Published online Apr 21, 2015. doi: 10.3748/wjg.v21.i15.4635
Peer-review started: October 29, 2014
First decision: November 26, 2014
Revised: December 4, 2014
Accepted: January 16, 2015
Article in press: January 16, 2015
Published online: April 21, 2015
Processing time: 173 Days and 7.9 Hours
AIM: To compare the outcomes of hepatic resection and transarterial chemoembolization (TACE) for solitary hepatocellular carcinoma (HCC) according to the Barcelona Clinic Liver Cancer (BCLC) staging system.
METHODS: A consecutive sample of 540 patients with solitary HCC who underwent liver resection (n = 312) or TACE (n = 128) were included in the present study. Baseline characteristics, tumor characteristics, and post-operative complications were compared between the two groups. The Kaplan-Meier method was used for long-term survival analysis. Independent prognostic predictors were identified using the Cox proportional hazards model (univariate and multivariate analyses).
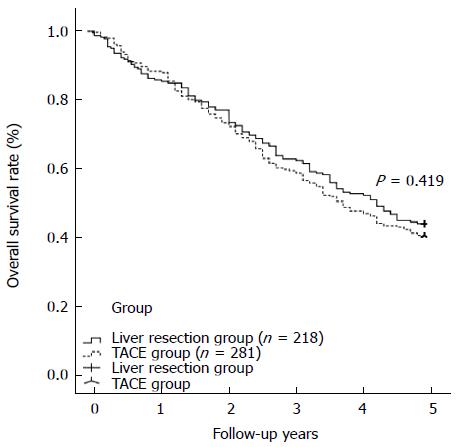
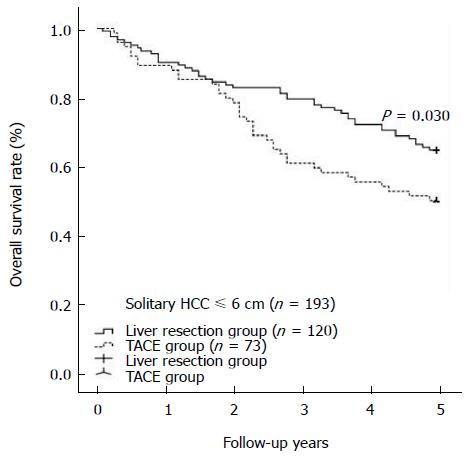
RESULTS: The TACE and liver resection groups had similar baseline demographic and clinicopathological characteristics. The TACE group showed a significantly lower rate of major complications than the liver resection group (3.9% vs 17.4%, P < 0.001). Univariate and multivariate analyses indicated that TACE did not contribute to poor overall survival compared with liver resection; however, a solitary tumor diameter of greater than 6 cm should be considered a risk factor for poor overall survival (HR = 1.328, 95%CI: 1.002-1.783, P = 0.048). The liver resection and TACE groups had comparable overall survival rates at 1 year, 3 years, and 5 years (86.2%, 62.8%, and 44.0% vs 88.3%, 59.8%, and 40.6%, respectively, P = 0.419). In cases with tumor diameters equal to or less than 6 cm, the liver resection group showed a survival benefit compared with the TACE group at 1 year, 3 years, and 5 years (P = 0.030). The 1-, 3-, and 5-year overall survival rates of HCC cases with tumor diameters of more than 6 cm were similar among the liver resection and TACE groups (P = 0.467).
CONCLUSION: A tumor diameter of 6 cm should be the cutoff for deciding between liver resection and TACE.
Core tip: In the present study, we tried to set a cutoff value for solitary hepatocellular carcinoma (HCC) according to the Barcelona Clinic Liver Cancer staging system. Univariate and multivariate analyses indicated that a tumor diameter greater than 6 cm should be considered a risk factor for poor overall survival (HR = 1.328, 95%CI: 0.902-1.783, P = 0.048). In cases with a tumor diameter equal to or less than 6 cm, the liver resection group showed a survival benefit compared with the transarterial chemoembolization (TACE) group. The 1-, 3-, and 5-year overall survival rates were similar among the liver resection and TACE groups regarding HCC cases with a tumor diameter of more than 6 cm.
- Citation: Zhang DZ, Wei XD, Wang XP. Comparison of hepatic resection and transarterial chemoembolization for solitary hepatocellular carcinoma. World J Gastroenterol 2015; 21(15): 4635-4643
- URL: https://www.wjgnet.com/1007-9327/full/v21/i15/4635.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i15.4635
Globally, hepatocellular carcinoma (HCC) is the sixth most common cancer and the third leading cause of cancer-related death[1]. According to the most recent report, liver cancer has the second highest mortality rate, immediately following lung cancer[2]. The HCC tumor burden is heavier in northeastern Asian countries, especially China, because of the high prevalence of hepatitis B virus infection. China accounts for 55% of all HCC cases worldwide[1].
The prognoses of patients with HCC are determined by the tumor status, liver function reserve, general health status and treatment efficacy[3]. A staging system that considers all of these factors is important for predicting the prognosis and for comparing the outcomes of patients with HCC. Several prognostic staging systems are used to predict the survival of patients with HCC. These include the Okuda system[4], tumor node metastasis (TNM) system[5], the Cancer of the Liver Italian Program (CLIP) score system[6], Japan Integrated Staging (JIS) system[7], the Chinese University Prognostic Index (CUPI)[8] and the Hong Kong Liver Cancer (HKLC) staging system[9]. However, there is no consensus as to the best system. The Barcelona Clinic Liver Cancer (BCLC) staging system combines the above 4 factors, links 5 different stages of HCC with the appropriate therapeutic treatment options, and is endorsed by the European Association of the Study of Liver Diseases (EASLD)[3] and the American Association for the Study of Liver Diseases (AASLD)[10]. The BCLC has recently been widely adopted as the staging system of choice in many Western countries, but it has not yet been adopted in Asia. There is substantial heterogeneity regarding the systems used in studies, especially those assessing the treatment of patients with BCLC-B HCC.
The BCLC staging classification is primarily based on the prognostic analysis of several small cohorts of predominantly hepatitis C virus (HCV)-infected patients with early HCC who were treated via resection, transplantation, or percutaneous ethanol injection[11]. The staging may not reflect the progression or prognosis of patients with HCC in whom hepatitis B virus (HBV) infection is the predominant etiologic factor. Additionally, more aggressive treatment approaches, especially surgical resection, have been adopted in most Asian centers due to higher case volumes and increased expertise[12,13]. Patients with BCLC-B HCC may also receive more benefits from liver resection than from the currently recommended TACE[14-16]. However, according to the BCLC system, all solitary target cases should be graded as BCLC stage A, and all of these BCLC-A cases should undergo curative treatments such as ablation, resection, or transplantation[17,18]. Although the effectiveness of ablation has been demonstrated in small liver cancers with diameters ≤ 3 cm, it is difficult to achieve effective radical treatment with radiofrequency ablation (RFA) in larger liver cancers (especially those with diameters > 5 cm)[17]. According to the Milan[19] and University of California, San Francisco (UCSF) criteria[20], the upper diameter of the solitary targets has been limited to 5 cm and 6.5 cm, respectively, and not all of these cases of solitary HCC are appropriate for liver transplantation. The shortage of donors and morbidity rate of nearly 10%[21] may also limit the use of LT for these HCC cases. Therefore, liver resection may be the most effective and most common approach for solitary HCC. However, not all cases of solitary HCC are suitable for liver resection due to considerations regarding liver function, remnant liver, postoperative complications, overall survival, and tumor recurrence.
Thus, in the present study, we compared the outcomes of TACE and hepatic resection for solitary BCLC stage A HCC with the aim of improving the BCLC staging system. A clear cutoff exists for cases of solitary HCC in which LT, RFA and resection are considered to be the most effective and most available approaches; therefore, we only included patients with solitary HCC who underwent liver resection or TACE.
We retrospectively assessed data from our hospital database for 1900 patients with HCC who underwent liver resection (312 cases) or TACE (128 cases). Our present study was approved by the institutional review board of the Gansu Provincial Hospital and was conducted in accordance with the Declaration of Helsinki and current ethical guidelines. Only cases with Child-Pugh scores of A or B who had liver function scores available and solitary target cases confirmed via preoperative enhanced CT or MRI were included in our present study; all of these patients underwent either TACE or liver resection as an initial therapy in our hospital and had routine postoperative follow-up in the outpatient department. The exclusion criteria included macroscopic vascular invasion and tumor metastases. Additionally, patients who underwent radical therapies such as RFA, liver resection, or LT following TACE were excluded from our study.
The diagnosis of HCC was confirmed via histology or cytology, increased α-fetoprotein levels (≥ 400 ng/mL), or typical radiological appearance in at least 2 clinical imaging modalities (i.e., ultrasonography, computed tomography, or magnetic resonance imaging). All patients with adequate liver function and radiologically resectable tumors were initially evaluated for partial hepatectomy. The remnant liver volume was at least 30% after liver resection in patients with HCC without cirrhosis and 50% in patients with HCC with liver cirrhosis or severe fatty liver. Our centers adopted the ICG-15 in 2006; the ICG-15 was not applied to the patients in this study. The choice of treatment protocol for solitary HCC was mainly based on the liver function, tumor diameter, tumor location and ECOG score.
The following variables were recorded for each patient: age, sex, cause of underlying liver disease, general conditions, main serological parameters, liver function, tumor radiological characteristics, tumor biological characteristics, primary treatment strategy, postoperative recovery, postoperative complications, long-term survival and tumor recurrence.
Portal hypertension (PHT) was defined as the presence of esophageal varices and/or a platelet count of less than 100000 per μL in association with splenomegaly[16].
TACE protocol: The indications for TACE should be a lack of main portal vein tumor thrombus and no severe renal dysfunction. All TACE procedures were performed by 1 of 2 experienced interventional radiologists under local anesthesia. Depending on the tumor size, the location and the arterial supply of the tumor, a 3 Fr microcatheter was advanced toward the tumor-feeding arteries for selective embolization via transfemoral access, and the tip of the catheter was directed toward tumor-feeding arteries (left or right branches) for the superselective embolization of all tumors. A mixture of doxorubicin hydrochloride and an iodized oil emulsion was injected until complete blockage of the tumor-feeding branch was achieved. The dose of the embolization agent was determined based on tumor size, tumor number, feeding vessels and liver function status. Meanwhile, the injection was continued until stasis was confirmed in the feeding artery.
Liver resection: All the surgical procedures were performed under general anesthesia by the chief hepatobiliary physician or by a deputy chief physician with at least 10-15 years of surgical experience. Partial hepatectomy was performed as an anatomical resection. The margins of the resection were at least 1-2 cm from the border of the tumor. Intraoperative in vivo radiotherapy and chemotherapy were not applied, and no portal vein chemotherapy was provided. During surgery, parenchymal dissection was performed with an ultrasonic surgical aspirator. The vessels were not directly pinched. When necessary, the liver pedicle was intermittently clamped in cycles of 10-15 min of clamping and 3-5 min of reperfusion.
Follow-up assessments: Ultrasonography, chest radiography, serum alpha-fetoprotein (AFP) assays and liver function tests were performed every 2-4 mo during the first postoperative year and every 6 mo in subsequent years. Enhanced CT or MRI was performed every 6 mo or when recurrence or progression was suspected via routine ultrasonography. The 1-, 3-, and 5-year overall survival rates were the primary criteria of the follow-up assessments. The treatment protocol for HCC recurrence was implemented according to the tumor location and size and the liver function of the patient. Re-resection, RFA, repeated TACE, and sorafenib were administered for most cases of recurrence. When lung metastasis was found, a gamma knife was the primary recommended treatment.
All data were analyzed using the SPSS statistical software package. Differences between the categorical data were analyzed using the χ2 test and Fisher’s exact test (2-tailed), if necessary. Survival curves were estimated using the Kaplan-Meier method and compared using the log-rank test. Univariate analyses were performed to identify factors that could predict overall and tumor-free survival. All variables with P < 0.05 were included in the multivariate analysis to assess independent predictive factors using Cox regression analyses. The Cox proportional hazards model was used to generate adjusted hazard ratios and 95% confidence intervals. A 2-tailed P value < 0.05 was considered statistically significant in all tests.
The baseline demographic and clinicopathological characteristics of the two groups of patients are listed and compared in Table 1. The mean number of TACE procedures was 2.3 ± 1.2 for patients in the TACE group. The patients in the TACE group had significantly larger tumors than the patients in the liver resection group (6.2 cm vs 7.6 cm, P < 0.001). No significant differences were observed with respect to age, gender, weight, height, body mass index (BMI), etiology of cirrhosis, MELD score, liver function (Child and MELD scores), the presence of portal hypertension, serum AFP level and grade, or neutrophil-lymphocyte ratio (NLR), among others (all P > 0.05). Most of the HCC cases were caused by HBV infection in the two groups; thus, 426 cases (85.4%) of portal hypertension were due to infection with HBV or HCV. Nevertheless, all of these patients demonstrated good liver function (grades A and B, no grade C).
| Liver resection group | TACE group | P value | |
| 218 | 281 | ||
| Age (yr) | 52.1 ± 12.5 | 52.0 ± 13.5 | 0.950 |
| Sex (M/F) | 162/56 | 210/71 | 0.915 |
| Weight (kg) | 67.6 ± 9.5 | 66.8 ± 9.4 | 0.358 |
| Height (cm) | 165.5 ± 8.7 | 164.9 ± 9.0 | 0.496 |
| BMI (kg/m2) | 23.6 ± 2.5 | 23.6 ± 2.4 | 0.870 |
| Cirrhosis etiology (HBV/HCV/negative/other) | 193/5/9/11 | 256/6/6/13 | 0.352 |
| MELD score | 5.4 ± 1.8 | 5.5 ± 1.7 | 0.607 |
| Child score (A/B/C) | 162/56/0 | 222/59/0 | 0.218 |
| EOCG score (0/1/2) | 161/29/28 | 211/39/31 | 0.697 |
| PHT (yes/no) | 182/36 | 244/37 | 0.668 |
| Tumor diameter of the largest target (cm) | 6.2 ± 2.3 | 7.6 ± 2.2 | < 0.001 |
| AFP level (ng/mL) | 2388.5 ± 7916.0 | 2650.1 ± 8514.4 | 0.726 |
| 0-400 ng/mL | 120 | 136 | |
| 400-800 ng/mL | 11 | 26 | |
| 800-1200 ng/mL | 15 | 13 | |
| ≥ 1210 ng/mL | 72 | 106 | |
| NLR ≥ 4 (yes/no) | 109/109 | 160/121 | 0.123 |
| Obvious arterial phase enhancement (yes/no) | 175/43 | 222/59 | 0.727 |
| Diagnostic method (enhanced CT/MRI/biopsy) | 172/36/10 | 238/27/16 | 0.127 |
As shown in Table 2, the Clavien-Dindo classification was used to evaluate and compare the postoperative complications after liver resection or TACE. To compare the two groups, we evaluated the postoperative complications in the initial TACE; however, not all sessions of TACE were evaluated, although multiple sessions of TACE were performed for most of the patients in the TACE group. Additionally, the number of overall complications in the TACE group was significantly higher than that in the liver resection group (54.8% vs 31.2%, P < 0.001). Similarly, the number of minor complications according to the Clavien-Dindo system was also significantly higher in the TACE group than in the liver resection group (43.3% vs 13.8%, P < 0.001). However, a significantly lower rate of major complications was observed in the TACE group compared with the liver resection group (3.9% vs 17.4%, P < 0.001). Both groups showed comparable outcomes regarding in-hospital, 30-d, and 90-d morbidity (all P > 0.05). The most common complications in the TACE group were due to the toxicity of the TACE itself, including nausea/emesis, pain in the upper quadrant, and fever.
| Resection group | TACE group | P value | |
| 218 | 281 | ||
| Complications (Clavien-Dindo classification) | 68 (31.2) | 154 (54.8) | < 0.001 |
| Grade I (without drugs, conservative treatment) | 18 (8.3) | 77 (27.4) | |
| Grade II (simple medicine treatment) | 12 (5.5) | 56 (19.9) | |
| Grade IIIa (therapeutic operation under local anesthesia) | 7 (3.2) | 5 (1.8) | |
| Grade IIIb (operational treatment under general anesthesia) | 5 (2.3) | 2 (0.7) | |
| Grade IVa (single organ function failure) | 5 (2.3) | 1 (0.4) | |
| Grade IVb (multiple organ failure) | 4 (1.8) | 1 (0.4) | |
| Grade V (In-hospital death) | 4 (1.8) | 2 (0.7) | 0.254 |
| Minor complications (I-II) | 30 (13.8) | 133 (43.3) | < 0.001 |
| Major complications (III-V) | 38 (17.4) | 11 (3.9) | < 0.001 |
| 30-d mortality | 5 (2.3) | 3 (1.1) | 0.280 |
| 90-d mortality | 8 (3.7) | 7 (2.5) | 0.445 |
Univariate and multivariate analyses were performed, and the results are shown in Tables 3 and 4; these analyses included factors that are associated with postoperative survival, including age, gender, BMI, the cause of liver disease, the Child-Pugh score, the ECOG score, portal hypertension, the albumin level, the platelet count, hemoglobin, creatinine, the NLR, the AFP level, the tumor diameter, obvious arterial phase enhancement, the diagnostic method, and the type of therapy. Univariate analyses identified the following prognostic factors as indicative of poor overall survival: age < 60, the presence of portal hypertension, NLR ≥ 4, and a tumor diameter > 6 cm. Multivariate Cox regression analyses were performed for these significant factors and revealed that a tumor diameter > 6 cm was a significant risk factor for overall survival in patients with solitary HCC. Interestingly, the mode of therapy (i.e., TACE) did not contribute to overall survival in the univariate and multivariate analyses for solitary HCC.
| Variables | n | Overall survival rate |
| 499 | P value | |
| Age < 60 (yes/no) | 341/158 | 0.041 |
| Gender (M/F) | 372/127 | 0.596 |
| BMI ≥ 26 (yes/no) | 72/427 | 0.268 |
| Cause of liver diseases (HBV/HCV/no/others) | 449/11/15/24 | 0.779 |
| Child-Pugh Score (A/B) | 384/115 | 0.897 |
| ECOG score (0/1/2) | 372/68/59 | 0.594 |
| PHT (yes/no) | 425/74 | < 0.001 |
| ALB ≥ 35 g/L (yes/no) | 275/224 | 0.221 |
| Platelets ≥ 100 × 109/L (yes/no) | 177/322 | 0.923 |
| HB ≥ 120 g/L (yes/no) | 331/168 | 0.602 |
| Creatinine ≥ 100 μmol/L (yes/no) | 49/450 | 0.675 |
| NLR ≥ 4 (yes/no) | 269/230 | < 0.001 |
| AFP level (1-400/400-800/800-1200/ > 1200 ng/mL) | 256/37/28/178 | 0.218 |
| Tumor diameter > 6 cm (yes/no) | 306/193 | < 0.001 |
| Obvious arterial phase enhancement (yes/no) | 397/102 | 0.361 |
| Diagnostic method (enhanced CT/MRI/biopsy) | 410/63/26 | 0.587 |
| Therapy (liver resection/TACE) | 218/281 | 0.437 |
| Variables | HR | 95%CI | P value |
| Age < 60 | 1.129 | 0.981-1.282 | 0.121 |
| PHT | 1.228 | 0.908-1.556 | 0.262 |
| NLR ≥ 4 | 1.431 | 0.992-1.781 | 0.128 |
| Tumor diameter > 6 cm | 1.328 | 1.002-1.783 | 0.048 |
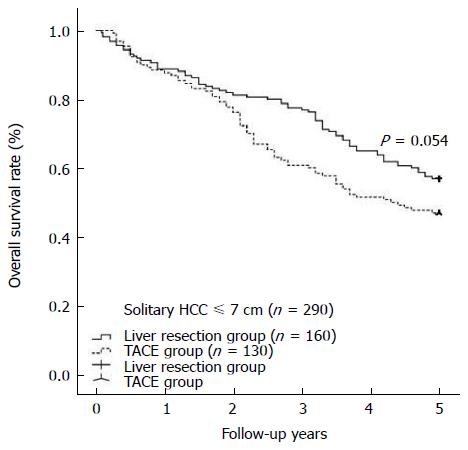
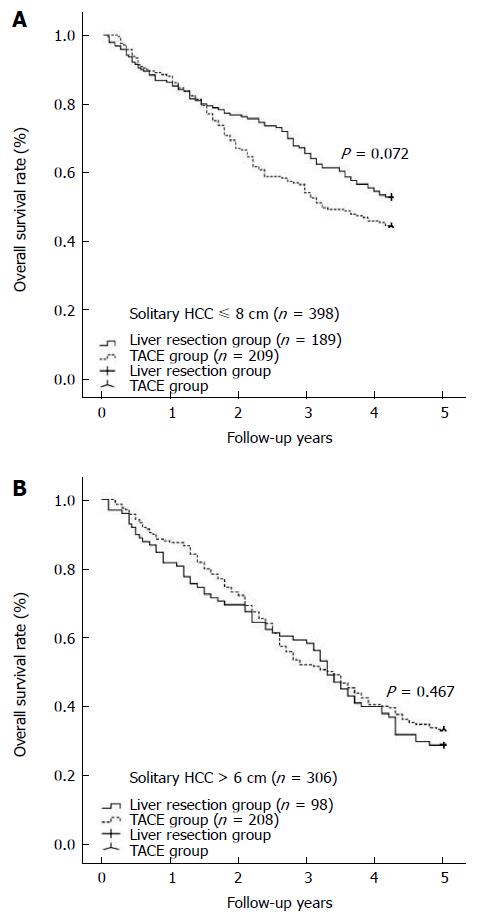
During at least 5 years of follow-up, 113 patients (51.8%) in the liver resection group and 176 patients (62.6%) in the TACE group died. The overall survival was comparable between the liver resection and TACE groups (1 year: 86.2% vs 88.3%; 3 years: 62.8% vs 59.8%; 5 years: 44.0% vs 40.6%) (shown in Figure 1, P = 0.419). In cases of solitary HCC with diameter equal to or less than 6 cm, a survival benefit was noted in the liver resection group compared with the TACE group at 1 year, 3 years, and 5 years (shown in Figure 2, P = 0.030). However, for patients with solitary HCC with a diameter of no more than 7 cm, there was a similar rate of long-term overall survival between the liver resection and TACE groups (shown in Figure 3, P = 0.054). A similar outcome was observed for the two groups of patients with solitary HCC with a diameter of no more than 8 cm (shown in Figure 4A, P = 0.072). The 1-, 3-, and 5-year overall survival rates were similar among the liver resection and TACE groups for cases of solitary HCC with a diameter of more than 6 cm (shown in Figure 4B, P = 0.467).
The major finding of the present study was that 6 cm is the optimal cutoff for BCLC stage A in solitary HCC. A solitary HCC equal to or less than 6 cm in diameter should be classified as BCLC stage A (early stage), and a solitary HCC larger than 6 cm should be classified as BCLC stage B (intermediate stage). According to the guidelines of the European Association for the Study of the Liver[3] and the American Association for the Study of Liver Disease[22] (which are based on the BCLC classification), radical therapies such as ablation, liver resection, and liver transplantation are indicated for patients with early-stage HCC, including cases of solitary HCC. However, radical therapies are not appropriate for all cases of solitary HCC, even with compensatory liver function and ECOG score, and there is no consensus as to the upper limit of the definition of BCLC stage A solitary HCC[17,18]. Zhong et al[16] defined BCLC stage B as the presence of 1 lesion greater than 5 cm in diameter; thus, a solitary HCC equal to or smaller than 5 cm in diameter would be graded as BCLC stage A. However, Zhong et al[16] compared the efficacy and safety of liver resection and TACE for BCLC stage B HCC. No studies have focused on the cutoff value according to the diameter of BCLC stage A solitary HCC. Additionally, few studies have compared TACE and liver resection in BCLC stage A HCC[23].
According to the Milan criteria for LT in HCC, the upper limit with respect to the diameter of solitary HCC cases was 5 cm[19]. However, with the development of transplantation technology and with increasing experience, some groups have argued that the Milan criteria are too restrictive and thus exclude some HCC patients from LT despite the possible survival benefit. The first report was published by Yao et al[20] at the University of California in 2001, who used the University of California, San Francisco (UCSF) criteria for LT. These authors found an excellent 5-year survival of 75%, and the upper limit for the diameter of solitary HCC cases for LT was extended to 6.5 cm. The Milan group (Mazzaferro et al[24]) further attempted to expand the Milan criteria to the Up-to-Seven criteria (now called the “new Milan criteria”): hepatocellular carcinoma with 7 as the sum of the size of the largest tumor (in cm) and the number of tumors. The Hangzhou criteria limit the upper diameter to 8 cm[25]. We established a 6-cm cutoff for BCLC stage A solitary HCC; the same cutoff is currently applied to liver transplantation for HCC.
Radiofrequency ablation is considered a safe method for small HCCs. The efficiency of RFA was very clear for cases with a diameter ≤ 3 cm[26], but some centers perform RFA in tumors with diameters up to 5 cm or greater[17]. Therefore, RFA should be adopted for tumor diameters no greater than 5 cm[17]. Our cutoff of 6 cm may have also included all of the cases that were appropriate for RFA according to the existing criteria.
Due to the shortage of donor liver grafts, the high risk of intraoperative and postoperative complications in LT cases, and the high strict upper limit of the diameter, liver resection should be considered the most acceptable and effective treatment for most patients with HCC[27]. However, perioperative morbidity and mortality have traditionally posed significant risks, and the short time to postoperative recurrence may limit the effectiveness of liver resection in some patients who could benefit from other therapies such as TACE. Although the postoperative complication rate in the TACE group (54.8%) was significantly higher than that in the liver resection group (31.2%), most of the complications in the TACE group were associated with the toxicity of TACE itself (e.g., nausea/emesis, pain in the upper quadrant, and fever). The rate of major complications (grades III-V) in the TACE group (3.9%) was significantly lower than that in the liver resection group (17.4%). Additionally, the 30-d and 90-d morbidities were similar between the two groups. Improvements in surgical technique and perioperative care have made liver resection a relatively safe method for patients with HCC, despite the potential risks. A balance between safety and efficiency should be considered in the treatment of patients with HCC.
Our study compared the long-term outcomes of patients who received liver resection and those who received TACE. As shown in Figure 1, liver resection showed no benefits compared with TACE; however, this may reflect imprecision in the BCLC staging system regarding solitary HCC. We compared the outcomes of the two groups and the subgroups according to tumor diameter. The liver resection group showed a significantly better long-term outcome, especially with respect to 3- and 5-year overall survival, when the diameter of the solitary HCC was no larger than 6 cm in the preoperative imaging scan. Meanwhile, the survival of patients with a single HCC < 6 cm in diameter was comparable with that of patients who had undergone resection according to the Milan criteria[17]. However, liver resection for solitary HCC was no longer advantageous for patients with tumors with diameters larger than 6 cm. These two groups showed comparable long-term outcomes when the solitary target was larger than 6 cm; however, due to the higher rate of major complications, greater potential risk, decreased potential for recurrence and increased cost[17], liver resection should not be routinely recommended to these patients. Although most episodes of recurrence occurred within the first 1-3 years, most tumor-related deaths occurred after this time frame. This may explain why the long-term survival of the two groups was comparable in the first year even for tumors with diameters of no more than 6 cm. Additionally, our univariate and multivariate analyses of overall survival indicated that TACE was not a risk factor for patients with solitary HCC. A solitary HCC with a diameter larger than 6 cm was a risk factor that affected overall survival.
This study has several limitations. The primary limitation is the nature of our patient population, which has one of the highest incidences of HCC in the world. Secondly, this study was limited by a possible selection bias that may have resulted from the comparison of these non-randomized groups and retrospective profiles. Thirdly, this was a single-center study, and the results may not be generalizable. Furthermore, this study included some patients with a performance status of 1-2, which should be graded as B or C. Lastly, there is a 30%-60% discrepancy between the tumor patterns determined preoperatively (via imaging) and the final pattern determined based on the evaluation of the specimen[28,29]. Therefore, a multi-center randomized comparative study with a large sample is warranted; we intend to focus on this in our future work.
In conclusion, if the preoperative imaging evaluation shows a solitary HCC tumor with a diameter less than or equal to 6 cm, the patient should be recommended for liver resection; if resection is not appropriate, TACE should be considered.
The Barcelona Clinic Liver Cancer (BCLC) staging classification is primarily based on the prognostic analysis of several small cohorts of predominantly hepatitis C virus (HCV)-infected patients with early hepatocellular carcinoma (HCC) who were treated via resection, transplantation, or percutaneous ethanol injection. According to the BCLC staging system, all cases of solitary HCC should be classified as stage A (early stage). However, not all solitary HCC patients may benefit from radical therapies. In the present study, the authors attempted to compare the outcomes of hepatic resection and transarterial chemoembolization (TACE) for treating solitary HCC.
If the preoperative imaging evaluation shows a solitary HCC tumor with a diameter less than or equal to 6 cm, the patient should be recommended for liver resection; if resection is not appropriate, TACE should be considered. This should guide the clinical treatment for solitary HCC.
These results revealed that a tumor diameter of 6 cm should be the cutoff for deciding between liver resection and TACE.
For solitary HCC with a diameter of less than 6 cm, radical therapy such as hepatic resection should be the first choice. If the diameter is larger than 6 cm, TACE should be recommended. Thus, a modified BCLC HCC system should be considered based on these results.
The BCLC staging classification is primarily based on the prognostic analysis of several small cohorts of predominantly HCV-infected patients with early HCC who were treated via resection, transplantation, or percutaneous ethanol injection.
The authors analyzed the prognosis of the solitary HCC treated with either TACE or surgery. In cases with a tumor diameter equal to or less than 6 cm, the liver resection group showed longer survival than TACE group. However, there were no differences in survival time between the two groups in cases with a tumor diameter more than 6 cm. The authors advocated that solitary HCC more than 6 cm in diameter should be classified as BCLC B. The manuscript was well organized and well written.
P- Reviewer: Miki K S- Editor: Ma YJ L- Editor: Logan S E- Editor: Liu XM
| 1. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. [PubMed] |
| 2. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25541] [Article Influence: 1824.4] [Reference Citation Analysis (7)] |
| 3. | Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430. [PubMed] |
| 4. | Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, Hasegawa H, Nakajima Y, Ohnishi K. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56:918-928. [PubMed] |
| 5. | Faria SC, Szklaruk J, Kaseb AO, Hassabo HM, Elsayes KM. TNM/Okuda/Barcelona/UNOS/CLIP International Multidisciplinary Classification of Hepatocellular Carcinoma: concepts, perspectives, and radiologic implications. Abdom Imaging. 2014;39:1070-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Prospective validation of the CLIP score: a new prognostic system for patients with cirrhosis and hepatocellular carcinoma. The Cancer of the Liver Italian Program (CLIP) Investigators. Hepatology. 2000;31:840-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 374] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 7. | Kudo M, Chung H, Osaki Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score). J Gastroenterol. 2003;38:207-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 501] [Cited by in RCA: 537] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 8. | Leung TW, Tang AM, Zee B, Lau WY, Lai PB, Leung KL, Lau JT, Yu SC, Johnson PJ. Construction of the Chinese University Prognostic Index for hepatocellular carcinoma and comparison with the TNM staging system, the Okuda staging system, and the Cancer of the Liver Italian Program staging system: a study based on 926 patients. Cancer. 2002;94:1760-1769. [PubMed] |
| 9. | Yau T, Tang VY, Yao TJ, Fan ST, Lo CM, Poon RT. Development of Hong Kong Liver Cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology. 2014;146:1691-1700.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 543] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 10. | Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4333] [Cited by in RCA: 4507] [Article Influence: 225.4] [Reference Citation Analysis (0)] |
| 11. | Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2645] [Cited by in RCA: 2874] [Article Influence: 110.5] [Reference Citation Analysis (1)] |
| 12. | Chan SL, Mo FK, Johnson PJ, Liem GS, Chan TC, Poon MC, Ma BB, Leung TW, Lai PB, Chan AT. Prospective validation of the Chinese University Prognostic Index and comparison with other staging systems for hepatocellular carcinoma in an Asian population. J Gastroenterol Hepatol. 2011;26:340-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 13. | Kudo M, Han KH, Kokudo N, Cheng AL, Choi BI, Furuse J, Izumi N, Park JW, Poon RT, Sakamoto M. Liver Cancer Working Group report. Jpn J Clin Oncol. 2010;40 Suppl 1:i19-i27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Zhong JH, Xiang BD, Gong WF, Ke Y, Mo QG, Ma L, Liu X, Li LQ. Comparison of long-term survival of patients with BCLC stage B hepatocellular carcinoma after liver resection or transarterial chemoembolization. PLoS One. 2013;8:e68193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 131] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 15. | Hsu CY, Hsia CY, Huang YH, Su CW, Lin HC, Pai JT, Loong CC, Chiou YY, Lee RC, Lee FY. Comparison of surgical resection and transarterial chemoembolization for hepatocellular carcinoma beyond the Milan criteria: a propensity score analysis. Ann Surg Oncol. 2012;19:842-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 106] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 16. | Zhong JH, Ke Y, Gong WF, Xiang BD, Ma L, Ye XP, Peng T, Xie GS, Li LQ. Hepatic resection associated with good survival for selected patients with intermediate and advanced-stage hepatocellular carcinoma. Ann Surg. 2014;260:329-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 375] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 17. | Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut. 2014;63:844-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 929] [Cited by in RCA: 1106] [Article Influence: 100.5] [Reference Citation Analysis (1)] |
| 18. | Vitale A, Morales RR, Zanus G, Farinati F, Burra P, Angeli P, Frigo AC, Del Poggio P, Rapaccini G, Di Nolfo MA. Barcelona Clinic Liver Cancer staging and transplant survival benefit for patients with hepatocellular carcinoma: a multicentre, cohort study. Lancet Oncol. 2011;12:654-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 127] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 19. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5110] [Cited by in RCA: 5309] [Article Influence: 183.1] [Reference Citation Analysis (0)] |
| 20. | Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1594] [Cited by in RCA: 1695] [Article Influence: 70.6] [Reference Citation Analysis (0)] |
| 21. | Dutkowski P, Linecker M, DeOliveira ML, Müllhaupt B, Clavien PA. Challenges to liver transplantation and strategies to improve outcomes. Gastroenterology. 2015;148:307-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 202] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 22. | Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6572] [Article Influence: 469.4] [Reference Citation Analysis (1)] |
| 23. | Forner A, Gilabert M, Bruix J, Raoul JL. Treatment of intermediate-stage hepatocellular carcinoma. Nat Rev Clin Oncol. 2014;11:525-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 360] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 24. | Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi GL. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1572] [Article Influence: 92.5] [Reference Citation Analysis (1)] |
| 25. | Zheng SS, Xu X, Wu J, Chen J, Wang WL, Zhang M, Liang TB, Wu LM. Liver transplantation for hepatocellular carcinoma: Hangzhou experiences. Transplantation. 2008;85:1726-1732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 379] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 26. | Imai K, Beppu T, Chikamoto A, Doi K, Okabe H, Hayashi H, Nitta H, Ishiko T, Takamori H, Baba H. Comparison between hepatic resection and radiofrequency ablation as first-line treatment for solitary small-sized hepatocellular carcinoma of 3 cm or less. Hepatol Res. 2013;43:853-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 27. | Truty MJ, Vauthey JN. Surgical resection of high-risk hepatocellular carcinoma: patient selection, preoperative considerations, and operative technique. Ann Surg Oncol. 2010;17:1219-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 28. | Otto G, Schuchmann M, Hoppe-Lotichius M, Heise M, Weinmann A, Hansen T, Pitton MP. How to decide about liver transplantation in patients with hepatocellular carcinoma: size and number of lesions or response to TACE? J Hepatol. 2013;59:279-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 29. | Sotiropoulos GC, Malagó M, Molmenti E, Paul A, Nadalin S, Brokalaki E, Kühl H, Dirsch O, Lang H, Broelsch CE. Liver transplantation for hepatocellular carcinoma in cirrhosis: is clinical tumor classification before transplantation realistic? Transplantation. 2005;79:483-487. [PubMed] |