A | 86.80% | 91.70% |
| Waage et al[56] | 69 | Rectal lesions | RTE | 94% | 96% | 93% |
| Hocke et al[5] | 58 | Pancreatic lesions | RTE | N/A | 94.7%(RTE) | 33.4%(RTE) |
| CE | 89.5%(CE) | 92.3%(CE) | ||||
| Dawwas et al[31] | 104 | Pancreatic masses | RTE | 86.50% | 16.70% | 100% |
| Kitano et al[39] | 277 | Pancreatic lesions | CH | N/A | 94.40% | 91.20% |
| Gong et al[41] | 1139 | Pancreatic masses | CE | N/A | 93% | 93% |
| Knabe et al[3] | 40 | LN lesions | RTE | 51.5 | 86.70% | 88.90% |
| Lee et al[43] | 37 | Pancreatic lesions | CH | 92% | N/A | 93% |
| Havre et al[34] | 39 | Pancreatic lesions | RTE | N/A | 71% | 67% |
| Imazu et al[59] | 36 | GB lesions | CH | 94.40% | 98% | 89.60% |
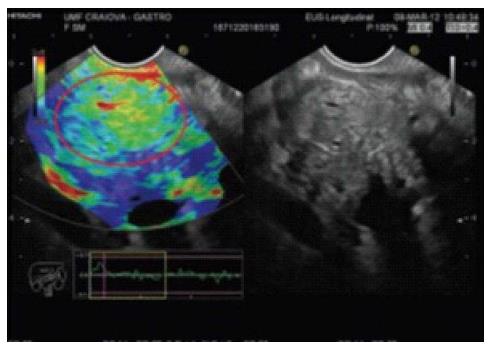
Elasticity varies in different types of tissues and in the same tissue affected by different pathologic states[1]. Elastography can evaluate the hardness of tissue by measuring its elasticity[2]. The principle of elastography is that tissue compression produces strain; alterations in strain can be detected and displayed in real time alongside conventional B-mode images with special software[3,4]. Elastography was developed in order to complement conventional EUS for the assessment of previously hard-to-reach tumors near the gastrointestinal tract, such as pancreatic masses[5,6] and lymph nodes[1,7].
Qualitative elastography: Less tissue deformation is caused by compression of hard tissue than of soft tissue[4]. The degree of deformation is represented by different colors[4,8]. Hard tissue is blue and soft tissue is red; tissues with an intermediate elasticity are in the green-yellow spectrum[6,9].
Hue/SH analysis: A histogram is used to represent the digital color distribution. Specialized software (Image J or SH) analyzes the color of the pixels inside the target lesions and each pixel color is represented by a value from 0 to 255 (soft to hard)[4,8]. Histograms produce an average value that represents the overall elasticity of tissues[6].
Strain ratio: Strain ratio (SR) is based on a different principle from histograms. The elasticity of the target tissue is expressed not as an absolute value, but as a relative ratio compared to the reference value provided by these tissues[2]. Two non-overlapping areas inside the region of interest (ROI) are selected: The lesion (area A) and the reference zone (area B). The B/A quotient yields the SR[10,11].
Elastography has been used to evaluate several organs including the breast, thyroid, prostate, cervix, liver and others[12,13]. Studies have demonstrated that primarily blue masses are malignant, whereas red and green masses are considered to be benign.
The contrast agents used in this new technique are gas-containing microbubbles that are covered by a protective shell[14]. The principles of contrast-enhanced EUS are as follows: when subjected to an ultrasonic signal, the microbubbles oscillate or break and generate components that can be detected and reconstructed on an ultrasound image[15,16], and components of a higher frequency are required for EUS enhancement[17].
Two generations of contrast agents have been developed. The first-generation agent was Levovist, which is composed of microbubbles of air covered by galactose and palmitic acid[18]. However, Levovist requires high acoustic power to oscillate the microbubbles. Second-generation contrast agents, such as Sonovue, Sonazoid and Definity, can be oscillated or broken by lower acoustic power[19,20]. The development of these contrast agents promoted the use of harmonic imaging in EUS[21].
The contrast microbubbles are restricted to the vascular system and do not lead to enhancement of the entire circulatory system[21]. They are generally safe, and adverse events have rarely been observed.
Contrast-enhanced color and power Doppler sonography (CD-EUS): CD-EUS allows the detection of intra-tumoral vasculature through the enhancement of tumor vessels[22,23]; it increases the sensitivity to signals from vessels by producing pseudo-Doppler signals from microbubbles[24]. However, CD-EUS technique has a limited ability to detect slow blood flow and it suffers from Doppler-related artifacts such as motion and blooming[14,25].
Contrast-enhanced harmonic EUS (CH-EUS): CH-EUS has been developed to overcome the limitations of CD-EUS. This technique allows microvessels and parenchymal perfusion to be visualized[26]. Moreover, by measuring the time-course of changes in the intensity of echogenicity (time-intensity curve), vascularity can be quantitatively analyzed[27,28].
Confocal endomicroscopy is an emerging technique and allows real-time optical biopsies to be performed in the gastrointestinal tract. The technique uses a EUS puncture needle in which the stylet is replaced by a confocal mini-probe. The mini-probe, which is preloaded into the EUS needle, is guided endosonographically into the target lesion. The intra-tumoral CM examination begins after the injection of fluorescein[29,30].
Many published studies have reported that a EUS-elastography finding of a blue (i.e., hard) pancreatic lesion is highly sensitive and specific for adenocarcinoma (Figure 1). Chronic pancreatitis is an intermediately soft (green) mass (Figure 2), and normal pancreatic tissue is homogeneously soft on EUS-elastography.
A prospective study conducted by Dawwas et al[31] which used elastography to differentiate pancreatic masses revealed that quantitative and qualitative EUS elastography techniques had a sensitivity of 100.0% and 95.7%, a specificity of 16.7% and 22.2%, a positive predictive value (PPV) of 86.1% and 86.4%, a negative predictive value (NPV) of 100.0% and 50.0%, and an overall accuracy of 86.5% and 83.8%, respectively. A recent meta-analysis that reviewed six studies showed that using the qualitative color pattern as the diagnostic standard, the pooled sensitivity was 99% (95%CI: 98%-100%) and the specificity was 74% (95%CI: 65%-82%)[32].
More recent studies have focused on quantitative elastography. A European multicenter study conducted by Săftoiu et al[6] demonstrated that Hue histogram elastography using 175 as the cut-off value had a sensitivity of 93.4%, a specificity of 66.0%, a PPV of 92.5%, an NPV of 68.9%, and an overall accuracy of 85.4 %. Another multicenter study conducted by Giovannini et al[33] yielded similar results. A study conducted by Havre et al[34] showed that the median SR in malignant lesions was 7.05 (3.02-27.57) and was 1.56 (0.07-35.55) (P < 0.001) in benign lesions. Iglesias-Garcia et al[8] reported that the SR was significantly higher among patients with pancreatic cancers than in those with inflammatory masses. An earlier study conducted by Săftoiu et al[35] in 2008 investigated the ability of quantitative EUS elastography to differentiate between benign and malignant pancreatic masses, and its sensitivity, specificity, PPV, NPV and accuracy were 91.4%, 87.9%, 88.9%, 90.6%, and 89.7%, respectively.
Ying et al[36] analyzed 10 studies including 893 pancreatic masses and found that the pooled sensitivity and specificity for the diagnosis of malignant pancreatic masses were 0.98 (95%CI: 0.93-1.00) and 0.69 (95%CI: 0.52-0.82) for qualitative EUS elastography, and 0.96 (95%CI: 0.86-0.99) and 0.76 (95%CI: 0.58-0.87) for quantitative EUS elastography, respectively. Another meta-analysis conducted by Li et al[37] yielded similar conclusions.
However, other elastography studies have reported less promising results. One study found overly similar color patterns between cancerous masses and pancreatitis[38]. One recently published large single-center study reported that quantitative elastography was not as accurate as was described in previous studies and meta-analyses[31].
There are four types of enhancement patterns in CH-EUS: non-enhancement, hypo-enhancement, iso-enhancement and hyper-enhancement[39]. A hypo-enhancing pattern has been considered to be one of the most common distinguishing characteristics of pancreatic adenocarcinoma (Figure 3), and is more diagnostically accurate than the finding of a hypoechoic lesion on conventional EUS (P < 0.001)[40]. A recent meta-analysis of CE-EUS showed that this method can identify pancreatic adenocarcinomas with a pooled sensitivity and specificity of 94% and 89%, respectively[41]. Hypo-vascularity which is a sign of ductal carcinomas in CH-EUS yielded a sensitivity of 89%-95% and a specificity of 64%-89%[36,40,42]. In particular, CH-EUS was significantly more accurate than CT in diagnosing small ductal carcinomas ≤ 2 cm (P < 0.034)[39].
Lee et al[43] demonstrated that pancreatic carcinomas and pancreatic neuroendocrine tumors showed different enhancement patterns on CE-EUS, suggesting that the enhancement pattern may be an important characteristic for diagnosis.
Differentiating between benign and malignant intraductal papillary mucinous neoplasms of the pancreas is challenging. Mural nodules have been identified as one of the most important signs predicting for malignancy. An earlier study conducted by Ohno et al[44] analyzed the enhancement pattern of mural nodules and found that papillary and invasive nodular patterns were more frequently related to invasive cancer. A recent study of CE-EUS in the differentiation of pancreatic cystic lesions showed that CE-EUS considerably increases the sensitivity of displaying cystic wall vascularization[45].
At present, the established standards indicating malignant involvement of lymph nodes (LN) include the following: round shape, hypo-echogenicity, diameter > 1 cm and distinguishing margin. However, all four features of malignant involvement are present in only one-fourth of malignant LNs[46] and the specificity of these findings is poor[8].
A recent meta-analysis conducted by Xu et al[7] found that EUS elastography demonstrated a pooled sensitivity of 88% and specificity of 85% for differentiating between benign and malignant LNs. A study conducted by Okasha et al[1] reached similar conclusions. However, a recent study by Larsen et al[47] delivered a disappointing result. The investigators concluded that EUS-elastography was not better than conventional EUS in differentiating between malignant and benign LNs.
On CD-EUS, the presence of a filling defect is a typical characteristic of malignant lymphadenopathy, with a sensitivity of 100% and a specificity of 86.4%[48]. In a study conducted by Xia et al[49], the sensitivity, specificity and accuracy rates of CD-EUS in diagnosing LN lesions with unknown origin were 96.3%, 100% and 97.6%,respectively.
The risk classifications for GISTs are based on size and the number of mitoses/50 high power fields. Immunohistochemical analysis should also be performed. Therefore, elastographic evaluation of malignancy in such lesions may be difficult.
A recent study conducted by Kannengiesser et al[50] demonstrated that the enhancement pattern of CH-EUS was able to distinguish between GISTs and other benign submucosal tumors such as leiomyoma or lipoma by the enhancement pattern. All histologically proven GISTs showed hyper-enhancement, while lipoma and leiomyoma both showed hypo-enhancement. A study conducted by Sakamoto et al[51] demonstrated that the overall sensitivity, specificity and accuracy of CH-EUS in prediction of malignant GISTs were 100%, 63% and 83%, respectively.
Elastography can help the user to select a site where FNA can be performed with improved diagnostic yield, particularly in patients with either necrotic tumors or possible cancers within diffuse inflammatory lesions.
CH-EUS clearly depicts subtle lesions that conventional EUS is unable to identify and, can be used to select targets for EUS-FNA[52]. Real-time CH-EUS-FNA can identify and avoid an avascular site, helping to prevent sampling of necrotic areas and allowing the selection of more suitable sites for biopsy[53].
The use of EUS-elastography has been investigated for the diagnosis and evaluation of prostate cancer, rectal cancer, and inflammatory bowel disease. In prostate cancer, EUS-elastography has been demonstrated to be better than conventional EUS[54], and it increases the specificity of prostate biopsies by highlighting areas that are highly suspicious for malignancy[55]. A study of transrectal elastography conducted by Waage et al[56] showed that the sensitivity, specificity and accuracy rates of SR were 93%, 96% and 94%, respectively. Dietrich et al[57] reported that left hepatic tumors can be differentiated by EUS-elastography.
Elastography of the hepatobiliary system is particularly useful for evaluation of the papilla of Vater and staging papillary carcinoma and papillomatosis[58].
A recent study of CH-EUS for the differential diagnosis of gallbladder wall thickening, which was conducted by Imazu et al[59], reported that the overall sensitivity, specificity and accuracy rates of CH-EUS for diagnosing malignant GB wall thickening were 89.6%, 98% and 94.4%, respectively.
CE-EUS has also been used in other gastrointestinal diseases, such as inflammatory bowel disease. A study published in 2012 showed that CE-EUS had excellent sensitivity and specificity for the diagnosis of postoperative recurrence in Crohn’s disease[60].
Studies of EUS-confocal microscopy are rare. A recent study conducted by Giovannini et al[61] demonstrated that EUS-confocal microscopy can effectively distinguish different pancreatic cystic lesions.
EUS-elastography is an operator-dependent technique, with a high image selection bias and, in some cases, a lack of reproducibility. Excessive compression of the tissue can artificially cause more deformation. The presence of certain tissues (e.g., vessels, cysts, and bone) in the ROI significantly influences elasticity measurements. Furthermore, the appropriate cut-off values for quantitative elastography remain controversial. Some authors have reported promising findings, while others noted disappointing results. Consequently, most authors have indicated that elastography is not ready to replace EUS-FNA, but may be a supplementary procedure in patients with negative or inconclusive EUS-FNA findings, if a strong suspicion of malignancy still exists[4].
CE-EUS has been criticized for its qualitative nature, and quantitative methods have been proposed to improve its reliability[62].
The therapeutic potential of CE-EUS is to selectively deliver medications and reduce side-effects using contrast microbubbles as carriers[63,64].
EUS-elastography and CH-EUS are emerging techniques. These techniques are simple and easy to perform (using a touch of a button for elastography), do not require extensive training and costly devices, have a low cost and low complication rate, do not add extra time to EUS procedures, and can provide valuable information regarding the characteristics of focal masses. Therefore, both are effective supplemental techniques in EUS-FNA and should be implemented in clinical practice. A combination of these emerging techniques can further increase the ability of EUS to diagnose pancreatic masses. However, these techniques should be performed in tertiary centers by experienced operators with expertise in EUS and EUS-FNA.
P- Reviewer: Amornyotin S, Figueiredo PN, Sureka B S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Zhang DN
| 1. | Okasha HH, Mansour M, Attia KA, Khattab HM, Sakr AY, Naguib M, Aref W, Al-Naggar AA, Ezzat R. Role of high resolution ultrasound/endosonography and elastography in predicting lymph node malignancy. Endosc Ultrasound. 2014;3:58-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Dietrich CF, Săftoiu A, Jenssen C. Real time elastography endoscopic ultrasound (RTE-EUS), a comprehensive review. Eur J Radiol. 2014;83:405-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 3. | Knabe M, Günter E, Ell C, Pech O. Can EUS elastography improve lymph node staging in esophageal cancer? Surg Endosc. 2013;27:1196-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Popescu A, Săftoiu A. Can elastography replace fine needle aspiration? Endosc Ultrasound. 2014;3:109-117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Hocke M, Ignee A, Dietrich CF. Advanced endosonographic diagnostic tools for discrimination of focal chronic pancreatitis and pancreatic carcinoma--elastography, contrast enhanced high mechanical index (CEHMI) and low mechanical index (CELMI) endosonography in direct comparison. Z Gastroenterol. 2012;50:199-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Săftoiu A, Vilmann P, Gorunescu F, Janssen J, Hocke M, Larsen M, Iglesias-Garcia J, Arcidiacono P, Will U, Giovannini M. Accuracy of endoscopic ultrasound elastography used for differential diagnosis of focal pancreatic masses: a multicenter study. Endoscopy. 2011;43:596-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 7. | Xu W, Shi J, Zeng X, Li X, Xie WF, Guo J, Lin Y. EUS elastography for the differentiation of benign and malignant lymph nodes: a meta-analysis. Gastrointest Endosc. 2011;74:1001-109; quiz 1001-109;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 8. | Iglesias-Garcia J, Lindkvist B, Lariño-Noia J, Domínguez-Muñoz JE. Endoscopic ultrasound elastography. Endosc Ultrasound. 2012;1:8-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Gheonea DI, Săftoiu A. Beyond conventional endoscopic ultrasound: elastography, contrast enhancement and hybrid techniques. Curr Opin Gastroenterol. 2011;27:423-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Iglesias-Garcia J, Larino-Noia J, Abdulkader I, Forteza J, Dominguez-Munoz JE. Quantitative endoscopic ultrasound elastography: an accurate method for the differentiation of solid pancreatic masses. Gastroenterology. 2010;139:1172-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 195] [Article Influence: 13.0] [Reference Citation Analysis (1)] |
| 11. | Itokawa F, Itoi T, Sofuni A, Kurihara T, Tsuchiya T, Ishii K, Tsuji S, Ikeuchi N, Umeda J, Tanaka R. EUS elastography combined with the strain ratio of tissue elasticity for diagnosis of solid pancreatic masses. J Gastroenterol. 2011;46:843-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 12. | Alam F, Naito K, Horiguchi J, Fukuda H, Tachikake T, Ito K. Accuracy of sonographic elastography in the differential diagnosis of enlarged cervical lymph nodes: comparison with conventional B-mode sonography. AJR Am J Roentgenol. 2008;191:604-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 187] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 13. | König K, Scheipers U, Pesavento A, Lorenz A, Ermert H, Senge T. Initial experiences with real-time elastography guided biopsies of the prostate. J Urol. 2005;174:115-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 149] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 14. | Reddy NK, Ioncică AM, Săftoiu A, Vilmann P, Bhutani MS. Contrast-enhanced endoscopic ultrasonography. World J Gastroenterol. 2011;17:42-48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Kaufmann BA, Lindner JR. Molecular imaging with targeted contrast ultrasound. Curr Opin Biotechnol. 2007;18:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 136] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 16. | Serrani M, Caletti G, Fusaroli P. Contrast enhancement and elastography in endoscopic ultrasound: an overview of clinical applications in pancreatic diseases. Minerva Med. 2014;105:353-361. [PubMed] |
| 17. | Yip HC, Teoh AY, Chong CC, Lau JY. Current status and future applications of contrast-enhanced endoscopic ultrasonography. World J Gastrointest Endosc. 2014;6:121-127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Kitano M, Sakamoto H, Kudo M. Contrast-enhanced endoscopic ultrasound. Dig Endosc. 2014;26 Suppl 1:79-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Sanchez MV, Varadarajulu S, Napoleon B. EUS contrast agents: what is available, how do they work, and are they effective? Gastrointest Endosc. 2009;69:S71-S77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Kitano M, Kudo M, Sakamoto H, Nakatani T, Maekawa K, Mizuguchi N, Ito Y, Miki M, Matsui U, Von Schrenck T. Preliminary study of contrast-enhanced harmonic endosonography with second-generation contrast agents. J Med Ultra. 2008;35:11-18. [RCA] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Săftoiu A, Dietrich CF, Vilmann P. Contrast-enhanced harmonic endoscopic ultrasound. Endoscopy. 2012;44:612-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 22. | Săftoiu A, Iordache SA, Gheonea DI, Popescu C, Maloş A, Gorunescu F, Ciurea T, Iordache A, Popescu GL, Manea CT. Combined contrast-enhanced power Doppler and real-time sonoelastography performed during EUS, used in the differential diagnosis of focal pancreatic masses (with videos). Gastrointest Endosc. 2010;72:739-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 23. | Ishikawa T, Itoh A, Kawashima H, Ohno E, Matsubara H, Itoh Y, Nakamura Y, Nakamura M, Miyahara R, Hayashi K. Usefulness of EUS combined with contrast-enhancement in the differential diagnosis of malignant versus benign and preoperative localization of pancreatic endocrine tumors. Gastrointest Endosc. 2010;71:951-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 24. | Iglesias-Garcia J, Lindkvist B, Cruz-Soares JB, Larino-Noia J, Dominguez-Munoz E. Does Contrast Enhancement Play a Role as an Adjunct to Endoscopic Ultrasound for the Diagnosis of Chronic Pancreatitis? a Pilot Study. Gastroenterology. 2012;142:S243-S244. |
| 25. | Kitano M, Sakamoto H, Komaki T, Kudo M. New techniques and future perspective of EUS for the differential diagnosis of pancreatic malignancies: contrast harmonic imaging. Dig Endosc. 2011;23 Suppl 1:46-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Kitano M, Sakamoto H, Matsui U, Ito Y, Maekawa K, von Schrenck T, Kudo M. A novel perfusion imaging technique of the pancreas: contrast-enhanced harmonic EUS (with video). Gastrointest Endosc. 2008;67:141-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 141] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 27. | Hirooka Y, Itoh A, Kawashima H, Ohno E, Itoh Y, Nakamura Y, Hiramatsu T, Sugimoto H, Sumi H, Hayashi D. Contrast-enhanced endoscopic ultrasonography in digestive diseases. J Gastroenterol. 2012;47:1063-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 28. | Fusaroli P, Saftoiu A, Mancino MG, Caletti G, Eloubeidi MA. Techniques of image enhancement in EUS (with videos). Gastrointest Endosc. 2011;74:645-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 29. | Dunbar K, Canto M. Confocal endomicroscopy. Curr Opin Gastroenterol. 2008;24:631-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Konda VJ, Aslanian HR, Wallace MB, Siddiqui UD, Hart J, Waxman I. First assessment of needle-based confocal laser endomicroscopy during EUS-FNA procedures of the pancreas (with videos). Gastrointest Endosc. 2011;74:1049-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 31. | Dawwas MF, Taha H, Leeds JS, Nayar MK, Oppong KW. Diagnostic accuracy of quantitative EUS elastography for discriminating malignant from benign solid pancreatic masses: a prospective, single-center study. Gastrointest Endosc. 2012;76:953-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 32. | Xu W, Shi J, Li X, Zeng X, Lin Y. Endoscopic ultrasound elastography for differentiation of benign and malignant pancreatic masses: a systemic review and meta-analysis. Eur J Gastroenterol Hepatol. 2013;25:218-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 33. | Giovannini M, Thomas B, Erwan B, Christian P, Fabrice C, Benjamin E, Geneviève M, Paolo A, Pierre D, Robert Y. Endoscopic ultrasound elastography for evaluation of lymph nodes and pancreatic masses: a multicenter study. World J Gastroenterol. 2009;15:1587-1593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 204] [Cited by in RCA: 201] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 34. | Havre RF, Ødegaard S, Gilja OH, Nesje LB. Characterization of solid focal pancreatic lesions using endoscopic ultrasonography with real-time elastography. Scand J Gastroenterol. 2014;49:742-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 35. | Săftoiu A, Vilmann P, Gorunescu F, Gheonea DI, Gorunescu M, Ciurea T, Popescu GL, Iordache A, Hassan H, Iordache S. Neural network analysis of dynamic sequences of EUS elastography used for the differential diagnosis of chronic pancreatitis and pancreatic cancer. Gastrointest Endosc. 2008;68:1086-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 174] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 36. | Ying L, Lin X, Xie ZL, Hu YP, Tang KF, Shi KQ. Clinical utility of endoscopic ultrasound elastography for identification of malignant pancreatic masses: a meta-analysis. J Gastroenterol Hepatol. 2013;28:1434-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 37. | Li X, Xu W, Shi J, Lin Y, Zeng X. Endoscopic ultrasound elastography for differentiating between pancreatic adenocarcinoma and inflammatory masses: a meta-analysis. World J Gastroenterol. 2013;19:6284-6291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 38. | Janssen J, Schlörer E, Greiner L. EUS elastography of the pancreas: feasibility and pattern description of the normal pancreas, chronic pancreatitis, and focal pancreatic lesions. Gastrointest Endosc. 2007;65:971-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 178] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 39. | Kitano M, Kudo M, Yamao K, Takagi T, Sakamoto H, Komaki T, Kamata K, Imai H, Chiba Y, Okada M. Characterization of small solid tumors in the pancreas: the value of contrast-enhanced harmonic endoscopic ultrasonography. Am J Gastroenterol. 2012;107:303-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 242] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 40. | Fusaroli P, Spada A, Mancino MG, Caletti G. Contrast harmonic echo-endoscopic ultrasound improves accuracy in diagnosis of solid pancreatic masses. Clin Gastroenterol Hepatol. 2010;8:629-34.e1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 153] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 41. | Gong TT, Hu DM, Zhu Q. Contrast-enhanced EUS for differential diagnosis of pancreatic mass lesions: a meta-analysis. Gastrointest Endosc. 2012;76:301-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 42. | Napoleon B, Alvarez-Sanchez MV, Gincoul R, Pujol B, Lefort C, Lepilliez V, Labadie M, Souquet JC, Queneau PE, Scoazec JY. Contrast-enhanced harmonic endoscopic ultrasound in solid lesions of the pancreas: results of a pilot study. Endoscopy. 2010;42:564-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 128] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 43. | Lee TY, Cheon YK, Shim CS. Clinical role of contrast-enhanced harmonic endoscopic ultrasound in differentiating solid lesions of the pancreas: a single-center experience in Korea. Gut Liver. 2013;7:599-604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 44. | Ohno E, Hirooka Y, Itoh A, Ishigami M, Katano Y, Ohmiya N, Niwa Y, Goto H. Intraductal papillary mucinous neoplasms of the pancreas: differentiation of malignant and benign tumors by endoscopic ultrasound findings of mural nodules. Ann Surg. 2009;249:628-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 146] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 45. | Hocke M, Cui XW, Domagk D, Ignee A, Dietrich CF. Pancreatic cystic lesions: The value of contrast-enhanced endoscopic ultrasound to influence the clinical pathway. Endosc Ultrasound. 2014;3:123-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 46. | Strongin A, Singh H, Eloubeidi MA, Siddiqui AA. Role of endoscopic ultrasonography in the evaluation of extrahepatic cholangiocarcinoma. Endosc Ultrasound. 2013;2:71-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 47. | Larsen MH, Fristrup C, Hansen TP, Hovendal CP, Mortensen MB. Endoscopic ultrasound, endoscopic sonoelastography, and strain ratio evaluation of lymph nodes with histology as gold standard. Endoscopy. 2012;44:759-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 48. | Kanamori A, Hirooka Y, Itoh A, Hashimoto S, Kawashima H, Hara K, Uchida H, Goto J, Ohmiya N, Niwa Y. Usefulness of contrast-enhanced endoscopic ultrasonography in the differentiation between malignant and benign lymphadenopathy. Am J Gastroenterol. 2006;101:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 64] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 49. | Xia Y, Kitano M, Kudo M, Imai H, Kamata K, Sakamoto H, Komaki T. Characterization of intra-abdominal lesions of undetermined origin by contrast-enhanced harmonic EUS (with videos). Gastrointest Endosc. 2010;72:637-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 50. | Kannengiesser K, Mahlke R, Petersen F, Peters A, Ross M, Kucharzik T, Maaser C. Contrast-enhanced harmonic endoscopic ultrasound is able to discriminate benign submucosal lesions from gastrointestinal stromal tumors. Scand J Gastroenterol. 2012;47:1515-1520. [RCA] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 51. | Sakamoto H, Kitano M, Matsui S, Kamata K, Komaki T, Imai H, Dote K, Kudo M. Estimation of malignant potential of GI stromal tumors by contrast-enhanced harmonic EUS (with videos). Gastrointest Endosc. 2011;73:227-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 52. | Romagnuolo J, Hoffman B, Vela S, Hawes R, Vignesh S. Accuracy of contrast-enhanced harmonic EUS with a second-generation perflutren lipid microsphere contrast agent (with video). Gastrointest Endosc. 2011;73:52-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 53. | Kitano M, Sakamoto H, Komaki T, Kudo M. FNA Guided By Contrast-Enhanced Harmonic EUS in Pancreatic Tumors. Gastrointestinal Endosc. 2009;69:Ab328-Ab329. |
| 54. | Kamoi K, Okihara K, Ochiai A, Ukimura O, Mizutani Y, Kawauchi A, Miki T. The utility of transrectal real-time elastography in the diagnosis of prostate cancer. Ultrasound Med Biol. 2008;34:1025-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 55. | Kapoor A, Kapoor A, Mahajan G, Sidhu BS. Real-time elastography in the detection of prostate cancer in patients with raised PSA level. Ultrasound Med Biol. 2011;37:1374-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 56. | Waage JE, Havre RF, Odegaard S, Leh S, Eide GE, Baatrup G. Endorectal elastography in the evaluation of rectal tumours. Colorectal Dis. 2011;13:1130-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 57. | Dietrich CF. Real Time Elastography Indications Not Only in the Gastrointestinal Tract. Endoskopie Heute. 2010;23:177-212. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 58. | Cui XW, Ignee A, Braden B, Woenckhaus M, Dietrich CF. Biliary papillomatosis and new ultrasound imaging modalities. Z Gastroenterol. 2012;50:226-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 59. | Imazu H, Mori N, Kanazawa K, Chiba M, Toyoizumi H, Torisu Y, Koyama S, Hino S, Ang TL, Tajiri H. Contrast-enhanced harmonic endoscopic ultrasonography in the differential diagnosis of gallbladder wall thickening. Dig Dis Sci. 2014;59:1909-1916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 60. | Paredes JM, Ripollés T, Cortés X, Moreno N, Martínez MJ, Bustamante-Balén M, Delgado F, Moreno-Osset E. Contrast-enhanced ultrasonography: usefulness in the assessment of postoperative recurrence of Crohn’s disease. J Crohns Colitis. 2013;7:192-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 61. | Giovannini M, Caillol F, Lemaistre A, Monges G, Napoleon B, Pujol B. Endoscopic ultrasound guided confocal microscopy: Atlas of cystic pancreatic lesions. Endosc Ultra. 2014;3:S19-S21. |
| 62. | Fusaroli P, Kypraios D, Mancino MG, Spada A, Benini MC, Bianchi M, Bocus P, De Angelis C, De Luca L, Fabbri C. Interobserver agreement in contrast harmonic endoscopic ultrasound. J Gastroenterol Hepatol. 2012;27:1063-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 63. | Hernot S, Klibanov AL. Microbubbles in ultrasound-triggered drug and gene delivery. Adv Drug Deliv Rev. 2008;60:1153-1166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 778] [Cited by in RCA: 679] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 64. | Kitano M, Sakamoto H, Kudo M. Endoscopic ultrasound: contrast enhancement. Gastrointest Endosc Clin N Am. 2012;22:349-58, xi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 65. | Kwek BE, Ang TL, Seo DW, Imazu H. Contrast-enhanced harmonic endoscopic ultrasonography of solid pancreatic lesions. Endosc Ultrasound. 2013;2:142-147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |