Published online Apr 14, 2015. doi: 10.3748/wjg.v21.i14.4397
Peer-review started: September 18, 2014
First decision: December 2, 2014
Revised: January 28, 2015
Accepted: March 19, 2015
Article in press: March 19, 2015
Published online: April 14, 2015
Processing time: 209 Days and 17.7 Hours
We report an unusual case of Clostridium perfringens liver abscess formation after transcatheter arterial chemoembolization (TACE) for large hepatocellular carcinoma. Severe deterioration in liver and renal function accompanied with hemocytolysis was found on the 2nd day after TACE. Blood culture found Clostridium perfringens and abdominal computed tomography revealed a gas-containing abscess in the liver. Following antibiotics administration and support care, the infection was controlled and the liver and renal function turned normal. The 2nd TACE procedure was performed 1.5 mo later and no recurrent Clostridium perfringens infection was found.
Core tip: Transcatheter arterial chemoembolization (TACE) is a major treatment for patients with hepatocellular carcinoma (HCC) that is not eligible for curative treatment. Though rare, Clostridium perfringens liver abscess after TACE is fatal. We successfully treated a 71-year-old man with large HCC who had liver and renal dysfunction and developed Clostridium perfringens liver abscess after TACE. After timely identification of the patient’s deteriorating condition, aggressive treatments, including antibiotics administration, anticoagulation and intensive care, were provided.
-
Citation: Li JH, Yao RR, Shen HJ, Zhang L, Xie XY, Chen RX, Wang YH, Ren ZG.
Clostridium perfringens infection after transarterial chemoembolization for large hepatocellular carcinoma. World J Gastroenterol 2015; 21(14): 4397-4401 - URL: https://www.wjgnet.com/1007-9327/full/v21/i14/4397.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i14.4397
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide and more than 50% of HCCs are treated with transcatheter arterial chemoembolization (TACE). Since the first case was reported in 1958, few cases of Clostridium perfringens liver abscess have been reported, among which only two cases were associated with TACE therapy[1]. Though rare, Clostridium perfringens liver abscess can be rapidly fatal. We report a case of a 71-year-old man with large HCC who had liver and renal dysfunction and developed Clostridium perfringens liver abscess after TACE.
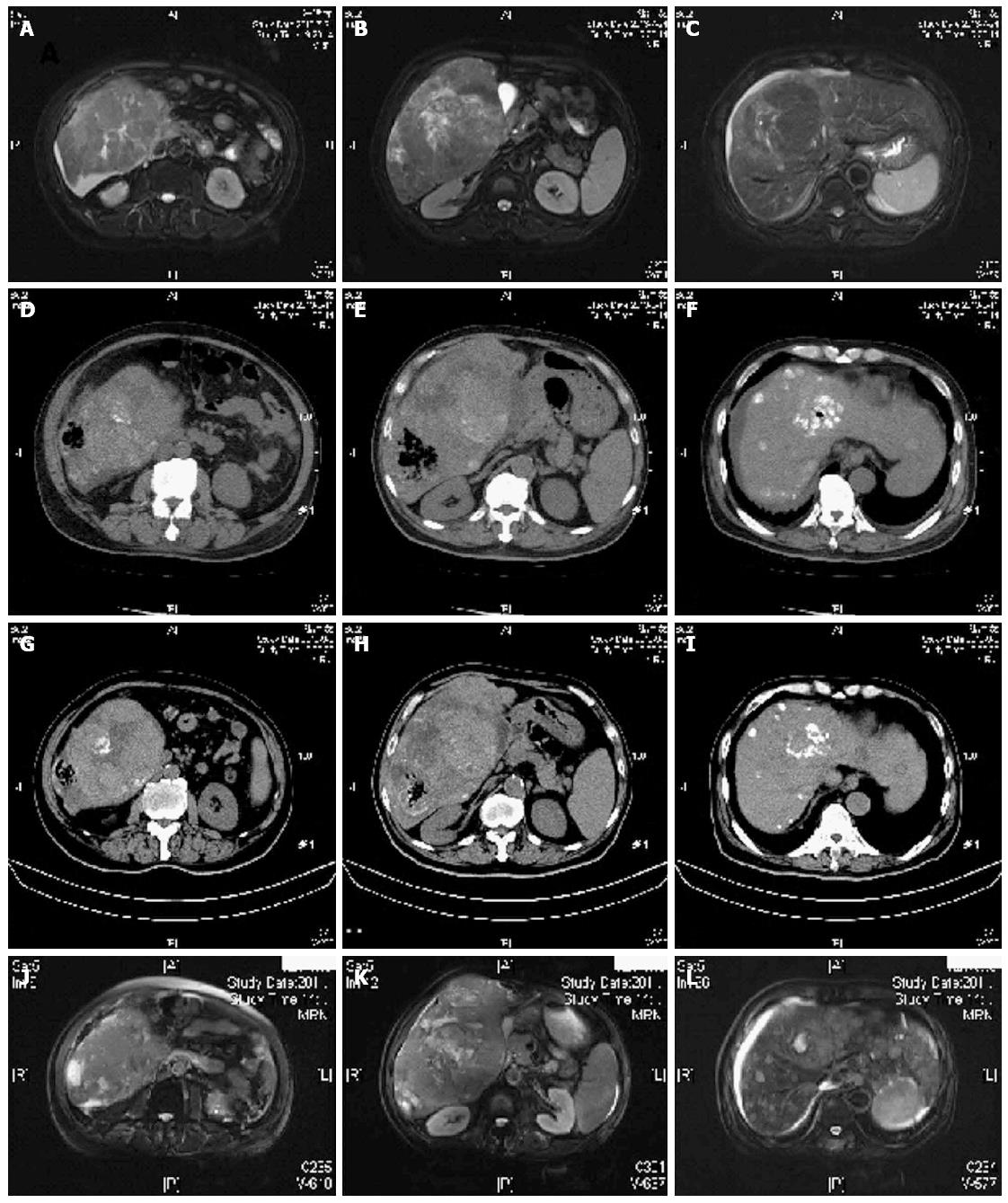
A previously healthy 71-year-old man was admitted due to abdominal distension. He was diagnosed with HCC based on the HBV infection history, and magnetic resonance imaging (MRI) findings. Dynamic MRI revealed a lesion of 179 mm in diameter surrounded by multiple satellite nodes and presented typical arterial enhancement and venous phase washout (Figure 1A-C), and the patient had elevated level of serum α-fetoprotein (AFP) (38653 ng/mL)[2]. Neither ascites nor portal vein embolus was observed. According to the laboratory findings, the patient was clinically staged as BCLC B, Child-Pugh A and ECOG 1. He had no history of surgery or other diseases, such as diabetes mellitus. After evaluating the general condition of the patient, we performed TACE with hepatic infusion of 5-fluorouracil 1000 mg and Oxaliplatin 150 mg followed by a mixture of 5 mg mitomycin C and 10 mL lipoidol chemoembolization.
Laboratory results indicated liver and renal dysfunction on the 1st day after TACE, but symptoms were mild which were attributed to the post-embolization syndrome (Table 1). However, on the 2nd day, the patient presented with deterioration in liver and renal function, anemia and dysfunction of coagulation. Total bilirubin and direct bilirubin increased to 323 μmol/L and 209 μmol/L, respectively. He had high fever (39.4 °C) accompanied with chill, and blood culture showed Clostridium perfringens. Then hematuria and hypotension occurred sequentially. Ultrasound and computed tomography (CT) both revealed a non-uniform abscess filled with gas but no liquid in the right lobe of the liver (Figure 1D-F).
| Baseline | Days after TACE | Follow-up | |||||||
| 1st | 2nd | 3rd | 8th | 13rd | 21st | 38th | |||
| Date | 27/7/2013 | 30/7/2013 | 1/8/2013 | 2/8/2013 | 6/8/2013 | 11/8/2013 | 19/8/2013 | 5/9/2013 | 11/10/2013 |
| TB (μmol/L) | 27.4 | 44.4 | 323 | 84.8 | 54.4 | 25 | 34.4 | 37.8 | |
| DB (μmol/L) | 10.9 | 14.6 | 209 | 65.7 | 37.4 | 18.6 | 18.7 | 24.3 | |
| ALB (g/L) | 31 | 35 | 27 | 27 | 29 | 30 | 32 | 31 | |
| ALT (U/L) | 145 | 1652 | 2312 | 261 | 82 | 45 | 62 | 53 | |
| AST (U/L) | 113 | 787 | 1953 | 120 | 73 | 68 | 75 | 87 | |
| UA | 362 | 454 | 393 | 229 | 201 | 230 | |||
| BUN (mmol/L) | 5.4 | 5.7 | 17.8 | 15 | 10.8 | 10.2 | 8.5 | 7.9 | |
| Cr (μmol/L) | 81 | 75 | 152 | 153 | 123 | 119 | 109 | ||
| Glucose(mmol/L) | 4.7 | 9 | 9.6 | 9.9 | 8.7 | 5.2 | |||
| K+ (mmol/L) | 4.7 | 4.1 | 3.8 | 3.4 | 3.4 | 3.9 | |||
| PT (s) | 10.4 | 11.6 | 17 | 14.9 | 13.4 | 11.8 | 12.6 | ||
| D-Dimer (mg/L) | 2.39 | 2.13 | 4.46 | 8.72 | 8.68 | 4.58 | 3.56 | ||
| INR | 0.91 | 1.02 | 1.48 | 1.31 | 1.17 | 1.03 | 1.1 | ||
| RBC (1012/L) | 4.46 | 4.79 | 4.7 | 3.68 | 2.72 | 2.47 | 2.63 | 3.29 | 3.6 |
| HEMO (g/L) | 140 | 153 | 148 | 118 | 87 | 76 | 83 | 101 | 111 |
| PLT (109/L) | 201 | 154 | 120 | 128 | 81 | 158 | 192 | 264 | 294 |
| WBC(109/L) | 3.82 | 7.96 | 6.1 | 11.52 | 4.38 | 4.76 | 3.89 | 4.59 | 7.67 |
| NEU% | 64.2 | 90.1 | 84.4 | 92.7 | 85.6 | 82.4 | 68.1 | 69.9 | 84.5 |
| CPR (mg/L) | 65.9 | 128.9 | 89.6 | 56 | |||||
| PCT (ng/mL) | 0.35 | 0.39 | 1.72 | 51.61 | 13.39 | 2.52 | 0.95 | 0.697 | |
| AFP (ng/mL) | 36895 | 37104 | 24962 | 11129 | 10069 | 13310 | 38935 | > 60500 | |
| Blood culture1 | + | - | |||||||
| Urine RBC2 | - | +/- | +/- | - | |||||
| Urine-bilirubin | + | + | - | ||||||
| Urinary-protein | + | + | +/- | ||||||
Empirical piperacillin/tazobactam was used right after the blood sample was taken for culture, which was later combined with Levofloxacin when the bacterium was reported. Other treatments, including hydration, urinary alkalinisation and diuretics, were given at the same time. To tackle the dysfunction of coagulation and prevent disseminated intravascular coagulation, low molecular heparin and plasma transfusion were administrated.
Laboratory indexes turned normal in 2 or 3 d after intensive care, except D-dimer which peaked on the 11th day after TACE. The temperature was lowered to about 38 °C without chilling, while the blood culture yielded no more pathogenic bacterium 1 and 2 wk later, respectively. Mild ascites and pitting edema over both legs developed on the 6th day after TACE, which was relieved gradually in the following 10 d. Follow-up ultrasound demonstrated a significant reduction in both the size of the liver abscess and gas volume, although the gas-containing mass still existed. The patient was discharged on the 26th day after TACE in a generally good condition. CT scanning undertaken two weeks after the patient discharge showed that both the HCC lesion and gas-containing liver abscess became smaller (Figure 1G-I). However, serum AFP levels increased to 39868 ng/mL which was proximal to the initial level.
The patient received the 2nd TACE 1.5 mo after the 1st procedure. No infection was observed. Enhanced MRI on October 18, 2014 showed the gas-containing liver abscess was stable. However, tumor progress and lung metastasis were demonstrated (Figure 1J-L).
HCC is the fifth most common cancer and the second cause of cancer related deaths in men worldwide[3]. Though the surveillance of the disease is enhanced, only a small fraction of patients are eligible for curative treatment[4]. TACE, one of palliative treatments for HCC, has been shown to achieve partial responses in 15%-55% of patients, significantly delay tumor progression and vascular invasion, and prolong survival among HCC patients who are not candidates for curative treatment[5]. TACE is also the major treatment for unresectable HCC and is performed in 58% of patients with recurrence[4]. Even in elderly patients (≥ 75 years of age), this procedure was proved to be safe and effective, and was not associated with decreased survival or increased complication rate according to a recent prospective cohort study[6].
The reported rate of major complications associated with TACE procedure ranged from 2.68% to 12.5%[7,8]. Post-TACE liver abscess is one of these complications and sometimes life threatening. The incidence of liver abscess formation was low, ranging from 0.1% to 4.6%. However, high mortality rate from post-TACE liver abscess, ranging from 13.3% to 50%, has complicated the TACE management[9-11].
Generally, for patients receiving TACE treatment, abdominal surgery history may increase the risk of liver abscess formation. This patient had no history of abdominal surgery. Old age, large HCC, and the infusion of chemotherapeutic agents might lead to an immune suppression status which is vulnerable to infection. Under this condition, the pathogen Clostridium perfringens, an anaerobic bacillus which is naturally distributed in the alimentary tracts of humans, could superimpose upon the embolic and necrosis lesion after TACE.
Though the incidence of Clostridium perfringens bacteremia is low (0.97/100000 in patients who were hospitalized[12] and 0.7/100000 in non-selected populations[13]), and the Clostridium perfringens liver abscess is even rare[14], and increasing age, poorly controlled diabetic mellitus, and presence of malignancies, especially gastrointestinal and genitourinary malignancies, could increase the risk for Clostridium septicemia development[14].
The patient presented typical clinical features of Clostridium perfringens liver abscess with fever, abdominal pain, gas-containing lesion in imaging examinations, intravascular hemolysis (anemia, high Lactate dehydrogenase level, hyperbilirubinemia, and secondary renal injury) and positive blood culture. Clostridium perfringens with a short doubling time could produce a large amount of potent hemolysin, such as alpha-toxin, which could result in severe intravascular hemolysis[15]. A hospital-based study showed that intra-abdominal is the major source of Clostridium perfringens infection[12]. In the present case, the patient had chill and fever on the 2nd day after TACE. Thus, TACE was an important predisposing cause. Considering the operating room was routinely monitored with bacteria culture and re-checked after the patient was diagnosed with sepsis, and no other patient presented the same symptoms who received TACE simultaneously by the same physician group, it is likely an autogenous infection, although its specific origin was not clear.
The 30-d mortality of Clostridium perfringens infection is high, ranging from 26% to 100%[12,15]. Drainage combined with antibiotics is the basic treatment of liver abscess[16]. Law et al[13] reviewed 20 cases of Clostridium perfringens liver abscess between 1990 and 2011, and there were only 6 cases survived and 5 cases had the primary focus of infection removed.
But in the case we reported here, abscess excision or drainage is unavailable because of the risk of tumor rupture existing in the patient. We attribute our successful treatment to the timely identification of the patient’s deteriorating condition, and aggressive treatments, including antibiotics administration, anticoagulation and intensive care. Given the presence of large tumor in this patient, hydration and urinary alkalinisation were administrated as soon as the TACE procedure was performed. Though his temperature was 39 °C on the 1st day after TACE, blood culture was sampled in the morning of the 2nd day when the patient had high fever along with chilling and obvious listlessness. At the same time, blood was tested; Piperacillin/tazobactam was used as prophylactic antibiotics. And Levofloxacin was added when the blood culture result was reported. Anticoagulation was administrated when the hematuria was first observed in the evening of the 2nd day (Table 1).
In conclusion, there are various risk factors in patients receiving TACE for Clostridium perfringens infection, such as old age, malignancy, and necrosis due to embolization. Timely identification of the liver and renal dysfunction and aggressive treatments including both antibiotics administration and intensive care, are crucial in post-TACE management.
Mild symptoms were found on the first day after transcatheter arterial chemoembolization (TACE), whereas high fever, chill and abdominal pain were observed on the second day after TACE in a 71-year-old man with large hepatocellular carcinoma (HCC).
High fever, severe jaundice, hematuria, and hypotension were observed after TACE in the 71-year-old man with large HCC.
Because of the non-specific symptoms, early diagnosis of Clostridium perfringens infection is difficulty, and its typical features included fever, abdominal pain, gas-containing lesion in imaging examinations, intravascular hemolysis and positive blood culture.
Positive blood culture of Clostridium perfringens is confirmatory for diagnosis and other laboratory findings include anemia, dysfunction of liver and renal, disorder of coagulation and hematuria.
Ultrasound and computed tomography imaging both indicated a non-uniform abscess filled with gas but no liquid in the right lobe of the liver.
Blood culture revealed Clostridium perfringens.
Upon identification of the patient’s deteriorating condition, the patient was treated with antibiotics, anticoagulation and intensive care.
“Two cases of liver abscess caused by Clostridium perfringens after transcatheter arterial chemoembolization” by Oshima et al.
TACE is an invasive procedure performed in interventional radiology to restrict a tumor’s blood supply. Small embolic particles combined with chemotherapeutic agents are injected selectively into an artery directly supplying a tumor.
The authors attribute their successful treatment in this patient to the timely identification of his deteriorating condition, and aggressive treatments, including antibiotics administration, anticoagulation and intensive care.
The authors reported an unusual case of Clostridium perfringens liver abscess formation after TACE for large HCC. They well reviewed the literature and discussed the results.
P- Reviewer: Zhu X S- Editor: Yu J L- Editor: A E- Editor: Liu XM
| 1. | Oshima S, Takaishi K, Tani N, Hirano M, Ikeda K, Makari Y, Hoshi M, Doi T, Kobori Y, Kurokawa E. [Two cases of liver abscess caused by Clostridium perfringens after transcatheter arterial chemoembolization]. Gan To Kagaku Ryoho. 2013;40:1795-1797. [PubMed] |
| 2. | Maluccio M, Covey A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA Cancer J Clin. 2012;62:394-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 698] [Article Influence: 53.7] [Reference Citation Analysis (0)] |
| 3. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25541] [Article Influence: 1824.4] [Reference Citation Analysis (7)] |
| 4. | Takayasu K. Transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: recent progression and perspective. Oncology. 2013;84 Suppl 1:28-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Llovet JM. Updated treatment approach to hepatocellular carcinoma. J Gastroenterol. 2005;40:225-235. [PubMed] |
| 6. | Cohen MJ, Bloom AI, Barak O, Klimov A, Nesher T, Shouval D, Levi I, Shibolet O. Trans-arterial chemo-embolization is safe and effective for very elderly patients with hepatocellular carcinoma. World J Gastroenterol. 2013;19:2521-2528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Jang ES, Yoon JH, Chung JW, Cho EJ, Yu SJ, Lee JH, Kim YJ, Lee HS, Kim CY. Survival of infiltrative hepatocellular carcinoma patients with preserved hepatic function after treatment with transarterial chemoembolization. J Cancer Res Clin Oncol. 2013;139:635-643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Xia J, Ren Z, Ye S, Sharma D, Lin Z, Gan Y, Chen Y, Ge N, Ma Z, Wu Z. Study of severe and rare complications of transarterial chemoembolization (TACE) for liver cancer. Eur J Radiol. 2006;59:407-412. [PubMed] |
| 9. | Woo S, Chung JW, Hur S, Joo SM, Kim HC, Jae HJ, Park JH. Liver abscess after transarterial chemoembolization in patients with bilioenteric anastomosis: frequency and risk factors. AJR Am J Roentgenol. 2013;200:1370-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 10. | Meyer T, Kirkwood A, Roughton M, Beare S, Tsochatzis E, Yu D, Davies N, Williams E, Pereira SP, Hochhauser D. A randomised phase II/III trial of 3-weekly cisplatin-based sequential transarterial chemoembolisation vs embolisation alone for hepatocellular carcinoma. Br J Cancer. 2013;108:1252-1259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 122] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 11. | Pua U, Merkle EM. Case report. Spontaneous cholecystocolic fistula and locoregional liver tumour ablation: a cautionary tale. Br J Radiol. 2011;84:e243-e245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Yang CC, Hsu PC, Chang HJ, Cheng CW, Lee MH. Clinical significance and outcomes of Clostridium perfringens bacteremia--a 10-year experience at a tertiary care hospital. Int J Infect Dis. 2013;17:e955-e960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Ngo JT, Parkins MD, Gregson DB, Pitout JD, Ross T, Church DL, Laupland KB. Population-based assessment of the incidence, risk factors, and outcomes of anaerobic bloodstream infections. Infection. 2013;41:41-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Law ST, Lee MK. A middle-aged lady with a pyogenic liver abscess caused by Clostridium perfringens. World J Hepatol. 2012;4:252-255. [PubMed] |
| 15. | Pita Zapata E, Sarmiento Penide A, Bautista Guillén A, González Cabano M, Agulla Budiño JA, Camba Rodríguez MA. [Massive intravascular hemolysis secondary to sepsis due to Clostridium perfringens]. Rev Esp Anestesiol Reanim. 2010;57:314-316. [PubMed] |
| 16. | Lai KC, Cheng KS, Jeng LB, Huang CC, Lee YT, Chang HR, Chen CC, Chen SC, Lee MC. Factors associated with treatment failure of percutaneous catheter drainage for pyogenic liver abscess in patients with hepatobiliary-pancreatic cancer. Am J Surg. 2013;205:52-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |