Published online Apr 14, 2015. doi: 10.3748/wjg.v21.i14.4402
Peer-review started: October 31, 2014
First decision: November 14, 2014
Revised: November 29, 2014
Accepted: January 16, 2015
Article in press: January 16, 2015
Published online: April 14, 2015
Processing time: 166 Days and 20.2 Hours
Therapy-related acute myeloid leukemia (t-AML) refers to a heterogeneous group of myeloid neoplasms that develop in patients following extensive exposure to either cytotoxic agents or radiation. The development of t-AML has been reported following treatment of cancers ranging from hematological malignancies to solid tumors; however, to our knowledge, t-AML has never been reported following treatment of gastric cancer. In this study, we report the development of t-acute promyelocytic leukemia in a cT4N1M0 gastric cancer patient after an approximate 44 mo latency period following treatment with 4 cycles of oxaliplatin (OXP) (85 mg/m2 on day 1) plus capecitabine (1250 mg/m2 orally twice daily on days 1-14) in combination with recombinant human granulocyte-colony stimulating factor treatment. Karyotype analysis of the patient revealed 46,XY,t(15;17)(q22;q21)[15]/46,idem,-9,+add(9)(p22)[2]/46,XY[3], which, according to previous studies, includes some “favorable” genetic abnormalities. The patient was then treated with all-trans retinoic acid (ATRA, 25 mg/m2/d) plus arsenic trioxide (ATO, 10 mg/d) and attained complete remission. Our case illuminated the role of certain cytotoxic agents in the induction of t-AML following gastric cancer treatment. We recommend instituting a mandatory additional evaluation for patients undergoing these therapies in the future.
Core tip: In the current study, t-acute promyelocytic leukemia (t-APL) was likely induced by treatment with oxaliplatin, capecitabine and recombinant human granulocyte-colony stimulating factor. The gastric cancer patient, classified as clinical stage cT4N1M0, had a rare karyotype: 46,XY,t(15;17)(q22;q21)[15]/46,idem,-9,+add(9)(p22)[2]/46,XY[3]. This case demonstrates that certain cytotoxic agents can induce t-APL in gastric cancer. We recommend mandatory additional evaluation for patients undergoing this treatment regimen.
- Citation: Zhang YC, Zhou YQ, Yan B, Shi J, Xiu LJ, Sun YW, Liu X, Qin ZF, Wei PK, Li YJ. Secondary acute promyelocytic leukemia following chemotherapy for gastric cancer: A case report. World J Gastroenterol 2015; 21(14): 4402-4407
- URL: https://www.wjgnet.com/1007-9327/full/v21/i14/4402.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i14.4402
Therapy-related acute myeloid leukemia (t-AML) refers to a heterogeneous group of myeloid neoplasms that develop in patients following extensive exposure to either cytotoxic agents or radiation[1]. In the past few decades, survival rates for cancer patients have improved, resulting in an increased risk of t-AML[2]. However, it is worth noting that while t-AML shares common phenotypic features with de novo AML, t-AML is relatively resistant to conventional therapies for leukemia. Additionally, t-AML has an overall poor prognosis with a median life expectancy of 8-10 mo following diagnosis[3].
While the underlying cause of t-AML remains to be elucidated, the development of t-AML has been confirmed to correlate with certain cytotoxic drugs. According to previous reports, alkylating agents (busulfan, carboplatin), topoisomerase-2 inhibitors (doxorubicin, mitoxantrone), antimetabolites (5-fluorouracil, fludarabine), antimicrotubule agents (docetaxel, paclitaxel) and growth factors (granulocyte-macrophage colony-stimulating factor; G-CSF) associate with the development of t-AML, although often with a varying latency period[4-6]. Interestingly, the chemical structure and dosage of these agents greatly affect the t-AML profile. For example, treatment with an alkylating agent usually results in a common subtype of t-AML, which is observed in approximately 75% of patients after 5-7 years of exposure. This subtype is often characterized by the loss of all or part of chromosomes 5 or 7[7]. Contrastingly, treatment with topoisomerase II inhibitors often result in gene rearrangements involving 21q22 with a latency ranging from 1-3 years[7].
Recently, a relatively distinct subgroup of t-AML was reported as “good” leukemia. This “good” leukemia refers to acute promyelocytic leukemia (APL) characterized with inv(16)/t(15;17) or more rarely t(8;21)[8]. To our knowledge, t-acute promyelocytic leukemia (t-APL) has yet to be reported following treatment of gastric cancer.
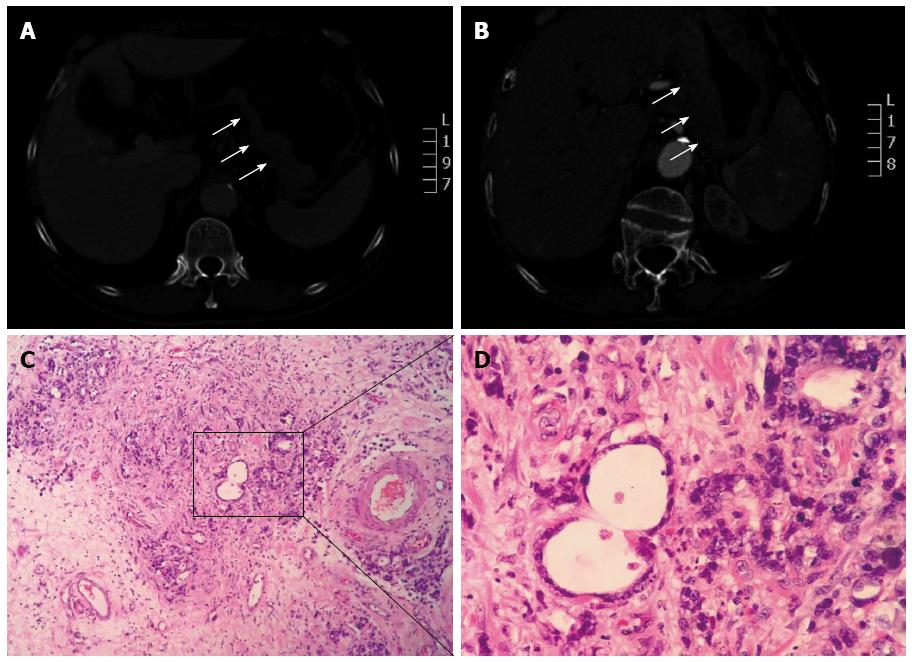
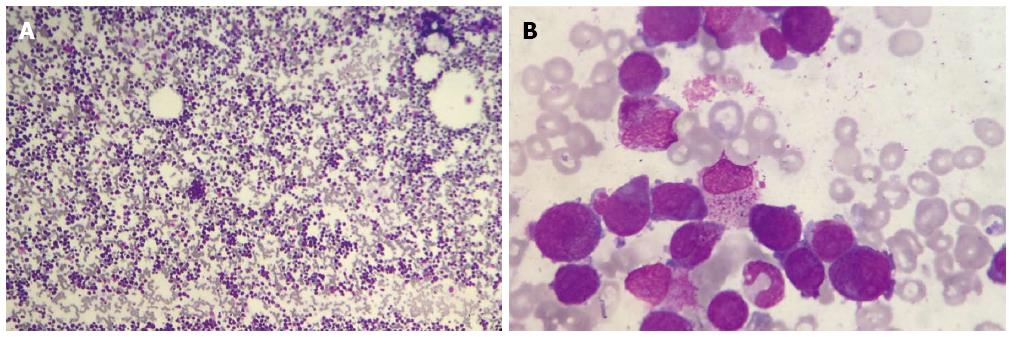
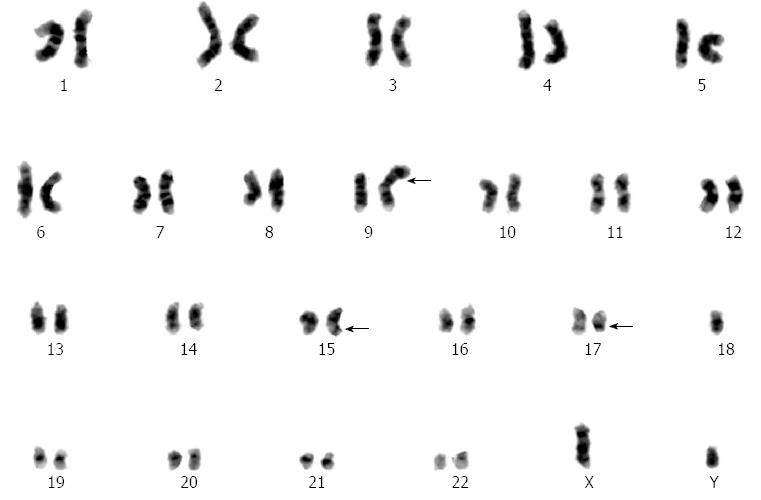
A 68-year-old man, diagnosed with gastric cardia cancer, was referred to Shanghai Changzheng Hospital (Shanghai, China) in March 2007 (Figure 1A, B). Post-operative analysis confirmed a poorly differentiated adenocarcinoma with penetrating invasion of the gastric wall. One out of the 29 regional lymph nodes was positive for cancer cell infiltration (Figure 1C, D). According to the TNM/UICC staging system, the patient classified as cT4N1M0 (IIIC). The patient then underwent 4 cycles of chemotherapy with oxaliplatin (OXP) (85 mg/m2 on day 1) and capecitabine (1250 mg/m2 orally twice daily on days 1-14) until August 2007. Treatment ended when the patient developed violent emesis. In the subsequent visit, the patient had recovered well, with no additional complications. On April 15, 2011, the patient suddenly presented fatigue and high fever (39.2 °C). A blood examination indicated pancytopenia. The patient was treated with recombinant human granulocyte-colony stimulating factor (rHu-G-CSF), followed by a bone marrow biopsy. Peripheral smear analysis revealed an abnormal increase in the amount of myeloblasts and promyelocytes (91.5%), with leukemic hiatus (Figure 2A, B). Chromosome-based analysis revealed structural rearrangements involving chromosomes 9, 15 and 17. The patient’s karyotype was 46,XY,t(15;17)(q22;q21)[15]/46, idem,-9,+add(9)(p22)[2]/46, XY[3]. Additionally, the Bcr1 subtype of the promyelocytic leukemia/retinoic acid receptor-alpha (PML/RARα) fusion gene was positive, whereas the Bcr2 and Bcr3 subtypes of the PML/RARα fusion were negative (Figure 3). According to WHO-based classification, these results verified the diagnosis as t-AML (M3a, which is also referred as t-APL). Routine blood examination indicated the following: red blood cells at 2.35 × 1012/L, white blood cells at 1.8 × 109/L, absolute neutrophil count at 0.67 × 109/L, hemoglobin at 75 g/L, and platelets at 81 g/L. The patient then underwent induction therapy with all-trans retinoic acid (ATRA, 25 mg/m2 per day) plus arsenic trioxide (ATO, 10 mg/d). Other supportive therapies were provided when necessary; these therapies included platelet and fresh frozen plasma transfusions as well as antibiotic administration. The patient presented complete remission (CR) in June 2011, and thus far, no other complications have been recorded.
As a relatively distinct subgroup of t-AML, t-APL is usually characterized with excellent prognosis. Regarding t-APL development, epirubicin and mitoxantron represent the most commonly implicated cytotoxic drugs[9]. In the current study, we investigate the occurrence of t-APL in gastric cancer, which was likely induced by OXP and capecitabine treatment. The patient was finally cured with a treatment regimen of ATRA plus ATO. To our knowledge, this study represents the first report of t-APL diagnosed following treatment of gastric cancer (Table 1).
| Ref. | Patient, age (yr)/gender | Primary cancer | Chemotherapy regimen | TRLs | Karyotype |
| Merrouche et al[12] | 65/female | Colon | LVFU2, Irinotecan, OXP | APL | 46,XX,add(6)(p23),-13,add(14)(p11),-16,add(17)(q?),-21,+3 mar |
| Carneiro et al[13] | 56/female | Cecum | FOLFOX-4, FOLFOX-6 | AML | Partial deletions of chromosomes 5, 7, 20, and 21, as well as trisomy 8 and loss of chromosomes 3 and 1 |
| Merlin et al[14] | 65/female | Colorectal | FOLFOX-4 | ALL | Not available |
| Damodaran et al[15] | 63/male | Esophagus | Capecitabine, OXP | AML | 47,X,der(Y)t(Y;3)(q12;q21),+8(21) |
| Buxhofer-Ausch et al[16] | 56/male | Colon | Capecitabine, 5-FU, Irinotecan, OXP | CML | Positive for Philadelphia chromosome |
| Kadikoylu et al[17] | 66/male | Rectum | Cetuximab, OXP, Irinotecan, Capecitabine | CML | Positive for Philadelphia chromosome |
| Shapiro et al[18] | 63/female | Cecum | Capecitabine | MLL | t(6;11) with breakpoint 11q23 |
| Tansley et al[19] | 66/male | Colon | Capecitabine | AML | Not available |
| Rashidi et al[20] | 58/male | Rectum | Capecitabine, Radiation | APL | 46,XY, t(15;17)(q24;q21) |
While the underlying cause of t-AML remains to be fully elucidated, we have established the importance of DNA damage, which includes methylation and intra- and inter-strand DNA cross links[10]. Platinum-based agents kill cancer cells through the formation of DNA adducts; these adducts lead to the formation of intra- and inter-strand DNA cross links that ultimately disrupt the processes of DNA replication and transcription[11]. Previous studies have reported an association of cisplatin and carboplatin treatment with the onset of therapy-related leukemia (TRL); however, the role of OXP in TRL was overlooked. As shown in Table 1, t-AML was diagnosed in 3 of the 6 reports regarding OXP-related TRL. Furthermore, only 1 report of OXP-related TRL was confirmed as t-APL[12-17]. In Merrouche et al[12], a female patient was treated with LVFU2, irinotecan and OXP 12 mo prior to t-APL diagnosis. The karyotype examination indicated (46,XX,add(6)(p23),-13,add(14)(p11),-16,add(17)(q?),-21,+3mar), which differed from the commonly reported t(15;17)(q22;q21) translocation[12]. Furthermore, Carneiro et al[13] reported a study of a female patient, first treated by FOLFOX-4 (OXP plus 5-fluorouracil [5-FU]) followed by FOLFOX-6 plus bevacizumab. Importantly, 28 mo prior to t-AML development, her karyotype revealed trisomy 8, loss of chromosomes 3 and 11, as well as partial deletions in chromosomes 5, 7, 20 and 21[13]. In Damodaran et al[15], a male patient with esophageal cancer received OXP plus capecitabine, with additional radiation treatment 29 mo prior to the emergence of t-AML. His karyotype was 47,X,der(Y)t(Y;3)(q12;q21),+8(21). Merlin et al[14] reported t-ALL diagnosis in a female patient 12 mo after administration of FOLFOX-4. Unfortunately, the corresponding karyotype analysis was not performed in this study[14]. In colorectal cancer, 2 cases of therapy-related chronic myeloid leukemia (t-CML) following OXP treatment tested positive for Philadelphia chromosome[16,17]. In Buxhofer-Ausch et al[16], a male patient was treated by cetuximab, OXP, irinotecan and capecitabine 18 mo prior to t-CML diagnosis. Contrastingly, in Kadikoylu et al[17], a male patient was administered with capecitabine, 5-FU, irinotecan and OXP 12 mo prior to detection of t-CML.
However, the aforementioned case studies involved complex chemotherapy schedules, which complicate the process of defining the tumorigenic potential of a single drug. As shown in Table 1, capecitabine-induced t-AML was previously reported in 2 studies[18,19]. Furthermore, Rashidi et al[20] reported a case involving capecitabine-induced t-APL with a karyotype of 46,XY,t(15;17)(q24;q21). Surprisingly, treatment with G-CSF was correlated with the development of TRL[21]. The results from these studies make it difficult to determine which agent is imperative in the development of TRL. Additionally, these studies highlight the considerable variation in the average time between treatment and onset of TRL. In our study, the patient was treated by OXP plus capecitabine and G-CSF, with a 44 mo latency period prior to onset of t-APL. The karyotype analysis of our patient presented 46,XY,t(15;17)(q22;q21)[15]/46,idem,-9,+add(9)(p22)[2]/46,XY[3], which was unlike previous studies. Balanced chromosome translocations were observed, although at low frequency, in t(15;17)(q22;q11). This genetic abnormality is often associated with treatments involving topoisomerase-2 inhibitors[22,23]; however, in an uncommon occurrence, balanced chromosome translocations were observed in our patient who was treated with OXP, capecitabine and G-CSF.
The prognosis of t-AML is thought to be worse than de novo AML, with a reported 5-year survival rate of less than 10%[24]. In Kayser et al[25], patients with t-AML were characterized with significantly inferior 4-year relapse-free survival (24.5% vs 39.5%) and 4-year overall survival (25.5% vs 37.9%) than de novo AML. Interestingly, karyotype variation provides key insight on the final outcome of t-AML patients[26]. As reported by Kern et al[27], patients with a favorable karyotype were characterized with a significantly higher median survival time (26.7 mo) compared to patients with an unfavorable karyotype (5.6 mo). However, in t-APL, these parameters are distinct from that of t-AML. t-APL patients with t(15;17)/PML-RARα have a complete remission rate of 63.6% compared to de novo APL (92.5%). Accordingly, the overall survival in t-APL is inferior to de novo APL (51% vs 84%)[28]. In our case, the patient partially displayed a favorable karyotype (t(15;17)/PML-RARα) and attained complete remission. However, further study is required to elucidate the function of other chromosomal abnormalities (for example, chromosome 9) in this outcome.
Our study presents the novel case of t-AML/t-APL following treatment of gastric cancer. We suggest establishing an additional evaluation process for patients undergoing treatment with certain cytotoxic therapies. For patients with t-APL, our case emphasized the importance of certain “favorable” genetic abnormalities. While standard treatment protocol is likely to yield a favorable outcome for patients, additional studies are required to elucidate the underlying process of t-APL development.
A 68-year-old man was diagnosed with gastric cardia cancer and was classified as cT4N1M0 (or IIIC), according to the TNM/UICC staging system.
After surgery, post-operative examination of the patient confirmed a poorly differentiated adenocarcinoma with penetrating invasion of the gastric wall. Out of 29 regional lymph nodes, 1 tested positive for cancer cell infiltrate.
Based on clinical symptoms, imageological examination and post-operative pathological analysis, the patient diagnosis was definitive.
Routine hematological examination indicated the following: red blood cells (2.35 × 1012/L), white blood cells (1.8 × 109/L), absolute neutrophil count (0.67 × 109/L), hemoglobin (75 g/L), and platelets (81 g/L).
Computed tomography scan indicated a localized, irregularly-shaped mass in the stomach. No obvious lymph node metastasis was observed.
Post-operative pathological examination confirmed gastric cancer. Bone marrow biopsy indicated acute myeloid leukemia. Leukemic hiatus was diagnosed as the total number of primitive cells plus immature leukemia cells accounted for 91.5% (2% + 89.5%) of the total cell population.
Oxaliplatin, capecitabine and recombinant human granulocyte-colony stimulating factor was administered for the treatment of gastric cancer. All-trans retinoic acid and arsenic trioxide was delivered to treat t-acute promyelocytic leukemia (t-APL).
In the literature, development of t-AML is rarely associated with treatment regimens involving oxaliplatin, capecitabine and recombinant human granulocyte-colony stimulating factor. The role of these cytotoxic agents in t-AML development remains unclear.
Therapy-related acute myeloid leukemia (t-AML) refers to a heterogeneous group of myeloid neoplasms that develops in patients who were extensively exposed to cytotoxic agents or radiation during prior treatment.
This case study elucidated that specific cytotoxic agents induce t-APL in gastric cancer patients. We recommend additional evaluation and follow-up for patients undergoing these therapies in the future.
This case report adds some information to the current literature. The case report and the discussion are well-written. They successfully managed a case of therapy-related acute myeloid leukemia. This case report provides an important update on therapy-related acute myeloid leukemias and presents an interesting patients who after diagnosis including karyotype analysis and treatment entered complete remission. The report is well illustrated and the authors well summarized current literature.
P- Reviewer: Iizuka T, Ozkan OV, Tarnawski A S- Editor: Ma YJ L- Editor: A E- Editor: Liu XM
| 1. | Kwong YL. Azathioprine: association with therapy-related myelodysplastic syndrome and acute myeloid leukemia. J Rheumatol. 2010;37:485-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 2. | Travis LB. The epidemiology of second primary cancers. Cancer Epidemiol Biomarkers Prev. 2006;15:2020-2026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 209] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 3. | Smith SM, Le Beau MM, Huo D, Karrison T, Sobecks RM, Anastasi J, Vardiman JW, Rowley JD, Larson RA. Clinical-cytogenetic associations in 306 patients with therapy-related myelodysplasia and myeloid leukemia: the University of Chicago series. Blood. 2003;102:43-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 498] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 4. | Casorelli I, Bossa C, Bignami M. DNA damage and repair in human cancer: molecular mechanisms and contribution to therapy-related leukemias. Int J Environ Res Public Health. 2012;9:2636-2657. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Czader M, Orazi A. Therapy-related myeloid neoplasms. Am J Clin Pathol. 2009;132:410-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Godley LA, Larson RA. Therapy-related myeloid leukemia. Semin Oncol. 2008;35:418-429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 257] [Cited by in RCA: 229] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 7. | Larson RA, Le Beau MM. Prognosis and therapy when acute promyelocytic leukemia and other «good risk» acute myeloid leukemias occur as a therapy-related myeloid neoplasm. Mediterr J Hematol Infect Dis. 2011;3:e2011032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Andersen MK, Larson RA, Mauritzson N, Schnittger S, Jhanwar SC, Pedersen-Bjergaard J. Balanced chromosome abnormalities inv(16) and t(15; 17) in therapy-related myelodysplastic syndromes and acute leukemia: report from an international workshop. Genes Chromosomes Cancer. 2002;33:395-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 102] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Mistry AR, Felix CA, Whitmarsh RJ, Mason A, Reiter A, Cassinat B, Parry A, Walz C, Wiemels JL, Segal MR. DNA topoisomerase II in therapy-related acute promyelocytic leukemia. N Engl J Med. 2005;352:1529-1538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 214] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 10. | Leone G, Pagano L, Ben-Yehuda D, Voso MT. Therapy-related leukemia and myelodysplasia: susceptibility and incidence. Haematologica. 2007;92:1389-1398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 153] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 11. | Raymond E, Faivre S, Woynarowski JM, Chaney SG. Oxaliplatin: mechanism of action and antineoplastic activity. Semin Oncol. 1998;25:4-12. [PubMed] |
| 12. | Merrouche Y, Mugneret F, Cahn JY. Secondary acute promyelocytic leukemia following irinotecan and oxaliplatin for advanced colon cancer. Ann Oncol. 2006;17:1025-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Carneiro BA, Kaminer L, Eldibany M, Sreekantaiah C, Kaul K, Locker GY. Oxaliplatin-related acute myelogenous leukemia. Oncologist. 2006;11:261-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Merlin F, Prochilo T, Kildani B, Tucci A, Ferrari S, Rossi G, D’Adda P, Beretta GD. Secondary acute lymphoblastic leukaemia following oxaliplatin for adjuvant chemotherapy in colon cancer. Acta Oncol. 2008;47:464-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Damodaran S, Bellavia T, Sait SN, Wang ES, Wetzler M, Khushalani NI. Acute myeloid leukemia secondary to oxaliplatin treatment for esophageal cancer. Clin Colorectal Cancer. 2012;11:151-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Buxhofer-Ausch V, Hinterberger-Fischer M, Hinterberger W. Bcr-abl positive blast crisis of chronic myeloid leukemia emerging in a case of metastatic colorectal cancer 3 months after completion of an 8-month course of cetuximab and irinotecan. Eur J Haematol. 2006;76:447-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Kadikoylu G, Yavasoglu I, Barutca S, Meydan N, Bolaman Z. Chronic myeloid leukemia following the treatment of rectal adenocarcinoma. Med Oncol. 2008;25:467-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Shapiro S, Hughes G, Al-Obaidi MJ, O’Reilly E, Ramesh S, Smith J, Ahmad R, Dawson C, Riddle P, Sekhar M. Acute myeloid leukaemia secondary to treatment with capecitabine for metastatic colorectal cancer. Eur J Haematol. 2007;78:543-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Tansley S, Gibbons S. Capecitabine-induced acute myeloid leukaemia. N Z Med J. 2009;122:118-119. [PubMed] |
| 20. | Rashidi A, Man L, Howard JR, Atienza D, Fisher SI. Therapy-related acute promyelocytic leukemia after chemoradiotherapy with capecitabine for rectal adenocarcinoma. Leuk Lymphoma. 2014;55:683-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Beekman R, Touw IP. G-CSF and its receptor in myeloid malignancy. Blood. 2010;115:5131-5136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 123] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 22. | Andersen MK, Johansson B, Larsen SO, Pedersen-Bjergaard J. Chromosomal abnormalities in secondary MDS and AML. Relationship to drugs and radiation with specific emphasis on the balanced rearrangements. Haematologica. 1998;83:483-488. [PubMed] |
| 23. | Joannides M, Grimwade D. Molecular biology of therapy-related leukaemias. Clin Transl Oncol. 2010;12:8-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Singh ZN, Huo D, Anastasi J, Smith SM, Karrison T, Le Beau MM, Larson RA, Vardiman JW. Therapy-related myelodysplastic syndrome: morphologic subclassification may not be clinically relevant. Am J Clin Pathol. 2007;127:197-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 25. | Kayser S, Döhner K, Krauter J, Köhne CH, Horst HA, Held G, von Lilienfeld-Toal M, Wilhelm S, Kündgen A, Götze K. The impact of therapy-related acute myeloid leukemia (AML) on outcome in 2853 adult patients with newly diagnosed AML. Blood. 2011;117:2137-2145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 343] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 26. | Park SH, Chi HS, Cho YU, Jang S, Park CJ. Evaluation of prognostic factors in patients with therapy-related acute myeloid leukemia. Blood Res. 2013;48:185-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Kern W, Haferlach T, Schnittger S, Hiddemann W, Schoch C. Prognosis in therapy-related acute myeloid leukemia and impact of karyotype. J Clin Oncol. 2004;22:2510-2511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 96] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 28. | Elliott MA, Letendre L, Tefferi A, Hogan WJ, Hook C, Kaufmann SH, Pruthi RK, Pardanani A, Begna KH, Ashrani AA. Therapy-related acute promyelocytic leukemia: observations relating to APL pathogenesis and therapy. Eur J Haematol. 2012;88:237-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |