Published online Apr 21, 2015. doi: 10.3748/wjg.v21.i15.4673
Peer-review started: November 5, 2014
First decision: December 11, 2014
Revised: December 30, 2014
Accepted: January 30, 2015
Article in press: January 30, 2015
Published online: April 21, 2015
Processing time: 166 Days and 10.4 Hours
AIM: To evaluate discrepancies between biopsy and resected specimens using the Japanese Classification of Gastric Carcinoma (JCGC) and tumor-node-metastasis (TNM) classification.
METHODS: A total of 376 consecutive paired samples from biopsy and resected gastric specimens, which were derived from curative gastrectomy for gastric cancer between 2008 and 2011, were retrospectively analyzed.
RESULTS: (1) Discrepancies in the histologic type were observed between biopsy and resected specimens; 11.7% (44/376) in the JCGC and 18.1% (68/376) in TNM. In specimens diagnosed as the differentiated type from biopsy specimens, 14.4% (28/195) in the JCGC and 41.1% (67/163) in TNM were finally diagnosed as the undifferentiated type from resected specimens; and (2) the incidence of mixed-type gastric cancer was significantly higher in specimens with discrepancies than in those without in both the JCGC and TNM (both P < 0.0001); 93.2% (41/44) of specimens with discrepancies in the JCGC and 97.1% (66/68) of specimens with discrepancies in TNM were mixed-type gastric cancers.
CONCLUSION: Mixed-type gastric cancer was associated with a high incidence of histologic discrepancies between biopsy and resected specimens in both the JCGC and TNM definitions. Care should be taken in deciding treatments based on diagnosis of the histologic type for mixed-type gastric cancer from biopsy specimens.
Core tip: Little is known about diagnostic discrepancies in the histologic type between biopsy and resected specimens in gastric cancer. We demonstrate that mixed-type gastric cancer is associated with a high incidence of histologic discrepancy between biopsy and resected specimens in both the Japanese Classification of Gastric Carcinoma and tumor-node-metastasis definitions.
- Citation: Komatsu S, Ichikawa D, Miyamae M, Kosuga T, Konishi H, Shiozaki A, Fujiwara H, Okamoto K, Kishimoto M, Otsuji E. Discrepancies in the histologic type between biopsy and resected specimens: A cautionary note for mixed-type gastric carcinoma. World J Gastroenterol 2015; 21(15): 4673-4679
- URL: https://www.wjgnet.com/1007-9327/full/v21/i15/4673.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i15.4673
Gastric cancer has various histologic types, each of which exhibits different characteristics. In early gastric cancer, the histologic type affects the extent of lymph node metastasis. The undifferentiated type, in particular, was shown to be one of the independent risk factors of lymph node metastasis[1-3]. The histologic type is an important factor that is used to predict prognosis, recurrence patterns, and chemosensitivity in patients with advanced gastric cancer[4-7]. Therefore, the histologic type of gastric cancer has been regarded as a pivotal factor that may have a potentially useful role in making decisions regarding several treatment strategies for gastric cancer.
The histologic type has already been defined as one of the indicative factors for endoscopic treatment and limited lymphadenectomy with gastrectomy according to gastric cancer treatment guidelines in Japan[8,9]. Although a pre-treatment diagnosis of the histologic type using endoscopic biopsy is indispensable, it may be difficult to accurately diagnose and confirm tumor differentiation in restricted biopsy samples because gastric cancer tissues often present histologic heterogeneity; tumor tissue does not necessarily consist of a single histologic type and sometimes contains a mixture of several different types. Therefore, we hypothesized that discrepancies in the histologic type of gastric cancer between biopsy and postoperative resected specimens from some patients could lead to inadequate treatments, which increase the risk of recurrence.
To verify this hypothesis, we investigated the extent of discrepancies in the histologic type between biopsy and resected specimens by comparing two different classifications of the histologic type; the 14th Japanese Classification of Gastric Carcinoma (JCGC)[10] and the 7th tumor-node-metastasis (TNM) classification[11]. Furthermore, we clarified possible problems and potential risks associated with diagnostic histologic discrepancies. Our results provide novel evidence and a cautionary note for histologic diagnoses using endoscopic biopsies.
A consecutive series of 376 patients with paired samples from biopsy and resected gastric specimens, which were derived from curative gastrectomy for gastric cancer, were enrolled in this study. The mean age of patients was 66.0 years (range: 35-94 years), and the male: female ratio was 1.98:1.0. The cohort included patients with T1 (n = 212), T2 (n = 43), T3 (n = 62), and T4 (n = 59) stage gastric cancer. All patients underwent curative gastrectomy with radical lymphadenectomy in the Division of Digestive Surgery, Kyoto Prefectural University of Medicine, between 2008 and 2011. Patients who were treated with chemotherapy before surgery and/or had multiple gastric cancer lesions were excluded from this study.
The resected stomach was opened and placed on a flat board with the mucosal side up and was fixed in a 10% buffered formalin solution. After fixation, tumors in the resected stomach were sectioned in the maximum cross-sectional plane parallel to the lesser curvature line based on the general rules of the JCGC[10]. Tumors were generally sectioned in their entirety parallel to the reference line at intervals of 5 mm. Biopsy and resected specimens were embedded in paraffin and stained by hematoxylin and eosin. The clinicopathologic factors of these patients were obtained from hospital records on the basis of the 14th JCGC[10], which is similar to the 7th TNM classification[11], excluding the definition for the histologic type.
Histologic quantitative predominance was recorded in stained biopsy specimens by examination from at least two pathologists in our hospital, though some specimens were examined by other hospital pathologists because the patients were referred to us for gastrectomy. The histologic types of biopsy and resected tumor specimens were divided into two major categories: (1) intestinal, expanding, or differentiated type; and (2) diffuse, infiltrative, or undifferentiated type[12,13]. The JGCC 2010 guidelines state that the differentiated type includes tubular and papillary adenocarcinomas, which arise from the gastric mucosa with intestinal metaplasia, and also that the undifferentiated type includes poorly differentiated adenocarcinoma, signet ring cell carcinoma, and mucinous adenocarcinoma, which arise from ordinary gastric mucosa without intestinal metaplasia[14].
There has been no universal standard regarding the definition for the histologic type, particularly in mixed-type gastric cancer. There are small differences in the definitions for the histologic type according to the JCGC and TNM classifications, with mixed-type gastric cancer defined by the quantitative predominance in JCGC classification, and by the qualitative predominance in the TNM classification. In other words, the histologic type is classified on the basis of the predominant component by the JCGC, on the basis of the poorest differentiated component in TNM classification. To define the histologic type according to both the JCGC and TNM classifications, the differentiated type of gastric cancer was divided into four subgroups in this study: (1) a pure differentiated component with no undifferentiated component (pure D group); (2) a differentiated-predominant mixed type with a < 50% undifferentiated component (D > U group); (3) an undifferentiated-predominant mixed type with a > 50% undifferentiated component (U > D group); and (4) a pure undifferentiated component with no differentiated component (pure U group). In the JCGC, pure D and D > U groups were classified as the differentiated type, whereas U > D and pure U groups were classified as the undifferentiated type. In the TNM classification, pure D was only classified as the differentiated type and the remaining D > U, U > D, and pure U groups were all classified as the undifferentiated type. Histologic mixed-type gastric cancer includes both differentiated and undifferentiated components, and is composed of the D > U or U > D group[15].
The χ2 and Fisher’s exact probability tests were performed for comparison of categorical variables between the two groups. A P < 0.05 was considered significant.
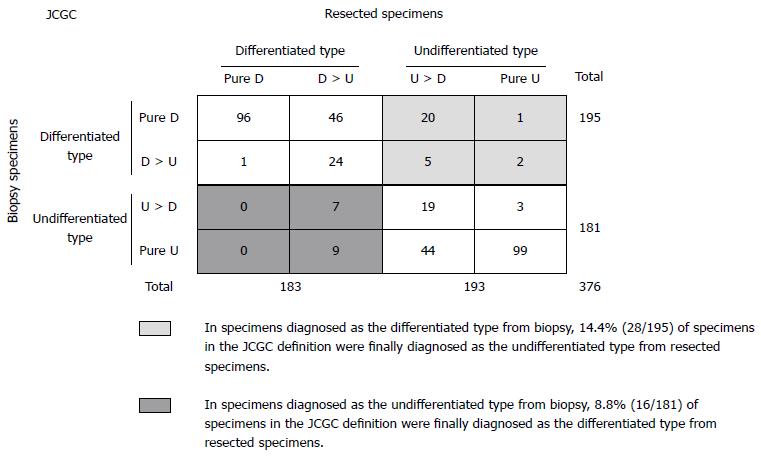
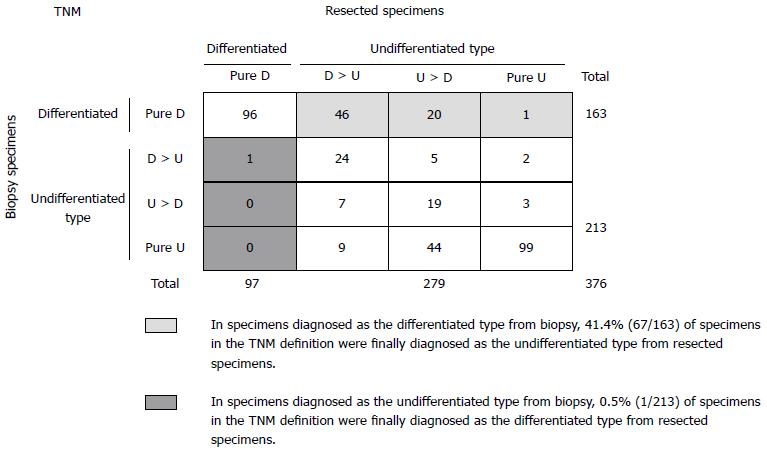
The four subgroups for histologic differentiation comprised the pure D group in 97/376 (26%) patients, the D > U group in 86/376 (23%) patients, the U > D group in 88/376 (23%) patients, and the pure U group in 105/376 (28%) patients. According to the criteria of the JCGC, 183/376 (49%) patients were diagnosed with differentiated gastric cancer (pure D and D > U group) and 193/376 (51%) patients were diagnosed with undifferentiated gastric cancer (U > D and pure U group) (Figure 1). On the other hand, 97/376 (26%) patients were diagnosed with differentiated gastric cancer (pure D group) and 279/376 (74%) were diagnosed with undifferentiated gastric cancer (D > U, U > D, and pure U group) according to the criteria of the TNM classification (Figure 2).
In Figure 1, there were 11.7% (44/376) discrepancies in the histologic type of gastric cancer between biopsy and postoperative resected specimens in the JCGC definition. Specifically, of 195 specimens diagnosed as the differentiated type from biopsy specimens, 28 (14.4%) specimens were finally diagnosed as the undifferentiated type from resected specimens. Sixteen of 181 (8.8%) specimens diagnosed as the undifferentiated type from biopsy specimens were finally diagnosed as the differentiated type. Therefore, the positive predictive values of the differentiated and undifferentiated type from biopsy specimens in the JCGC definition were 85.6% (167/195) and 91.2% (165/181), respectively.
However, in Figure 2, there were 18.1% (68/376) discrepancies in the TNM classification. Of 163 specimens diagnosed as the differentiated type from biopsy specimens, 67 (41.1%) specimens were finally diagnosed as the undifferentiated type from resected specimens. However, only 1/213 (0.5%) specimens diagnosed as the undifferentiated type from biopsy specimens was finally diagnosed as the differentiated type. Therefore, the positive predictive values of the differentiated and undifferentiated type from biopsy specimens in the TNM definition were 58.9% (96/163) and 99.5% (212/213), respectively.
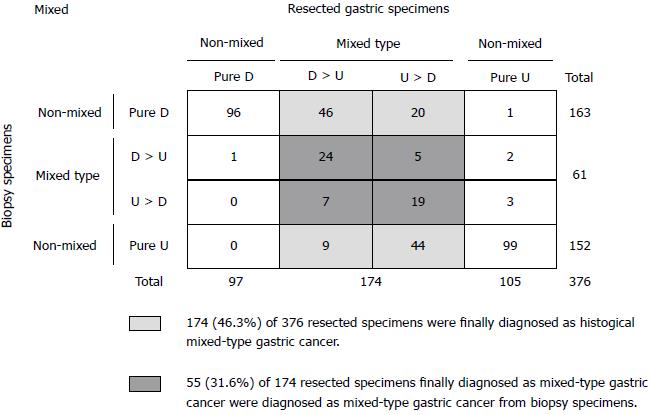
In Figure 3, a total of 174/376 (46.3%) specimens were finally diagnosed as histologic mixed-type gastric cancer. However, only 55/174 (31.6%) resected specimens finally diagnosed as mixed-type gastric cancer were diagnosed as mixed-type gastric cancer from biopsy specimens; 68.4% (119/174) of patients could not be diagnosed with mixed-type gastric cancer from pretreatment biopsy specimens. Therefore, the positive predictive value of the mixed-type from biopsy specimens was 90.2% (55/61).
Regarding the association with histologic type discrepancies, in the JCGC definition, 41/44 (93.2%) specimens with the histologic type discrepancy between biopsy and resected specimens were derived from mixed-type gastric cancers. However, 133/332 (40.1%) specimens without the histologic type discrepancy were derived from mixed-type gastric cancers (P < 0.0001) (Table 1). In the TNM definition, 66/68 (97.1%) specimens with the histologic type discrepancy between biopsy and resected specimens were derived from mixed-type gastric cancers, in contrast to only 108/308 (35.1%) specimens without the histologic type discrepancy (P < 0.0001).
| Classification | Histologic discrepancy | P value | ||
| Absence | Presence | |||
| JCGC definition | ||||
| Histologic | Absence | 199 (59.9) | 3 (6.8) | |
| Mixed-type | Presence | 133 (40.1) | 41 (93.2) | < 0.0001 |
| TNM definition | ||||
| Histologic | Absence | 200 (64.9) | 2 (2.9) | |
| Mixed-type | Presence | 108 (35.1) | 66 (97.1) | < 0.0001 |
Only a few studies have examined clinical features associated with the histologic mixture of differentiated and undifferentiated components in gastric cancer[14-22]; therefore, little is known about the clinical effects of a histologic mixture in diagnosing the histologic type of gastric cancer. In the present study, we clearly demonstrate that histologic type discrepancies between biopsy and resected specimens are strongly associated with a high incidence of histologic mixed-type gastric cancer and its diagnostic difficulty from biopsy specimens. Specifically, in patients with histologic type discrepancies, 93.2% and 97.1% of specimens in the JGCC and TMN classification, respectively, were due to mixed-type gastric cancers. These results suggest that care should be taken regarding histologic mixed-type gastric cancer in the pretreatment diagnosis of the histologic type.
The histologic type has been defined as one of the indicative factors for limited treatments according to Japanese gastric cancer treatment guidelines[8,9]. Therefore, endoscopic submucosal dissection with narrow band imaging magnifying endoscopy[23] and limited gastrectomy with laparoscopic surgery[24,25] have emerged as new, less-invasive technologies and are widely accepted as limited treatments for early gastric cancer. Gotoda et al[2] reported that, in 3016 patients with T1a gastric cancer, none of the 1230 differentiated cases < 30 mm in size had lymph node metastases (95%CI: 0%-0.4%); in contrast, 18/821 (2.2%) undifferentiated cases < 30 mm in size had lymph node metastases. These findings changed our concept and improved the treatment of early gastric cancer. However, these findings were based on a final pathologic diagnosis of the histologic type from resected specimens following gastrectomy. Therefore, some patients may undergo inadequate treatments according to the treatment guidelines and consequently need additional treatment options and/or intensive follow-up if pretreatment biopsies are diagnosed as the differentiated type of gastric cancer and a post-treatment histologic examination using resected specimens reveals a different histologic diagnosis.
In this study, we investigated potentially high-risk cases that were initially diagnosed with the differentiated type by endoscopic biopsies and finally diagnosed with the undifferentiated type by resected specimens. As a result, 14.4% of specimens in the JCGC and 41.1% of specimens in TNM were included in this category. According to the Japanese gastric cancer treatment guidelines 2010 (Ver.3)[8], D1 lymphadenectomy is indicated as a limited treatment for cT1bN0 tumors that are histologically of the differentiated type and ≤ 1.5 cm in diameter. In the present study, all patients with these discrepancies underwent adequate lymphadenectomy and no related recurrent cases were noted. However, clinical doctors should always consider that the initial diagnosis of the histologic type, based on biopsy specimens, may change following an inspection of resected specimens obtained from gastrectomy.
In this study, we also demonstrate that the histologic mixture of differentiated and undifferentiated components adversely affects the pretreatment diagnostic accuracy of the histologic type. However, it is difficult for pathologists to accurately diagnose the histologic differentiation of mixed-type gastric cancer due to the restricted tumor volume, even from several biopsy specimens, which only reflects a part of the tumor, and the accuracy of the histologic type is apparently inferior to that of the final diagnosis from resected specimens. It may be easier for pathologists to diagnose whether biopsy specimens include histologic mixed-type gastric cancer. Therefore, in order to apply histologic differentiation to clinical settings, the presence of the histologic mixed-type itself could be a better indicative factor for limited treatments than the histologic type, as proposed by recent studies[15,18-20,22]. Indeed, the positive predictive value is a bit higher in the histologic mixed-type (90.2%) than in the differentiated type (85.6%) from biopsy specimens according to the JCGC definition. These data suggest that it may be not difficult for pathologists to diagnose the histologic mixed-type.
Only a few studies have examined the clinical behaviors of histologic mixed-type gastric cancer, which is associated with lymph node metastasis[18,19,22], a larger tumor size in early gastric cancer[20], and enhanced CpG island hypermethylation status[26]. We also previously demonstrated that the presence of undifferentiated predominant mixed-type gastric cancer is associated with tumor progression and lymph node metastasis in differentiated T1/T2 cancer; the histologic mixed-type was an independent prognostic factor (HR = 12.1, 95%CI: 1.4-107.0)[15]. These results indicate that the presence of the histologic mixed-type itself could present malignant clinical behaviors, and, because it is comparatively easier to diagnosis, may be a better indicative factor for limited treatments and treatment sensitivity than the histologic type. These issues are currently being evaluated from the viewpoints of pathology and molecular biology.
In conclusion, mixed-type gastric cancer is associated with a high incidence of histologic discrepancies between biopsy and resected gastric specimens by both the JCGC and TNM definitions. In mixed-type gastric cancer, care should be taken in making decisions regarding limited treatments based on the histologic type from biopsy specimens.
Little is known about diagnostic discrepancies in the histologic type between biopsy and resected specimens in gastric cancer. These discrepancies may lead to inadequate treatments, which increase the risk of recurrence.
This study was designed to clarify the incidence of discrepancies between histologic gastric cancer types determined from biopsy and resected specimens according to pathologic definitions by the Japanese Classification of Gastric Carcinoma (JCGC) and tumor-node-metastasis (TNM) classification.
A total of 376 consecutive paired samples from biopsy and resected gastric specimens, which were derived from curative gastrectomy for gastric cancer between 2008 and 2011, were used in this study. The incidence of mixed-type gastric cancer was significantly higher in specimens with discrepancies than in those without discrepancies in both the JCGC and TNM classification; 93.2% and 97.1% of specimens with discrepancies in JCGC and TNM, respectively, were mixed-type gastric cancers.
Care should be taken in making decisions regarding limited treatments based on the diagnosis of the histologic type for mixed-type gastric cancer from biopsy specimens.
Histologic mixed-type gastric cancer consists of both differentiated and undifferentiated components.
This was a good descriptive study showing that mixed-type gastric cancer is associated with a high incidence of histologic discrepancies between biopsy and resected specimens in both the JCGC and TNM definitions.
P- Reviewer: Bustamante-Balen M, Huerta-Franco MR S- Editor: Qi Y L- Editor: AmEditor E- Editor: Liu XM
| 1. | Popiela T, Kulig J, Kolodziejczyk P, Sierzega M. Long-term results of surgery for early gastric cancer. Br J Surg. 2002;89:1035-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Gotoda T, Sasako M, Ono H, Katai H, Sano T, Shimoda T. Evaluation of the necessity for gastrectomy with lymph node dissection for patients with submucosal invasive gastric cancer. Br J Surg. 2001;88:444-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 133] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 3. | Folli S, Morgagni P, Roviello F, De Manzoni G, Marrelli D, Saragoni L, Di Leo A, Gaudio M, Nanni O, Carli A. Risk factors for lymph node metastases and their prognostic significance in early gastric cancer (EGC) for the Italian Research Group for Gastric Cancer (IRGGC). Jpn J Clin Oncol. 2001;31:495-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 108] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Adachi Y, Yasuda K, Inomata M, Sato K, Shiraishi N, Kitano S. Pathology and prognosis of gastric carcinoma: well versus poorly differentiated type. Cancer. 2000;89:1418-1424. [PubMed] |
| 5. | Noda S, Soejima K, Inokuchi K. Clinicopathological analysis of the intestinal type and diffuse type of gastric carcinoma. Jpn J Surg. 1980;10:277-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Ribeiro MM, Sarmento JA, Sobrinho Simões MA, Bastos J. Prognostic significance of Lauren and Ming classifications and other pathologic parameters in gastric carcinoma. Cancer. 1981;47:780-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Maehara Y, Anai H, Kusumoto H, Sugimachi K. Poorly differentiated human gastric carcinoma is more sensitive to antitumor drugs than is well differentiated carcinoma. Eur J Surg Oncol. 1987;13:203-206. [PubMed] |
| 8. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1723] [Cited by in RCA: 1897] [Article Influence: 135.5] [Reference Citation Analysis (0)] |
| 9. | Nakajima T. Gastric cancer treatment guidelines in Japan. Gastric Cancer. 2002;5:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 418] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 10. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2390] [Cited by in RCA: 2872] [Article Influence: 205.1] [Reference Citation Analysis (0)] |
| 11. | Sobin L, Gospodarowicz M, Wittekind C, editors: International union against cancer. TNM classification of malignant tumours, 7th edition. New York: Wiley-Blackwell 2010; . |
| 12. | Nakamura K, Sugano H, Takagi K. Carcinoma of the stomach in incipient phase: its histogenesis and histological appearances. Gan. 1968;59:251-258. [PubMed] |
| 13. | Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31-49. [PubMed] |
| 14. | Tahara E, Semba S, Tahara H. Molecular biological observations in gastric cancer. Semin Oncol. 1996;23:307-315. [PubMed] |
| 15. | Shimizu H, Ichikawa D, Komatsu S, Okamoto K, Shiozaki A, Fujiwara H, Murayama Y, Kuriu Y, Ikoma H, Nakanishi M. The decision criterion of histological mixed type in “T1/T2” gastric carcinoma--comparison between TNM classification and Japanese Classification of Gastric Cancer. J Surg Oncol. 2012;105:800-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Watanabe G, Ajioka Y, Kato T, Nishikura K. Pathological characteristics of differentiated-type early gastric carcinoma mixed with undifferentiated-type-Status of lymph node metastasis and macroscopic features [in Japanese with English abstract]. Stomach Intestine. 2007;42:1577-1587. |
| 17. | Tanabe H, Iwashita A, Haraoka S, Ikeda K, Oshige K, Ohta A, Nishimata N, Futami K, Matsui T, Nagahama T. Clinicopathological characteristics of undifferentiated mixed-type early gastric carcinoma with lymph node metastasis [in Japanese with English abstract]. Stomach Intestine. 2007;42:1561-1576. |
| 18. | Hanaoka N, Tanabe S, Mikami T, Okayasu I, Saigenji K. Mixed-histologic-type submucosal invasive gastric cancer as a risk factor for lymph node metastasis: feasibility of endoscopic submucosal dissection. Endoscopy. 2009;41:427-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 19. | Tajima Y, Murakami M, Yamazaki K, Masuda Y, Aoki S, Kato M, Sato A, Goto S, Otsuka K, Kato T. Risk factors for lymph node metastasis from gastric cancers with submucosal invasion. Ann Surg Oncol. 2010;17:1597-1604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Iwamoto J, Mizokami Y, Ito M, Shomokobe K, Hirayama T, Honda A, Saito Y, Ikegami T, Matsuzaki Y. Clinicopathological features of undifferentiated mixed type early gastric cancer treated with endoscopic submucosal dissection. Hepatogastroenterology. 2010;57:185-190. [PubMed] |
| 21. | Takao M, Kakushima N, Takizawa K, Tanaka M, Yamaguchi Y, Matsubayashi H, Kusafuka K, Ono H. Discrepancies in histologic diagnoses of early gastric cancer between biopsy and endoscopic mucosal resection specimens. Gastric Cancer. 2012;15:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 22. | Takizawa K, Ono H, Kakushima N, Tanaka M, Hasuike N, Matsubayashi H, Yamagichi Y, Bando E, Terashima M, Kusafuka K. Risk of lymph node metastases from intramucosal gastric cancer in relation to histological types: how to manage the mixed histological type for endoscopic submucosal dissection. Gastric Cancer. 2013;16:531-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 23. | Nakayoshi T, Tajiri H, Matsuda K, Kaise M, Ikegami M, Sasaki H. Magnifying endoscopy combined with narrow band imaging system for early gastric cancer: correlation of vascular pattern with histopathology (including video). Endoscopy. 2004;36:1080-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 335] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 24. | Huscher CG, Mingoli A, Sgarzini G, Sansonetti A, Di Paola M, Recher A, Ponzano C. Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: five-year results of a randomized prospective trial. Ann Surg. 2005;241:232-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 650] [Cited by in RCA: 655] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 25. | Kitano S, Shiraishi N, Uyama I, Sugihara K, Tanigawa N. A multicenter study on oncologic outcome of laparoscopic gastrectomy for early cancer in Japan. Ann Surg. 2007;245:68-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 519] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 26. | Park SY, Kook MC, Kim YW, Cho NY, Kim TY, Kang GH. Mixed-type gastric cancer and its association with high-frequency CpG island hypermethylation. Virchows Arch. 2010;456:625-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |