Published online Apr 21, 2015. doi: 10.3748/wjg.v21.i15.4666
Peer-review started: May 21, 2014
First decision: July 8, 2014
Revised: July 24, 2014
Accepted: October 15, 2014
Article in press: October 15, 2014
Published online: April 21, 2015
Processing time: 335 Days and 11.2 Hours
AIM: To study anti-Epstein-Barr virus (EBV) IgG antibodies in Crohn´s disease in relation to treatment, immune cells, and prior tonsillectomy/appendectomy.
METHODS: This study included 36 CD patients and 36 healthy individuals (controls), and evaluated different clinical scenarios (new patient, remission and active disease), previous mucosa-associated lymphoid tissue removal (tonsillectomy and appendectomy) and therapeutic regimens (5-aminosalicylic acid, azathioprine, anti-tumor necrosis factor, antibiotics, and corticosteroids). T and B cells subsets in peripheral blood were analyzed by flow cytometry (markers included: CD45, CD4, CD8, CD3, CD19, CD56, CD2, CD3, TCRαβ and TCRγδ) to relate with the levels of anti-EBV IgG antibodies, determined by enzyme-linked immunosorbent assay.
RESULTS: The lowest anti-EBV IgG levels were observed in the group of patients that were not in a specific treatment (95.4 ± 53.9 U/mL vs 131.5 ± 46.2 U/mL, P = 0.038). The patients that were treated with 5-aminosalicylic acid showed the highest anti-EBV IgG values (144.3 U/mL vs 102.6 U/mL, P = 0.045). CD19+ cells had the largest decrease in the group of CD patients that received treatment (138.6 vs 223.9, P = 0.022). The analysis of anti-EBV IgG with respect to the presence or absence of tonsillectomy showed the highest values in the tonsillectomy group of CD patients (169.2 ± 20.7 U/mL vs 106.1 ± 50.3 U/mL, P = 0.002). However, in the group of healthy controls, no differences were seen between those who had been tonsillectomized and subjects who had not been operated on (134.0 ± 52.5 U/mL vs 127.7 ± 48.1 U/mL, P = 0.523).
CONCLUSION: High anti-EBV IgG levels in CD are associated with 5-aminosalicylic acid treatment, tonsillectomy, and decrease of CD19+ cells.
Core tip: The prevalence of anti-Epstein-Barr virus (EBV) IgG antibodies was studied in patients with Crohn’s disease. The lowest anti-EBV IgG levels were observed in the patients without specific treatment. 5-aminosalicylic acid treatment showed the highest anti-EBV IgG values. CD19+ cells had the largest decrease in Crohn’s disease patients that had received treatment. Crohn’s disease patients had the highest values of anti-EBV IgG.
- Citation: Andreu-Ballester JC, Gil-Borrás R, García-Ballesteros C, Catalán-Serra I, Amigo V, Fernández-Fígares V, Cuéllar C. Epstein-Barr virus is related with 5-aminosalicylic acid, tonsillectomy, and CD19+ cells in Crohn’s disease. World J Gastroenterol 2015; 21(15): 4666-4672
- URL: https://www.wjgnet.com/1007-9327/full/v21/i15/4666.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i15.4666
Crohn’s disease (CD) is one of the main types of inflammatory bowel disease (IBD). Although its etiology remains has not been described, it is known as a relapsing-remitting or progressive inflammatory condition that affects the gastrointestinal tract[1]. In a previous study, we observed a decrease of lymphocytes in the peripheral blood of patients with CD[2]. Lymphocyte populations did not differ between treated and untreated patients, with the exception of CD8+ T and CD19+ lymphocytes. Further, in a case control study, we concluded that CD patients are at risk for microsporidiasis, so we proposed that microsporidia, a group of opportunistic pathogens, may be involved in the etiology of CD[3]. The above-mentioned immune- deficiency may predispose individuals to be vulnerable to opportunistic infectious agents responsible for the disease.
Currently, the most common drugs used to treat CD are 5-aminosalicylic acid (5-ASA) drugs, corticosteroids, immunosuppressive agents, biologic therapies, and antibiotics[4]. Wagner et al[5] summarized the current literature surrounding the association between viruses and CD, and suggested that Epstein-Barr virus (EBV), a ubiquitous gamma herpes virus, may be associated with CD.
Viral transmission through the oropharyngeal mucosal epithelium is crucial for the entrance of the virus into the body. Mucosa associated lymphoid tissue (MALT) is essential in EBV infection because viral particles colonize the tongue, oropharyngeal mucosa, and salivary glands; later, they infect B cells, establishing the latent infection[6,7]. It has been suggested that extirpation of MALT, such as of tonsils and the appendix, could be related with CD, although there are many discrepancies about this theory[8-11].
The aim of this work was to study the prevalence of anti-EBV IgG antibodies in patients with CD, and its relation with different clinical situations, treatments, T and B cells subsets, and MALTectomy (tonsillectomy and appendectomy).
In this retrospective study we used the same population recruited in a previous work[2]. We collected serum samples from 36 CD patients and from 36 healthy individuals (controls). Serum samples were maintained at -80 °C until analytical determinations were performed. The 36 CD patients were selected following Lennard-Jones criteria for CD[12]. Both groups were similar regarding sex and age ± 5 years. CD patients were divided according to three clinical situations: “new patients,” with active CD presenting at diagnosis with no previous treatment for CD; “remission,” CD activity index (CDAI) < 150 for ≥ 12 mo; and “active disease,” CDAI > 150 and signs and symptoms of disease[13,14]. The activity of the disease was evaluated in accordance with CDAI[15]. Patients in remission were recruited among patients in follow-up at the outpatient clinic. On the other hand, new patients and patients with active disease were selected among patients admitted to the Gastroenterology Department at the Arnau de Vilanova Hospital (Valencia, Spain).
Although the number of subjects included in the study was not large, patients and controls were divided into three groups: those who had been appendectomized, those who had been tonsillectomized, and those without previous MALTectomy. The inclusion criteria of healthy controls were: absence of acute infections, and absence of inflammatory, autoimmune or immunodeficiency diseases, and no immunosuppressive or antibiotic treatment or any vaccine during the previous year.
Blood cell counts were performed using an automated hematology analyzer (LH750 and Cytomics FC 500; Beckman Coulter Inc., Brea, CA, United States) and later analyzed with CXP Software. The following monoclonal antibodies were used: CD45, CD4, CD8, CD3, and CD19 for the peripheral blood subpopulations, and CD4, CD8, CD56, CD2, CD3, CD19, TCRαβ, and TCRγδ for the γδ T cells study. γδ T cells subsets were analyzed with phycoerythrin-cyanine 5.1-conjugated anti-human TCRγδ (clone: IMMU 510; Beckman Coulter Inc.), and αβ T cells subsets were analyzed with phycoerythrin-cyanine 5.1-conjugated anti-human TCRαβ (clone: IP26A; Beckman Coulter Inc.).
Serum was separated from the blood after clotting and centrifugation. The detection and quantitative determination of specific IgG antibodies against EBV viral capsid antigen (VCA) in the serum of patients and controls were carried out using the DEMDITEC EBV VCA IgG Antibody ELISA Test Kit (Demeditec Diagnostics GmBH, Kiel, Germany) according to the manufacturer’s instructions. For testing, the samples were diluted 1/101 with sample diluent. After reading at 450 nm, the optical densities of the standards were plotted against their concentrations. Then, the concentrations of the samples, expressed in U/mL, were calculated from the standard curve. For the interpretation of the results, values > 12 U/mL were considered as positive.
Each participant in the study signed an informed consent form. The study was approved by the Ethics and Investigation Committee of the Arnau de Vilanova Hospital (Valencia, Spain).
Mann-Whitney U Test was used to compare the means of the quantitative variables for paired samples. Contingency tables were drawn up for the qualitative variables (χ2 or Fisher’s exact tests). The level of significance was set at two-tailed P < 0.05. Data were analyzed using the statistical software SPSS, version 19 (IBM Corp., Armonk, NY, United States).
Of the 36 CD patients included in the study, 22 were male (61.1%) and 14 were female (38.9%). The age mean was 41.9 ± 15.9 years. The clinical situations were: 13/36 (36.1%) new patients, 13/36 (36.1%) active disease, and 10/36 (27.8%) remissions. The sites of intestinal involvement were: ileal in 15/36 (41.7%) patients, colonic in 11/36 (30.6%), and ileocolonic in 10/36 (27.8%) patients. According to the evolution of the disease, 24/36 (66.7%) cases were inflammatory, 8/36 (22.2%) stenotic, and 4/36 (11.1%) had fistulas.
In the present study, 97.2% of the sera samples were IgG positive against EBV by ELISA. No differences in percentages of positive were seen between CD patients and healthy controls. Anti-EBV IgG levels were similar in CD and control groups (116.5 ± 52.1 U/mL vs 129.1 ± 49.6 U/mL).
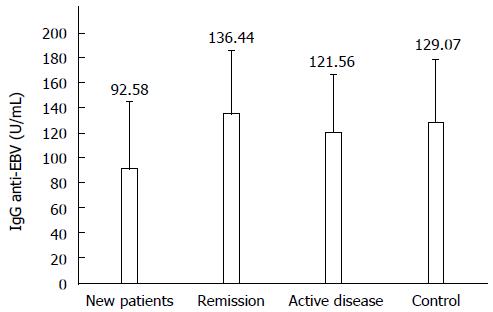
Anti-EBV IgG are described in accordance with the different clinical situations studied in the 36 CD patients and 36 healthy controls (Figure 1); new patients (92.6 ± 53.0 U/mL) were significantly different from patients in remission (136.4 ± 49.8 U/mL) and healthy controls (129.1 ± 49.6 U/mL) (both P < 0.05).
Table 1 shows anti-EBV IgG antibodies depending on the treatment. Twenty-one patients (58.3%) were in treatment at the moment of the sampling. As indicated above, the lowest levels of anti-EBV IgG were observed in the CD new patients. None of the 13 new patients were in treatment at the moment of the sampling. However, 10/12 patients with active disease and 9/13 patients in remission were taking specific treatment for CD. The lowest anti-EBV IgG levels were observed in the group of patients that were not in specific treatment.
| Treatment type | Treated | Anti-EBV IgG | Untreated | Anti-EBV IgG | P value |
| All drugs | 21 (58.3) | 131.5 ± 46.2 | 15 (41.7) | 95.4 ± 53.9 | 0.038 |
| Azathioprine | 8 (22.2) | 122.4 ± 45.8 | 28 (77.8) | 114.7 ± 54.3 | 0.720 |
| Anti-TNF | 3 (8.3) | 106.0 ± 67.4 | 33 (91.7) | 117.4 ± 51.7 | 0.722 |
| 5-ASA | 12 (33.3) | 144.3 ± 47.7 | 24 (66.7) | 102.6 ± 49.3 | 0.010 |
| Antibiotic | 5 (13.9) | 87.6 ± 45.3 | 31 (86.1) | 121.1 ± 52.2 | 0.161 |
| Corticosteroids | 6 (16.7) | 105.2 ± 45.7 | 30 (83.3) | 118.7 ± 53.7 | 0.538 |
| Drugs/patient | |||||
| 0 | 21 (100) | 95.4 ± 53.9 | |||
| 1 | 10 (47.6) | 146.5 ± 36.3 | 15 (41.7) | 95.4 ± 53.9 | 0.023 |
| 2 | 8 (38.1) | 132.1 ± 46.2 | 15 (41.7) | 95.4 ± 53.9 | 0.169 |
| 3 | 3 (14.3)a | 80.0 ± 53.5 | 15 (41.7) | 95.4 ± 53.9 | 0.824 |
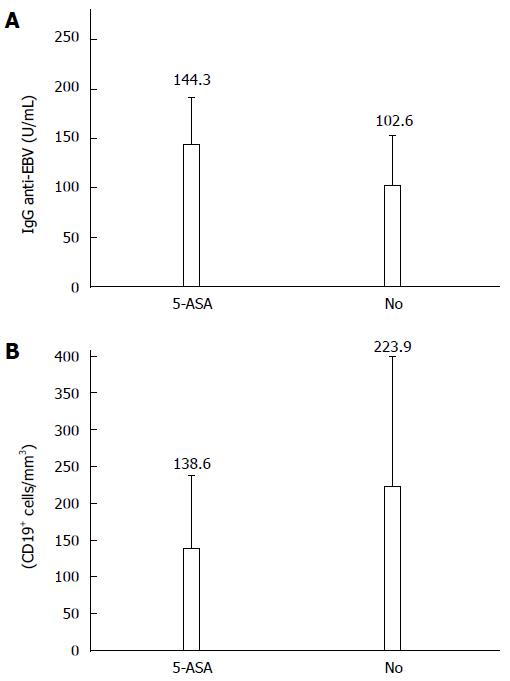
The increase of anti-EBV IgG levels was related to 5-ASA treatment. Thus, the patients that were treated with 5-ASA showed significantly higher anti-EBV IgG values that those observed in the group of CD patients that were not treated at the moment of the sampling (P < 0.05) (Figure 2).
The highest anti-EBV IgG levels from the 21 patients with treatment were observed in the groups treated with one drug only or with two drugs compared to the untreated group and the group of patients treated with three drugs (Ps < 0.05).
When significant differences between lymphocyte subsets were studied in CD patients depending on the presence or absence of treatment, CD19+ cells had the largest decrease in the group of CD patients that had received treatment compared to the untreated ones, which reached similar values to those observed in the control group. The frequencies of CD19+ cells were significantly lower in patients treated with 5-ASA than in untreated patients (138.6 ± 98.5/mm3vs 223.9 ± 155.8/mm3, P = 0.057) (Figure 2). CD3+CD8+ and CD3+CD56+γδ T cells were also reduced in 5-ASA treated patients (5.2 ± 4.1/mm3 and 3.3 ± 3.0/mm3, respectively) compared to the untreated group (7.5 ± 8.2/mm3 and 5.3 ± 8.3/mm3, respectively), though significant differences were not observed, likely due to the small size of these groups.
Although the subgroup analyses lead to a very small group size, we studied the relationship between anti-EBV IgG levels and MALTectomy in CD patients and healthy controls; 9.8% of the subjects included in the study were appendectomized. No differences between groups were seen (11.1% vs 8.6% in CD patients and healthy controls, respectively).
Twenty-two (31.9%) of the subjects were tonsillectomized and the highest percentage was observed in the control healthy group (45.7%) compared to the CD patients (17.6%) (OR = 0.55, 95%CI: 0.36-0.84; P = 0.019).
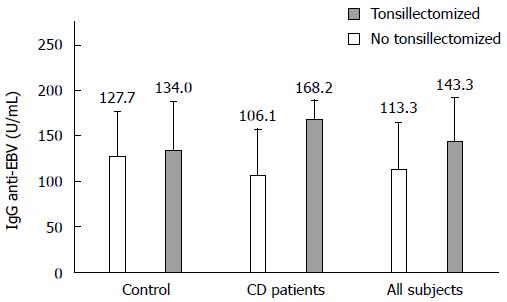
When anti-EBV IgG levels were compared with respect to the presence or absence of tonsillectomy in all studied patients (Figure 3), the highest values were observed in the tonsillectomy group (143.3 ± 48.0 U/mL vs 113.3 ± 49.8 U/mL, P = 0.015). In CD patients, the highest values were also observed in the tonsillectomy group (168.2 ± 20.7 U/mL vs 106.1 ± 50.3 U/mL, P = 0.002). However, in the group of healthy controls, no differences were seen between tonsillectomized patients and subjects who had not undergone a MALTectomy (134.0 ± 52.5 U/mL vs 127.7 ± 48.1 U/mL).
We determined IgG by ELISA against VCA of EBV in sera from 36 CD patients and 36 healthy controls. The development of VCA IgG antibodies usually takes place at the beginning of the disease, reaching the peak titers after two or four weeks and remaining positive thereafter[16].
It is interesting to highlight that the patients who were in the initial stages of the disease showed the lowest anti-EBV IgG levels. IgG levels progressively increased throughout the evolution of the disease, showing similar levels in the active disease until reaching the highest values in patients in remission, who showed similar levels to those obtained in the control group. In a previous study, we showed that a complex alteration of immune responses that affects the total numbers and function of γδ T cells was present in CD[2]. We also observed that CD4+ and CD8+γδ T cells were lower in new patients, and CD4+ and CD8+αβ T cells were significantly lower than their controls.
5-ASA, also known as mesalazine, is a structural analogue of aspirin that, along with other non-steroidal anti-inflammatory drugs (NSAIDs), is among the most conventional drugs currently used to treat CD. Mesalazine negatively regulates the cyclooxygenase-2/prostaglandin E2 axis[4,17], and also inhibits the synthesis of 5-lipoxygenase products, including leukotriene B4[18]. 5-ASA inhibits production of thromboxane A2 and consequently decreases adhesion to the endothelium and the aggregation of platelets and polymorphonuclear cells[19]. The pharmacologic profile of mesalazine includes an ability to inhibit intestinal macrophage chemotaxis. 5-ASA can exert antioxidant actions in vitro and in vivo[20]. These mechanisms could facilitate the entry of a virus through the mucous membranes and therefore they could increase the production of antibodies in treated patients.
We have previously demonstrated that levels of CD3+, CD4+, CD8+, and CD19+ lymphocytes were decreased in CD patients compared to the control group[2]. However, no correlations were observed among anti-EBV IgG levels and different lymphocyte subsets.
The lowest levels of anti-EBV IgG were observed in the new CD patients group and, according to the treatment, the lowest IgG levels were observed in the group of patients that were not receiving specific treatment. However, it is a well-known fact that the anti-inflammatory properties of 5-ASA reflect an inhibition of antibody synthesis[19]. On the other hand, it is interesting to know that other NSAIDs such as aspirin induce cell killing of EBV-positive B lymphocytes by reactivating EBV into lytic replication[21]. The induction of EBV lytic replication in host cells may produce the increase in anti-EBV IgG levels observed in the sera of 5-ASA-treated patients. Besides, the decrease of CD19+ cells in those patients may be the result of a cytotoxic effect of the drug on EBV-positive cells, which resulted in their death. Komatsuda et al[22] reported a case of sulfasalazine-induced hypersensitivity syndrome associated with reactivation of EBV with progressive cytopenia. It is important to note that not one of the new patients were in treatment, and as it was reported before, lymphocyte populations did not differ between treated and untreated patients, except in CD8+ T and CD19+ cells[2]. Consequently, there was an inverse relationship between CD19+ cells rates and anti-EBV IgG levels. In a study carried out by Magro et al[23] with IBD patients, the prevalence of EBV DNA was significantly higher in patients treated with 5-ASA compared to controls, though IgG-VCA positivity was not relevant for EBV DNA presence or load. In addition, the 5-ASA-treated group had a significantly higher percentage of patients with > 500 copies/mL compared to controls. This is in accordance with the induction of cell killing of EBV-positive B lymphocytes by reactivating EBV into lytic replication exerted by NSAIDs[21], which is reflected in the CD19+ decrease observed by us in the group of CD patients that had received treatment compared to the untreated ones.
Following a review of the literature, the relationship between appendectomy and CD is unclear. For example, Kurina et al[8] observed an association of prior appendectomy with a significantly increased risk of CD that was strongest in the year after the appendectomy. They concluded that CD could have been first misdiagnosed as appendicitis in these patients because they observed a reduction in risk of CD (considered as non-significant) when five or more years separated appendectomy and CD. Furthermore, Radford-Smith et al[9] showed an inverse association between appendectomy and CD and, conversely, Duggan et al[10] found no association with appendectomy for CD. In our study, only 9.8% of the subjects were appendectomized, and this number is too small to draw conclusions.
Our results indicate that tonsillectomy could act as a protective factor for CD. Although Kurina et al[8] did not observe an association between prior tonsillectomy and any increase or decrease of risk of CD, Duggan et al[10] reported that tonsillectomy showed a weak positive association with CD. However, Hansen et al[11] showed that tonsillectomy was negatively associated with the development of CD, which is in accordance with the results obtained in the present study, though they need to be analyzed with caution because of the small group sizes.
It is interesting to note that in the CD patient group, levels of anti-EBV IgG were significantly higher in the tonsillectomized group. In a previous work, we found that both specific antibody levels against Kudoa (a frequent fish parasite) and the number of patients with recognized Kudoa antigens were higher in tonsillectomy patients when compared to the control group[24]. This fact shows the role of tonsils as the first MALT defense line, suggesting that tonsils may play an important role in avoiding parasites and other infectious agents travelling along the digestive tract and preventing potential pathogenicity.
EBV has a tropism for the oral and nasopharyngeal tissues, and is one of the main viruses involved in the pathogenesis of recurrent tonsillitis and tonsillar hypertrophy[25]. We have previously demonstrated that MALTectomy significantly decreases secretory IgA levels in serum. The decrease of this IgG that plays a critical role in mucosal immunity was more pronounced when both surgeries (tonsillectomy and appendectomy) have been performed in the same patient[26].
Taking into account that tonsils exposed to EBV play an important role in controlling infection by EBV in the oropharynx it is not surprising that, in tonsillectomized patients, the dissemination of viruses takes place easily and, consequently, the antibody levels to EBV were higher in the sera from these patients.
In summary, high anti-EBV IgG levels in CD were associated with 5-ASA treatment, tonsillectomy, and decrease of CD19+ cells.
We would like to thank Anselmo Villar-Grimalt, MD, PhD, Head of Internal Medicine Department, Arnau de Vilanova Hospital and President of Association for Medical-Oncology Research, Valencia Spain, José Mayans, MD, Head of Hematology Department, and Miguel Bixquert-Jiménez, MD, PhD, for their unconditional support for our work.
The common conventional drugs currently use to treat Crohn’s disease (CD) involve 5-aminosalicylic acid. Epstein-Barr virus (EBV) may be associated with CD. Extirpation of mucosa associated lymphoid tissue could be related with CD.
CD may result from an immune deficiency. This situation may favor opportunistic infections in predisposed individuals.
5-aminosalicylic acid causes adverse effects on the immune system and therefore could facilitate the entry of the virus through the mucous membranes. Tonsillectomy may also contribute to the immune dysfunction.
The study sheds new light on anti-inflammatory effects on CD evolution.
CD is a type of inflammatory bowel disease that may affect any part of the gastrointestinal tract from mouth to anus. The EBV is a virus of the herpes family, and is one of the most common viruses in humans. B cells are a type of lymphocyte in the humoral immunity of the adaptive immune system. Tonsillectomy is a surgical procedure in which each tonsil is removed from a recess in the side of the pharynx called the tonsillar fossa. 5-aminosalicylic acid is an anti-inflammatory drug used to treat inflammatory bowel disease.
This manuscript is a cross-sectional analysis of anti-EBV antibodies in a group of patients with CD and a control group.
P- Reviewer: Day AS S- Editor: Gou SX L- Editor: AmEditor E- Editor: Liu XM
| 1. | Thompson AI, Lees CW. Genetics of ulcerative colitis. Inflamm Bowel Dis. 2011;17:831-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 2. | Andreu-Ballester JC, Amigó-García V, Catalán-Serra I, Gil-Borrás R, Ballester F, Almela-Quilis A, Millan-Scheiding M, Peñarroja-Otero C. Deficit of gammadelta T lymphocytes in the peripheral blood of patients with Crohn’s disease. Dig Dis Sci. 2011;56:2613-2622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Andreu-Ballester JC, Garcia-Ballesteros C, Amigo V, Ballester F, Gil-Borrás R, Catalán-Serra I, Magnet A, Fenoy S, del Aguila C, Ferrando-Marco J. Microsporidia and its relation to Crohn’s disease. A retrospective study. PLoS One. 2013;8:e62107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Pichai MV, Ferguson LR. Potential prospects of nanomedicine for targeted therapeutics in inflammatory bowel diseases. World J Gastroenterol. 2012;18:2895-2901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Wagner J, Sim WH, Lee KJ, Kirkwood CD. Current knowledge and systematic review of viruses associated with Crohn’s disease. Rev Med Virol. 2013;23:145-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Tugizov SM, Berline JW, Palefsky JM. Epstein-Barr virus infection of polarized tongue and nasopharyngeal epithelial cells. Nat Med. 2003;9:307-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 206] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 7. | Tugizov SM, Herrera R, Palefsky JM. Epstein-Barr virus transcytosis through polarized oral epithelial cells. J Virol. 2013;87:8179-8194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 8. | Kurina LM, Goldacre MJ, Yeates D, Seagroatt V. Appendicectomy, tonsillectomy, and inflammatory bowel disease: a case-control record linkage study. J Epidemiol Community Health. 2002;56:551-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Radford-Smith GL, Edwards JE, Purdie DM, Pandeya N, Watson M, Martin NG, Green A, Newman B, Florin TH. Protective role of appendicectomy on onset and severity of ulcerative colitis and Crohn’s disease. Gut. 2002;51:808-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 138] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 10. | Duggan AE, Usmani I, Neal KR, Logan RF. Appendicectomy, childhood hygiene, Helicobacter pylori status, and risk of inflammatory bowel disease: a case control study. Gut. 1998;43:494-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 128] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 11. | Hansen TS, Jess T, Vind I, Elkjaer M, Nielsen MF, Gamborg M, Munkholm P. Environmental factors in inflammatory bowel disease: a case-control study based on a Danish inception cohort. J Crohns Colitis. 2011;5:577-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 125] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 12. | Lennard-Jones JE. Classification of inflammatory bowel disease. Scand J Gastroenterol Suppl. 1989;170:2-6; discussion 16-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1396] [Cited by in RCA: 1445] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 13. | Stange EF, Travis SP, Vermeire S, Beglinger C, Kupcinkas L, Geboes K, Barakauskiene A, Villanacci V, Von Herbay A, Warren BF. European evidence based consensus on the diagnosis and management of Crohn’s disease: definitions and diagnosis. Gut. 2006;55 Suppl 1:i1-i15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 381] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 14. | Travis SP, Stange EF, Lémann M, Oresland T, Chowers Y, Forbes A, D’Haens G, Kitis G, Cortot A, Prantera C. European evidence based consensus on the diagnosis and management of Crohn’s disease: current management. Gut. 2006;55 Suppl 1:i16-i35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 413] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 15. | Best WR, Becktel JM, Singleton JW, Kern F. Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology. 1976;70:439-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | De Paschale M, Clerici P. Serological diagnosis of Epstein-Barr virus infection: Problems and solutions. World J Virol. 2012;1:31-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 180] [Cited by in RCA: 207] [Article Influence: 15.9] [Reference Citation Analysis (4)] |
| 17. | Stolfi C, De Simone V, Pallone F, Monteleone G. Mechanisms of action of non-steroidal anti-inflammatory drugs (NSAIDs) and mesalazine in the chemoprevention of colorectal cancer. Int J Mol Sci. 2013;14:17972-17985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 18. | Lauritsen K, Laursen LS, Bukhave K, Rask-Madsen J. Effects of topical 5-aminosalicylic acid and prednisolone on prostaglandin E2 and leukotriene B4 levels determined by equilibrium in vivo dialysis of rectum in relapsing ulcerative colitis. Gastroenterology. 1986;91:837-844. [PubMed] |
| 19. | Punchard NA, Greenfield SM, Thompson RP. Mechanism of action of 5-arninosalicylic acid. Mediators Inflamm. 1992;1:151-165. [PubMed] |
| 20. | Whittle BJ, Varga C. New light on the anti-colitic actions of therapeutic aminosalicylates: the role of heme oxygenase. Pharmacol Rep. 2010;62:548-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Liu SF, Wang H, Li ZJ, Deng XY, Xiang H, Tao YG, Li W, Tang M, Cao Y. Aspirin induces lytic cytotoxicity in Epstein-Barr virus-positive cells. Eur J Pharmacol. 2008;589:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Komatsuda A, Okamoto Y, Hatakeyama T, Wakui H, Sawada K. Sulfasalazine-induced hypersensitivity syndrome and hemophagocytic syndrome associated with reactivation of Epstein-Barr virus. Clin Rheumatol. 2008;27:395-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Magro F, Santos-Antunes J, Albuquerque A, Vilas-Boas F, Macedo GN, Nazareth N, Lopes S, Sobrinho-Simões J, Teixeira S, Dias CC. Epstein-Barr virus in inflammatory bowel disease-correlation with different therapeutic regimens. Inflamm Bowel Dis. 2013;19:1710-1716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Andreu-Ballester JC, Ballester F, Pérez-Griera J, Amigo V, Peñarroja-Otero C, Colomer-Rubio E, Ortiz-Tarín I, Pelayo V, García-Hernández P, Rodero M. Differential effect of appendectomy and tonsillectomy on anti-Kudoa sp. antibodies in patients with MALTectomy. Parasitol Int. 2009;58:401-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 25. | Endo LH, Ferreira D, Montenegro MC, Pinto GA, Altemani A, Bortoleto AE, Vassallo J. Detection of Epstein-Barr virus in tonsillar tissue of children and the relationship with recurrent tonsillitis. Int J Pediatr Otorhinolaryngol. 2001;58:9-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Andreu-Ballester JC, Pérez-Griera J, Ballester F, Colomer-Rubio E, Ortiz-Tarín I, Peñarroja Otero C. Secretory immunoglobulin A (sIgA) deficiency in serum of patients with GALTectomy (appendectomy and tonsillectomy). Clin Immunol. 2007;123:289-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |