Published online Apr 21, 2015. doi: 10.3748/wjg.v21.i15.4583
Peer-review started: September 7, 2014
First decision: October 14, 2014
Revised: November 29, 2014
Accepted: January 16, 2015
Article in press: January 16, 2015
Published online: April 21, 2015
Processing time: 224 Days and 22 Hours
AIM: To establish a prognostic formula that distinguishes non-hypervascular hepatic nodules (NHNs) with higher aggressiveness from less hazardous one.
METHODS: Seventy-three NHNs were detected in gadolinium ethoxybenzyl diethylene-triamine-pentaacetic-acid magnetic resonance imaging (Gd-EOB-DTPA-MRI) study and confirmed to change 2 mm or more in size and/or to gain hypervascularity. All images were interpreted independently by an experienced, board-certified abdominal radiologist and hepatologist; both knew that the patients were at risk for hepatocellular carcinoma development but were blinded to the clinical information. A formula predicting NHN destiny was developed using a generalized estimating equation model with thirteen explanatory variables: age, gender, background liver diseases, Child-Pugh class, NHN diameter, T1-weighted imaging/T2-weighted imaging detectability, fat deposition, lower signal intensity in arterial phase, lower signal intensity in equilibrium phase, α-fetoprotein, des-γ-carboxy prothrombin, α-fetoprotein-L3, and coexistence of classical hepatocellular carcinoma. The accuracy of the formula was validated in bootstrap samples that were created by resampling of 1000 iterations.
RESULTS: During a median follow-up period of 504 d, 73 NHNs with a median diameter of 9 mm (interquartile range: 8-12 mm) grew or shrank by 68.5% (fifty nodules) or 20.5% (fifteen nodules), respectively, whereas hypervascularity developed in 38.4% (twenty eight nodules). In the fifteen shrank nodules, twelve nodules disappeared, while 11.0% (eight nodules) were stable in size but acquired vascularity. A generalized estimating equation analysis selected five explanatories from the thirteen variables as significant factors to predict NHN progression. The estimated regression coefficients were 0.36 for age, 6.51 for lower signal intensity in arterial phase, 8.70 or 6.03 for positivity of hepatitis B virus or hepatitis C virus, 9.37 for des-γ-carboxy prothrombin, and -4.05 for fat deposition. A formula incorporating the five coefficients revealed sensitivity, specificity, and accuracy of 88.0%, 86.7%, and 87.7% in the formulating cohort, whereas these of 87.2% ± 5.7%, 83.8% ± 13.6%, and 87.3% ± 4.5% in the bootstrap samples.
CONCLUSION: These data suggest that the formula helps Gd-EOB-DTPA-MRI detect a trend toward hepatocyte transformation by predicting NHN destiny.
Core tip: This manuscript features a way of efficient prediction of fate for non-hypervascular hepatic nodules, which appear to be frequently detected after the introduction of gadolinium ethoxybenzyl diethylene-triamine-pentaacetic-acid in a magnetic resonance imaging study. A statistical analysis based on the interpretation of magnetic resonance imaging and regular clinicopathological factors lead to the development of a formula with an accuracy of 87.7% and 87.3% in the formulation and validation samples. Furthermore, the authors have developed a convenient application to evaluate the fate of non-hypervascular hepatic nodules through internet (http://www.med.niigata-u.ac.jp/in3/resident/NHN.html), which requires inputting only five factors.
- Citation: Kanefuji T, Takano T, Suda T, Akazawa K, Yokoo T, Kamimura H, Kamimura K, Tsuchiya A, Takamura M, Kawai H, Yamagiwa S, Aoyama H, Nomoto M, Terai S. Factors predicting aggressiveness of non-hypervascular hepatic nodules detected on hepatobiliary phase of gadolinium ethoxybenzyl diethylene-triamine-pentaacetic-acid magnetic resonance imaging. World J Gastroenterol 2015; 21(15): 4583-4591
- URL: https://www.wjgnet.com/1007-9327/full/v21/i15/4583.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i15.4583
According to the International Agency for Research on Cancer, primary liver cancer is the fifth and seventh most common cancer in men and women worldwide (7.9% and 6.5% of all cancers), respectively, and the third most common cause of cancer-related death. A periodic follow-up observation for patients with chronic liver diseases, especially for cirrhotic liver using imaging modalities and tumor markers, is considered to aid in the detection of hepatocellular carcinoma (HCC) at its early stages, leading to the improvement of overall survival[1]. With the goal of earlier detection of hepatocyte transformation, several hepatocyte-specific MRI contrast agents have been investigated. Gadolinium ethoxybenzyl diethylene-triamine-pentaacetic-acid (Gd-EOB-DTPA) was introduced in the clinic in 2007.
The lipophilic modification of Gd-DTPA, which is a contrast medium solely for extracellular perfusion[2], consisting of a covalent linkage with ethoxybenzyl moiety has allowed both extracellular hemodynamics and hepatocyte function to be explored using one contrast medium in MRI[3]. Gd-EOB-DTPA is actively taken up by hepatocytes, followed by excretion into bile juice. A phase I study has revealed that liver parenchyma is immediately enhanced after the injection of Gd-EOB-DTPA reaching a peak intensity 10 to 20 min after the injection, which is sustained for more than 2 h, the so-called hepatobiliary phase, in volunteers with normal functioning liver parenchyma[4]. Organic anion-transporting polypeptide 1B3 and multidrug-resistant proteins are responsible for hepatocyte uptake and excretion, respectively, of the contrast medium[5].
Imaging of hepatocytes by EOB uptake can detect a focal liver lesion with less signal intensity based on a functional deviation of hepatocytes through their transformation process[6]. Although most classical HCCs reveal less signal intensity in the hepatobiliary phase[7], it is still under debate whether non-hypervascular hepatic nodules (NHNs), which are detected in the hepatobiliary phase but not enhanced in the arterial phase (as is the case for classical HCC), are destined to result in overt HCC. This study aims to establish a prognosticator that allows clinicians to determine NHNs with a higher potential to become an overt HCC by means of grow and/or gain hypervascularity as a surrogate marker.
The institutional review board of our institution, which did not require informed consent for a retrospective study using medical records or imaging examinations, approved the present study. In total, among 236 consecutive patients, who underwent 375 Gd-EOB-DTPA-MRI studies in a screen of chronic liver diseases between May 2008 and January 2010, we qualified 73 NHNs of 29 patients (17 males, median age 68 years, and 12 females, median age 74 years) out of 141 candidate NHNs in 48 patients (see image analysis) that were valid for the present retrospective study. Our inclusion criteria were that a subsequent Gd-EOB-DTPA-MRI revealed a definite vascularity and/or size change without any treatment; hypervascularity in the arterial phase; and/or a size alteration of 2 mm or more. Because neither size increase nor gain hypervascularity in a certain period of time may not always connect with innocent nodules, a stable nodule in terms of size and vascularity were purposely excluded from further analyses. The characteristics of NHNs are summarized in supplementary Table 1. The modality to evaluate NHN size and vascularity in this study was restricted to Gd-EOB-DTPA-MRI in order to exclude a potential variation due to the modality used. The valid NHNs were further classified into one of two groups, progressed or resolved, in which NHNs showed enlargement/hypervascularity or size reduction without vascularization, respectively.
| Signa HDX 1.5T | Achiva Nova Dual 1.5T | Magnetom vario | |||
| Unenhanced | T1WI | TR (ms) | 175.79 | 189.93 | 190 |
| TE (OP/IP) (ms) | 2.2/4.6 | 2.3/4.6 | 1.49/2.64 | ||
| Number of exitation | 1 | 1 | 1 | ||
| Field of view (cm) | 35 | 35 | 35 | ||
| Matrix | 288 × 214 | 288 × 214 | 256 × 208 | ||
| Slice thickness (mm) | 6 | 6 | 6 | ||
| T2WI | TR (ms) | Breath synchronization | Breath synchronization | Breath synchronization | |
| TE (ms) | 88.62 | 90 | 85 | ||
| Number of exitation | 2 | 2 | 2 | ||
| Field of view (cm) | 35 | 35 | 35 | ||
| Matrix | 320 × 192 | 320 × 245 | 512 × 319 | ||
| Slice thickness (mm) | 6 | 6 | 6 | ||
| Enhanced | Dynamic | TR (ms) | 3.83 | 3.93 | 2.77 |
| TE (ms) | 1.65 | 1.93 | 1.14 | ||
| Number of exitation | 1 | 1 | 1 | ||
| Flip angle (degree) | 12 | 12 | 12 | ||
| Field of view (cm) | 35 | 35 | 35 | ||
| Matrix | 320 × 192 | 320 × 192 | 320 × 182 | ||
| Slice thickness (mm) | 2.5 | 2.5 | 2.5 | ||
MRIs were performed using a 1.5-T system [Signa HDxt 1.5T (GE Medical Systems, Waukesha, United States), an Achieva Nova Dual 1.5T (PHILIPS, Amsterdam, Netherlands)], or a 3-T system [Magnetom Verio (SIEMENS, Berlin, Germany)]. For all patients, unenhanced MRI evaluations [T1-weighted imaging (T1WI) and T2-weighted imaging (T2WI) with fat suppression] were performed prior to a dynamic study under the conditions described in Table 1. During the dynamic study, each patient was intravenously administered 25 μmol/kg (0.1 mL/kg) of Gd-EOB-DTPA (Primovist®, Bayer Holding Ltd., Osaka, Japan) in 5 seconds with the saline injection at the same rate for 35 seconds. Dynamic contrast-enhanced MRIs were initiated approximately 5, 60, and 100 s after the beginning of the aortic enhancement. The images at the hepatobiliary phase were obtained 20 min after the injection.
All images were interpreted independently by an experienced, board-certified abdominal radiologist and hepatologist; both knew that the patients were at risk for HCC development but were blinded to the clinical information. At first each doctor separately marked all hypointense lesions showing a round or oval distinct shape in the hepatobiliary phase. Then, candidate NHNs were decided after excluding the lesions corresponding to any of following criteria: (1) hypervascularity in an arterial phase; (2) delayed enhancement; (3) strong high intensity on T2WI; and/or (4) a size of less than 5 mm. If there was any discordance between two interpreters, the lesion was reviewed to reach a consensus. Finally, all candidate NHNs were recorded in three-dimensional coordinates of Gd-EOB-DTPA-MRI image space, so that NHNs could be precisely located in future MRI studies.
HBsAg and anti-HCV antibody were detected by a chemiluminescence immunoassay using ARCHITECT HBsAg QT and ARCHITECT HCV (Abbott Japan Co. Ltd., Chiba, Japan), respectively. Routine blood biochemistry was measured to determine Child-Pugh classification in the clinical laboratories of our hospital, where a quality control of each test is routinely performed every day.
Total and Lens culinaris agglutinin A-reactive α-fetoprotein (AFP) concentrations in the serum were quantified by a liquid-phase binding assay system (LiBASys; Wako Pure Chemical Indus-tries Ltd., Osaka, Japan). L3 was calculated as a percentage of Lens culinaris agglutinin A-reactive species against total AFP. Serum des-γ-carboxy prothrombin (DCP) was measured using an electro-chemiluminescence immunoassay (Wako Pure Chemical Industries Ltd, Osaka, Japan).
Two expert histologists separately gave a histological diagnosis of HCC based on microscopic observations of tissues stained with hematoxylin and eosin, silver, iron, periodic acid-Schiff, periodic acid-Schiff with diastase digestion, and azan.
Univariate comparisons of categorical data or metric variables between progressed and resolved NHNs were performed using a chi-square or Mann-Whitney U test, respectively. Generalized estimating equation (GEE) models were used for primary analyses that examined the relation between NHN fate and clinicopathological factors in association with either an individual or each nodule. An exchangeable matrix and logit were selected for a working correlation matrix structure and link function, respectively. For validation of the equation, a bootstrap randomization test was conducted on individual but not nodule data. We ran 1000 iterations to compare sensitivity, specificity, negative predictive value (NPV), positive predictive value (PPV), and accuracy for the prediction of NHN progression. All analyses were conducted using GraphPad Prism 6 software (GraphPad Software Inc., La Jolla, United States), except for the multivariate analysis, which was performed with PASW statics 17.0 (SPSS Inc., Chicago, United States). A two-sided P-value less than 0.05 was considered statistically significant.
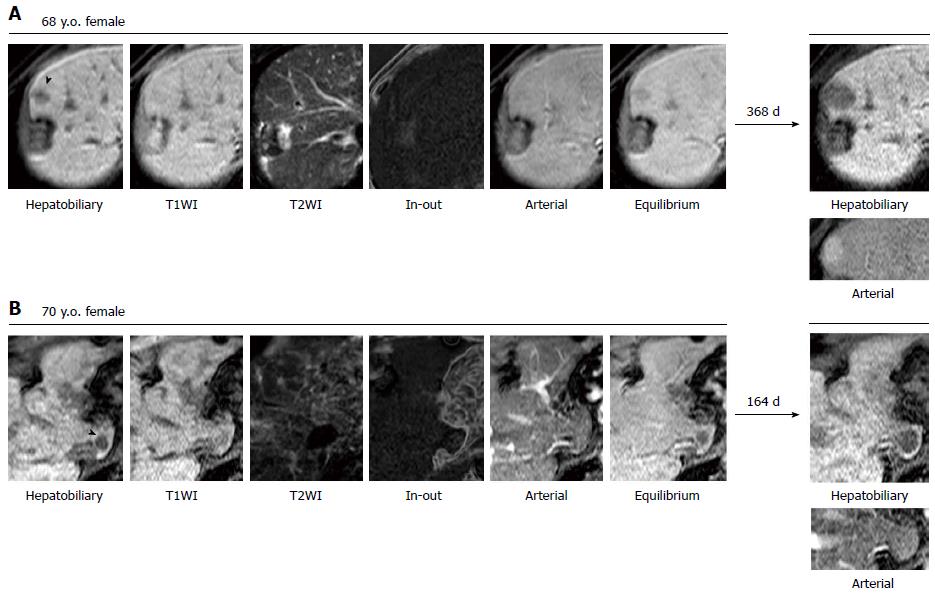
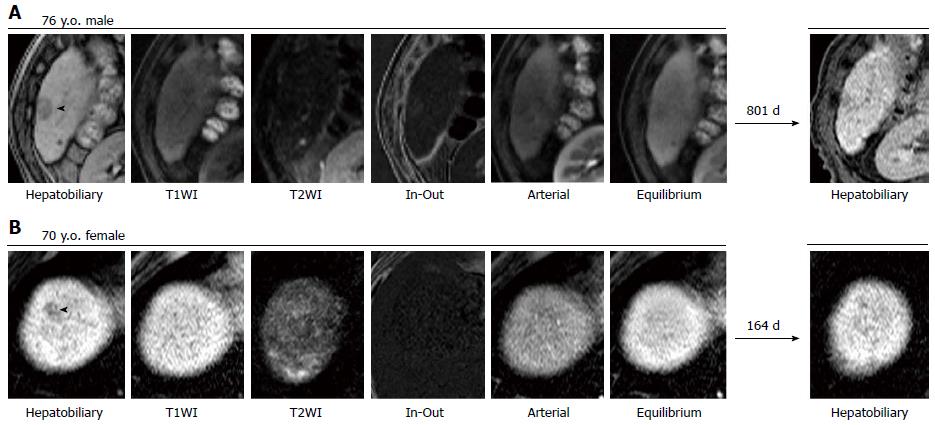
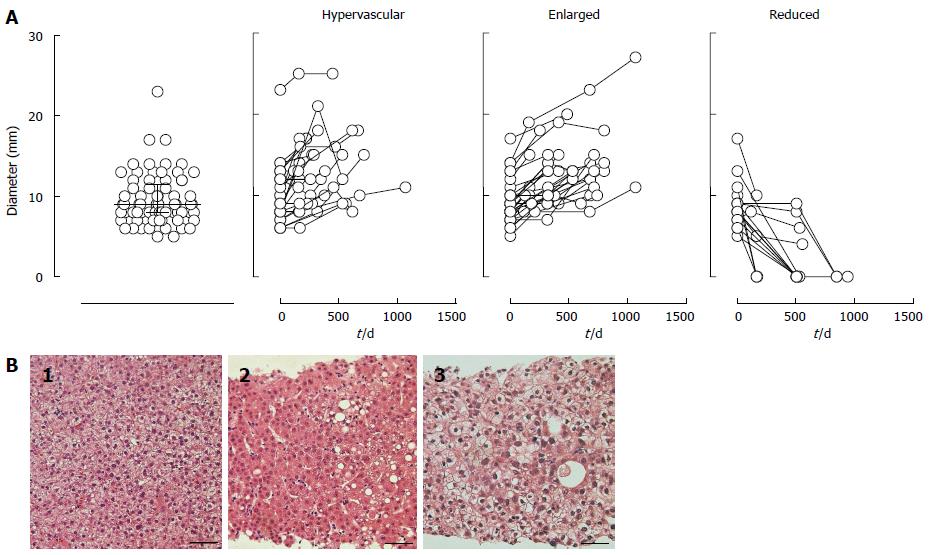
The basic characteristics of the NHNs are summarized in supplementary Table 1. In a median follow-up period of 504 d (IQR: 227-673 d), 68.5% or 20.5% of 73 NHNs were enlarged or reduced (including 16.4% that disappeared) by 2 mm or more in diameter, respectively, whereas the remaining 11.0% were stable in size but acquired vascularity. Representative images are shown in Figures 1 and 2. No NHN that decreased in size developed hypervascularity, whereas hypervascularity was depicted in 40.0% of the enlarged NHNs. Figure 3A shows the actual size distributions over the observation period. The median diameter of the 73 NHNs was 9 mm (IQR: 8-12 mm) at the initial detection. Tissue samples were obtained from 6 of 58 progressed NHNs, cases #1-3, 12-1, 14-1, 19-5, 21-1, and 22-1 allocated in supplementary Table 1, and all of these tissues revealed a histological diagnosis of HCC with high to moderate differentiation (Figure 3B).
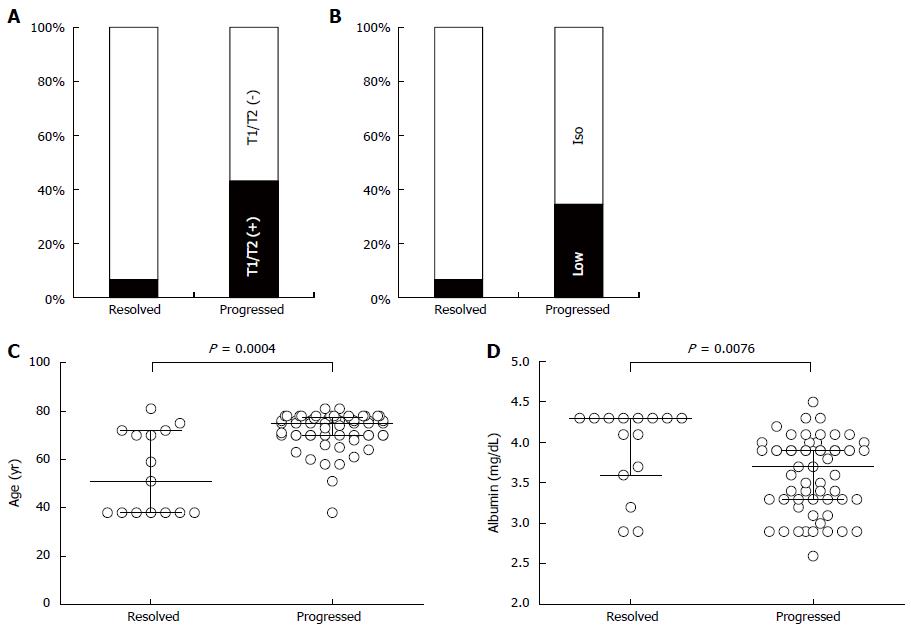
At first, we compared clinicopathological factors between progressed and resolved NHNs using univariate analyses. As shown in Figure 4A, NHNs were detected with a significantly higher frequency by T1WI and/or T2WI in progressed NHNs than in resolved NHNs (43.1% vs 6.7%, P = 0.01). In the arterial phase, progressed NHNs were also detected as less attenuated nodules with a significantly higher frequency than resolved NHNs (Figure 4B, 34.5% vs 6.7%, P = 0.05). When a characteristic feature was evaluated among patients and not among nodules, the patients carrying progressed NHNs were significantly older than the others (median: 75 years vs 51 years, P = 0.0004) (Figure 4C). Furthermore, serum albumin concentrations were 3.7 g/dL and 4.3 g/dL in the patients with progressed and resolved NHNs, respectively, and were significantly lower in the patients with progressed NHNs (Figure 4D, P = 0.008). Serum AFP concentration, which was expressed logarithmically, tended to be higher toward significance in patients with progressed NHNs than resolved NHNs (1.28 vs 1.10, P = 0.09).
As shown in Table 2, based on clinicopathological data from 73 NHNs, estimated regression coefficient was calculated by adapting a GEE model to predict a risk of NHN progression for 13 variables: (1) age (years); (2) gender (male/female); (3) background liver diseases (HBV/HCV/others); (4) Child-Pugh class (A/B); (5) NHN diameter (mm); (6) T1WI/T2WI detectability (detectable/ambiguous); (7) fat deposition (yes/no); (8) lower signal intensity in arterial phase (detectable/ambiguous); (9) lower signal intensity in equilibrium phase (detectable/ambiguous); (10) AFP (log10); (11) DCP (log10); (12) AFP-L3 (%); and (13) coexistence of classical HCC (never/it is or has been). As the final result of GEE model analysis, 5 variables (age, lower signal intensity in arterial phase, positivity of HBV or HCV, DCP, fat deposition) were selected as significant explanatory factors for NHN progression. The logit value of NHN progression is calculated by the following formula.
| Variable | Coefficient | 95%CI | Level of significance | Condition1 | Step2 |
| Age | 0.36 | 0.17-0.54 | < 0.01 | 8th | |
| EOB 1st | 6.51 | 2.93-10.09 | < 0.01 | Detectable | 8th |
| Background | 8.70 | 3.36-14.04 | < 0.01 | HBV | 8th |
| Background | 6.03 | 1.34-10.71 | 0.01 | HCV | 8th |
| Log DCP | 9.37 | 1.25-17.49 | 0.02 | 8th | |
| Fat | -4.05 | -7.75 - -0.36 | 0.03 | Yes | 8th |
| Size | -0.01 | -0.43-0.41 | 0.97 | 1st | |
| Log AFP | 0.28 | -2.67-3.24 | 0.85 | 2nd | |
| Gender | -0.87 | -5.69-3.94 | 0.72 | Male | 3rd |
| EOB 2nd | 0.82 | -1.05-2.69 | 0.39 | Detectable | 4th |
| T1/T2 | 2.52 | -1.75-6.79 | 0.25 | Detectable | 5th |
| L3 | -0.03 | -0.19-0.13 | 0.70 | 6th | |
| Child-Pugh | -1.74 | -5.94-2.45 | 0.42 | A | 7th |
| Classical HCC | 3.60 | -0.43-7.63 | 0.08 | Never | 8th |
λ = 0.36 × Age + lower signal intensity in arterial phase (detectable: 6.51) + Background (HBV: 8.70 HCV: 6.03: Others: 0) + 9.37 × log10 (DCP) + fat deposition (yes: -4.05) - 40.81
According to receiver-operator characteristic curve analysis showing 88.9% of area under the curve, a cut-off value of λ was set at 0.5, which made 2 false-positive and 7 false-negative judgments leading to sensitivity, specificity, NPV, PPV, and accuracy to pick up NHN that shows progressive character were 88.0%, 86.7%, 65.0%, 96.2%, and 87.7%, respectively. To validate the accuracy of the formula, all 29 cases were subjected to 1000 times resampling of a bootstrap method: a repeated sampling of the same number of cases with replacement. In the result, the averages of sensitivity, specificity, NPV, PPV, and accuracy of the resampled cohort were 87.2% ± 5.7%, 83.8% ± 13.6%, 61.8% ± 15.1%, 96.2% ± 2.6%, and 87.3% ± 4.5%, respectively.
A deviation of the equilibrium between the uptake and excretion of EOB due to hepatocarcinogenesis makes it possible to distinguish transforming hepatocytes as a lower-intensity nodule earlier than any other available imaging modality[8,9]. However, NHN, which is detected in the hepatobiliary phase without the characteristic hypervascularity of classical HCC, is not always destined to become an overt HCC. Akai et al[10] and Kumada et al[11] reported that 3.2% and 43.5% of NHNs, respectively, turned into hypervascular nodules in one year. Although the exact reason for the large difference between these incidences is unclear, the NHN size difference may be one of the causes, as the tumor doubling time and initial tumor diameter were correlated with each other[12,13]. The mean diameter in the Akai cohort was 8.1 mm, whereas median diameters of vascularized and non-vascularized NHNs were 20 and 14 mm, respectively, in Kumada’s report. The median diameter of the present study was 9.0 mm, and hypervascularity was confirmed in 37.0% of NHNs during a median follow-up period of 504 d. Because it is difficult to perform Gd-EOB-DTPA-MRI frequently in a large cohort, both from the patient’s point of view and because of the limited availability of hospital equipment, it is hard to define precisely when the vascularity appeared. However, it may be reasonable to assume from those 2 studies and the present study that roughly 30% of 10-mm NHNs would gain vascularity in a year. Either way, it is not likely that more than half of 10-mm NHNs progress into a classical HCC in a year. Therefore, it is critical to establish a strategy to differentiate progressive NHNs from relatively indolent ones, which would enable us to define a high-risk group and to provide a rationale for further intensive investigations, including histological evaluations.
It is important to define progressed and non-progressed features properly to determine the fate of NHNs. Previous studies solely employed hypervascularity as a hallmark for progression in NHN follow-up[10,11]. It is clear, however, that there are HCCs that lack a hypervascular phenotype. Bolondi et al[14] reported that the European Association for the Study of the Liver’s imaging criteria for the diagnosis of HCC, which require coincidental arterial hypervascularity in contrast-enhanced ultrasound and helical computed tomography, was satisfied in only 61% of 10- to 30-mm nodules detected by ultrasound in cirrhotic liver. In fact, well-differentiated HCCs were histologically diagnosed without hypervascular features in 10 and 4 NHNs from the 68 excluded and 73 included NHNs in our cohort, respectively. Because the sensitivity rather than the specificity is primary importance for a screening study of Gd-EOB-DTPA-MRI, the size increase should be incorporated into the criteria of progressive NHN irrespective of hypervascularity. Another important factor affecting the establishment of a predictive formula is how to handle NHNs that are stable in terms of vascularity and size over a follow-up period. In previous reports, stable NHNs were generally classified as non-progressive nodules. It is hard to determine, however, how much time is sufficient to judge a NHN as non-progressive when the NHN does not significantly change in size and/or vascularity. On the other hand, no NHN that decreased by 2 mm in size showed a progressed phenotype over the follow period in this study. To avoid the risk of misreading the NHN prognosis and mistakenly defining progressive NHNs as innocent nodules, it would be preferable to exclude stable NHNs from further analyses.
One limitation of our study is the relatively small number of cases. The limited case number may have resulted in an inadequate assessment of the biological variability. It seems reasonable that age, lower signal intensity in arterial phase, positivity of HBV or HCV, and DCP would affect NHN progression, based on our current understanding of the risk for HCC. For example, Shimada et al[15] reported that DCP level was significantly higher in non-cancerous parts of the liver when there were simultaneous multicentric HCCs. On the other hand, the tumor diameter was not incorporated in our NHN-progression prediction formula. While Kumada et al[11] reported that NHNs larger than 15 mm became a hypervascular nodule more frequently than smaller NHNs, the estimated regression coefficient for tumor diameter is small (-0.01) in multivariate analysis, and the tumor size was not a significant determinant even in univariate analysis in our cohort. Because the median diameter of NHNs in our cohort was 9 mm, substantially smaller than 15 mm, NHN size may not be a key determinant for NHN progression as long as the size is below a specified threshold. Smaller NHNs may have a higher potential for malignancy than larger NHNs because a strong contrast due to a larger functional deviation in EOB uptake and excretion should be required for the detection of small NHNs, for example, those less than 10 mm. Furthermore, differences in the definition of NHN progression may have caused some discrepancies with previous studies. To clarify the significance of these factors and to confirm the accuracy of our formula, it is essential to perform a prospective study using a larger cohort without any treatment except for pure regional therapies, such as resection and/or radiofrequency ablation. For convenient evaluation of NHN fate, we are providing a free application on our homepage as NHN Fate Predictor (http://www.med.niigata-u.ac.jp/in3/resident/NHN.html).
We developed a formula predicting whether NHNs will grow and/or gain vascularity in a couple of years. It is a hard task to differentiate two types of NHNs; NHNs turn into overt HCC within a short period and NHNs stay non-progressive for a substantial time. Our formula would be useful for the management of chronic liver diseases in guiding the necessity of successive examinations and treatments for NHNs detected by Gd-EOB-DTPA-MRI.
We thank Mr. Tsutomu Kanazawa and all radiologic technicians of our hospital for daily excellent MRI studies based on their expertise.
In general, it is crucial to find a malignant tumor at an earlier stage with smaller size to attain a preferable patient’s prognosis. A deviation of the equilibrium between the uptake and excretion of gadolinium ethoxybenzyl diethylene-triamine-pentaacetic-acid due to hepatocarcinogenesis makes it possible for magnetic resonance imaging study to visualize transforming hepatocytes as a lower-intensity nodule earlier than any other available imaging modality. At 20 min or later after an injection of the gadolinium with an ethoxybenzyl moiety, non-hypervascular hepatic nodules, which is detected a lower attenuation nodule without the characteristic hypervascularity of classical hepatocellular carcinoma, are frequently detected but are not always destined to become an overt hepatocellular carcinoma. Therefore, it is critical to establish a strategy to differentiate progressive non-hypervascular hepatic nodules from relatively indolent ones, which would enable us to define a high-risk group and to provide a rationale for further intensive investigations, including histological evaluations.
Although the larger size of non-hypervascular hepatic nodules such as 15 mm or larger was reported to be an indicative for the higher probability that a non-hypervascular hepatic nodule will gain vascularity in a near future. Because it was known that the tumor doubling time and tumor diameter were correlated with each other, it is reasonable to assume that a larger nodule would have the higher probability to show the distinct characteristics of classical hepatocellular carcinoma. However, the size of non-hypervascular hepatic nodules has been getting smaller and smaller such as less than 9 mm as the improvements of magnetic resonance imaging technologies. In this setting, the size cannot be a meaningful indicator any more, and a systematic approach is required to predict the fate of small non-hypervascular tumor.
By means of applying a generalized estimating equation model and bootstrap resampling strategy, the research team led by Takeshi Suda from Department of Gastroenterology and Hepatology of Niigata University Medical and Dental Hospital developed and validated a formula to predict the fate of no-hypervascular hepatic nodule in a near future using readily available 5 clinical factors. The authors included not only the gaining of hypervascularity but also the size enlargement in the criteria of progressive characteristics of non-hypervascular nodules, because a substantial number of non-hypervascular hepatic nodules were histologically proved to be hepatocellular carcinomas. Furthermore, only the nodules that were confirmed to gain hypervascularity and/or change more than 2 mm in size were subjected for the analyses to minimize a false positive or negative judgment as indolent due to an insufficient observation period.
Because the formula requests only five factors readily available in a regular clinic, a physician can decide if the non-hypervascular hepatic nodule should be simply followed or further intensively evaluated by using invasive modalities such as biopsy at an outpatient clinic. At the same time, this formula makes it possible to conduct a prospective evaluation of the fate of non-hypervascular hepatic nodules in a large cohort. For convenient evaluation of NHN fate, the authors are providing a free application of a NHN Fate Predictor on their homepage (http://www.med.niigata-u.ac.jp/in3/resident/NHN.html).
Gadolinium ethoxybenzyl diethylene-triamine-pentaacetic-acid (Gd-EOB-DTPA) was introduced in clinic on 2007 with lipophilic modification of Gd-DTPA, which is a contrast medium solely for extracellular perfusion. A covalent linkage with ethoxybenzyl moiety has allowed both extracellular hemodynamics and hepatocyte function to be explored using one contrast medium in a magnetic resonance imaging study. Gd-EOB-DTPA is actively taken up by hepatocytes, followed by excretion into bile juice.
This is a well-conducted study that developed a formula in helping to decide how to treat non-hypervascular hepatic nodules. It is encouraged a future study evaluating the prognostic value of the formula in a larger group of patients.
P- Reviewer: Bakoyiannis A, Buell JF, Morales-Ruiz M, Kaido T S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN
| 1. | Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6572] [Article Influence: 469.4] [Reference Citation Analysis (1)] |
| 2. | Schuhmann-Giampieri G, Schmitt-Willich H, Press WR, Negishi C, Weinmann HJ, Speck U. Preclinical evaluation of Gd-EOB-DTPA as a contrast agent in MR imaging of the hepatobiliary system. Radiology. 1992;183:59-64. [PubMed] |
| 3. | Reimer P, Rummeny EJ, Shamsi K, Balzer T, Daldrup HE, Tombach B, Hesse T, Berns T, Peters PE. Phase II clinical evaluation of Gd-EOB-DTPA: dose, safety aspects, and pulse sequence. Radiology. 1996;199:177-183. [PubMed] |
| 4. | Hamm B, Staks T, Mühler A, Bollow M, Taupitz M, Frenzel T, Wolf KJ, Weinmann HJ, Lange L. Phase I clinical evaluation of Gd-EOB-DTPA as a hepatobiliary MR contrast agent: safety, pharmacokinetics, and MR imaging. Radiology. 1995;195:785-792. [PubMed] |
| 5. | Kitao A, Zen Y, Matsui O, Gabata T, Kobayashi S, Koda W, Kozaka K, Yoneda N, Yamashita T, Kaneko S. Hepatocellular carcinoma: signal intensity at gadoxetic acid-enhanced MR Imaging--correlation with molecular transporters and histopathologic features. Radiology. 2010;256:817-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 281] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 6. | Vogl TJ, Kümmel S, Hammerstingl R, Schellenbeck M, Schumacher G, Balzer T, Schwarz W, Müller PK, Bechstein WO, Mack MG. Liver tumors: comparison of MR imaging with Gd-EOB-DTPA and Gd-DTPA. Radiology. 1996;200:59-67. [PubMed] |
| 7. | Golfieri R, Renzulli M, Lucidi V, Corcioni B, Trevisani F, Bolondi L. Contribution of the hepatobiliary phase of Gd-EOB-DTPA-enhanced MRI to Dynamic MRI in the detection of hypovascular small (≤ 2 cm) HCC in cirrhosis. Eur Radiol. 2011;21:1233-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 165] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 8. | Kim MJ. Current limitations and potential breakthroughs for the early diagnosis of hepatocellular carcinoma. Gut Liver. 2011;5:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Ichikawa T, Saito K, Yoshioka N, Tanimoto A, Gokan T, Takehara Y, Kamura T, Gabata T, Murakami T, Ito K. Detection and characterization of focal liver lesions: a Japanese phase III, multicenter comparison between gadoxetic acid disodium-enhanced magnetic resonance imaging and contrast-enhanced computed tomography predominantly in patients with hepatocellular carcinoma and chronic liver disease. Invest Radiol. 2010;45:133-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 197] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 10. | Akai H, Matsuda I, Kiryu S, Tajima T, Takao H, Watanabe Y, Imamura H, Kokudo N, Akahane M, Ohtomo K. Fate of hypointense lesions on Gd-EOB-DTPA-enhanced magnetic resonance imaging. Eur J Radiol. 2012;81:2973-2977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 11. | Kumada T, Toyoda H, Tada T, Sone Y, Fujimori M, Ogawa S, Ishikawa T. Evolution of hypointense hepatocellular nodules observed only in the hepatobiliary phase of gadoxetate disodium-enhanced MRI. AJR Am J Roentgenol. 2011;197:58-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 132] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 12. | Sheu JC, Sung JL, Chen DS, Yang PM, Lai MY, Lee CS, Hsu HC, Chuang CN, Yang PC, Wang TH. Growth rate of asymptomatic hepatocellular carcinoma and its clinical implications. Gastroenterology. 1985;89:259-266. [PubMed] |
| 13. | Barbara L, Benzi G, Gaiani S, Fusconi F, Zironi G, Siringo S, Rigamonti A, Barbara C, Grigioni W, Mazziotti A. Natural history of small untreated hepatocellular carcinoma in cirrhosis: a multivariate analysis of prognostic factors of tumor growth rate and patient survival. Hepatology. 1992;16:132-137. [PubMed] |
| 14. | Bolondi L, Gaiani S, Celli N, Golfieri R, Grigioni WF, Leoni S, Venturi AM, Piscaglia F. Characterization of small nodules in cirrhosis by assessment of vascularity: the problem of hypovascular hepatocellular carcinoma. Hepatology. 2005;42:27-34. [PubMed] |
| 15. | Shimada M, Yamashita Y, Hamatsu T, Hasegawa H, Utsunomiya T, Aishima S, Sugimachi K. The role of des-gamma-carboxy prothrombin levels in hepatocellular carcinoma and liver tissues. Cancer Lett. 2000;159:87-94. [PubMed] |