Published online Apr 14, 2015. doi: 10.3748/wjg.v21.i14.4391
Peer-review started: August 20, 2014
First decision: September 27, 2014
Revised: October 20, 2014
Accepted: December 16, 2014
Article in press: December 16, 2014
Published online: April 14, 2015
Processing time: 238 Days and 12.2 Hours
Myoepithelioma/myoepithelial carcinomas are not commonly found in soft tissues and are especially rare at visceral sites. This report describes a case of a rare low-grade myoepithelial carcinoma of the stomach. A 61-year-old female patient presented with postprandial abdominal discomfort. Endoscopy revealed a 1.1 cm submucosal lesion. Local excision was performed after malignancy was confirmed by biopsy. The resection margin is free of tumor and she received no adjuvant therapy. The tumor was characterized by multinodular growth with biphasic epithelioid and spindle components. Infiltrative margin and nuclear pleomorphism are seen. Tumor cells were positive for both epithelial and myoepithelial markers. Evidence of epithelial differentiation was confirmed by electron microscopy. No EWSR1 rearrangement was detected. The final diagnosis was low-grade myoepithelial gastric carcinoma. The patient is currently well, and no evidence of recurrence or metastasis was found after ten-month of follow-up. Myoepithelial carcinoma should be considered in the differential diagnosis of a biphasic gastric tumor.
Core tip: Myoepithelial tumor can cause diagnostic pitfall due to its rarity and various morphology. This report reveals a rare low-grade myoepithelial carcinoma of stomach and its morphological, immunohistochemical and molecular characteristics.
- Citation: Tseng CE, Hsieh YH, Wei CK, Huang HY, Chi CL. Myoepithelial carcinoma of the stomach: A diagnostic pitfall. World J Gastroenterol 2015; 21(14): 4391-4396
- URL: https://www.wjgnet.com/1007-9327/full/v21/i14/4391.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i14.4391
Myoepithelioma, myoepithelial carcinoma, mixed tumor, and the synonym parachordoma are terms placed into the same category in the recent edition of the World Health Organization classification of soft tissue tumors[1]. “Myoepithelial tumor” is an uncommon entity and refers to a tumor exhibiting immunohistochemical or ultrastructural evidence of myoepithelial differentiation. In addition to salivary glands, myoepithelial tumors have been reported to arise from cutaneous or soft tissues[2-5]. However, myoepithelial tumors of visceral organs are especially rare; very few case reports of such tumors have been published with most (8 of 11) describing tumors of pulmonary origin[6-10]. The present report describes a second case of a myoepithelial tumor of the stomach and the immunohistochemical, ultrastructural, and molecular findings for this tumor.
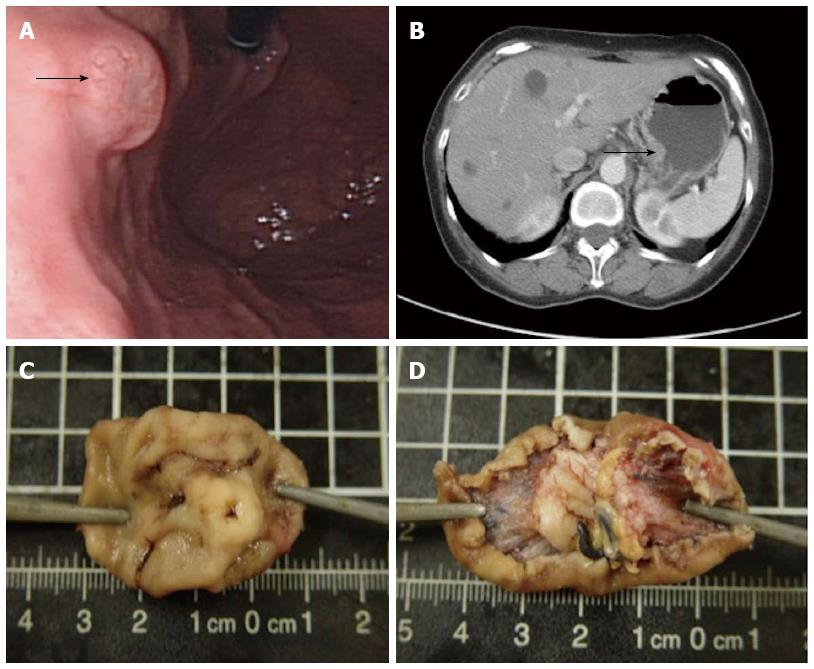
A 61-year-old female with a history of hypertension and grade A gastroesophageal reflux disease presented to our hospital with abdominal pain of one year duration. The abdominal pain was intermittent but gradually increased in intensity. The peak of her discomfort was usually observed 30-60 min after a meal. She denied nausea, vomiting, hematemesis, hematochezia, constipation, diarrhea, or weight loss. She also denied a family history of cancer or hereditary diseases. Panendoscopy revealed a 1.1 cm submucosal lesion at the posterior wall of the gastric high body (Figure 1A). Biopsy was performed, and the pathology report was positive for a malignant tumor. Computed tomography revealed a localized lesion without regional lymph node enlargement or distant metastasis (Figure 1B). Local excision was performed. Grossly, the mucosa overlying the lesion appeared ulcerated. Upon excision, the submucosal lesion appeared well-circumscribed, grayish-white, and elastic. The size of the tumor was 1.3 cm × 1.1 cm × 0.7 cm (Figure 1C and D).
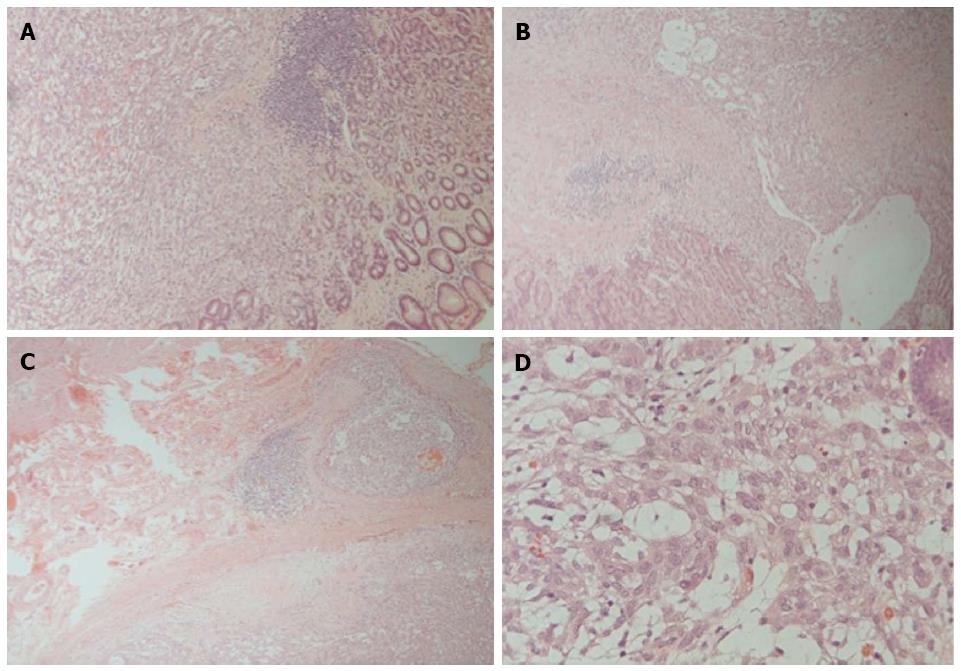
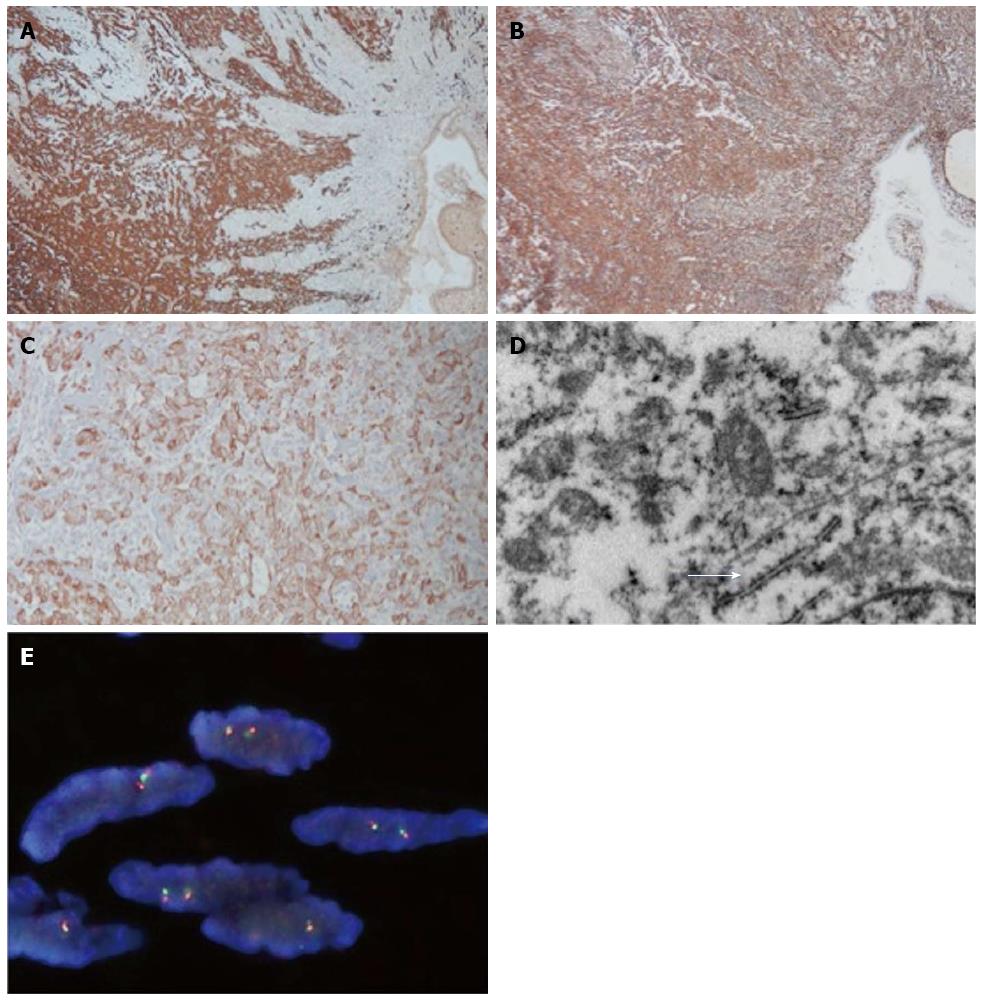
Microscopically, the tumor was seen to be delineated by a fibrous capsule, and occasional lymphocytic cuffing was present (Figure 2A). Some satellite nodules were seen adjacent to the main tumor. A biphasic pattern of cells composed of epithelioid and oval to spindle cells embedded in myxoid to fibrous stroma was observed (Figure 2B). The tumor cells displayed pleomorphism, mitosis (1-2/10 HPFs), and visible nucleoli (Figure 2D). The epithelioid cells tended to form sheets, cords, or glandular structures whereas the spindle cells were aligned in a parallel fashion. The tumor cells penetrated the fibrous capsule focally (Figure 2C) and invaded the mucosa (Figure 2B). The deep margin was close (< 1 mm) but not involved with the tumor. No necrosis, neural invasion, or lymphovascular permeation was found. Immunohistochemically, the tumor cells were diffusely positive for vimentin (Dako, 1:200) and S-100 protein (Dako, 1:400) and were focally positive for cytokeratin (Genemed, 1:200), AE1/AE3 (Dako, 1:200), epithelial membrane antigen (Leica, 1:100), synaptophysin (Leica, 1:100), DOG1 (Leica, 1:100), CD10 (spotty; Novocastra, 1:50) and melan-A (weak; Leica, 1:50) but were negative for CD117 (Genemed, 1:300), HMB-45 (Leica, 1:100), calretinin (Leica, 1:100), CD34 (Leica, 1:100), glial fibrillary acidic protein (GFAP) (Dako, 1:100), CD56 (Leica, 1:50), desmin (Dako, 1:100), or actin-M851 (Leica, 1:100) (Figure 3). The tumor cells were positive for p53 (30%; Dako, 1:200) and Ki-67 (5%; Genemed, 1:300) (Figure 3A-C). Ultrastructurally, the tumor cells appeared epithelioid- to spindle-shaped, with irregular nuclei and abundant cytoplasm; the latter contained abundant rough endoplasmic reticulum, intermediate filaments, and small aggregates of dense bodies. Some cell junctions were also present (Figure 3D). For detection of EWSR1 gene rearrangement, the break-apart FISH (fluorescence in situ hybridization) assay was performed on formalin-fixed, paraffin-embedded tissue sections of 4-μm thickness according to the instructions of the manufacturer. No break-apart signals were detected (Figure 3E). The final diagnosis was low-grade myoepithelial carcinoma based on the presence of mild pleomorphism, considerable mitotic activity, and the invasive nature of the borders.
No adjuvant chemotherapy was administered. The patient was alive without disease after 10 mo of follow-up.
This report describes a rare case of a low-grade myoepithelial carcinoma of the stomach. Only one other comparable case, describing a “parachordoma of the gastric serosa”, has been reported in the literature (Table 1). The differential diagnosis of a myoepithelial tumor is challenging and dependent on the location of the tumor. Differential diagnoses of gastric biphasic tumors include carcinosarcoma, synovial sarcoma, gastrointestinal stromal tumor, mesothelioma, subtypes of neurogenic tumor (reticular schwannoma or epithelioid malignant peripheral nerve sheath tumor), and gastroblastoma. Rendering a diagnosis of carcinosarcoma requires identification of marked pleomorphism of both the carcinoma and the sarcoma components. Although synovial sarcomas are generally positive for cytokeratin and occasionally positive for S100 protein, the most sensitive marker for these sarcomas is TLE1[11,12]. Neither CD117 nor DOG-1 expression supports a diagnosis of gastrointestinal stromal tumor. The absence of calretinin expression by a tumor disfavors a mesothelial origin. Although lymphocytic cuffing was observed for the tumor described in the present report, the absence of Antoni structures and of cytokeratin expression disfavors a diagnosis of schwannoma. The cytological features of the tumor exclude a diagnosis of epithelioid malignant peripheral nerve sheath tumor. Gastroblastoma, a recently identified entity described in only five reports to date, is also a biphasic tumor of the stomach[13-15]. Certain features of a gastroblastoma overlap with those of a myoepithelial neoplasm, such as a variable mixture of epithelial and mesenchymal components, a bland-appearing cytology, and a multinodular growth pattern with a potentially malignant behavior. Additionally, gastroblastomas and myoepithelial neoplasms share certain immunohistochemical and ultrastructural features including positivity for cytokeratins and vimentin and, indicative of epithelial differentiation, the presence of desmosomes and microvilli[14]. The epithelial components of gastroblastomas express cytokeratin and the mesenchymal components of these tumors express vimentin; however, the tumor described in the present case was diffusely positive for vimentin expression. Furthermore, the most striking difference between gastroblastomas and myoepithelial neoplasms is that gastroblastomas do not express myoepithelial markers including S100 protein, p63, calponin, GFAP, or smooth muscle actin. The only exception to the above is the expression of smooth muscle actin and desmin by an epitheliomesenchymal biphasic tumor/duodeno blastoma[16]. In the limited number of publications describing gastroblastomas, positivity for CD10 and CD56 of unknown significance was reported; in contrast, the tumor described in the present case was negative for CD56 expression and positivity for CD10 was spotty. Interestingly, the duodenoblastoma described by Poizat et al[16] was found to be negative for both markers. Forty-five percent of soft tissue myoepithelioma and myoepithelial carcinomas, including four pulmonary tumors, in a series were found to display EWSR1 rearrangement[10]. Translocation of the gene with such known fusion partners as PBX1, ZNF444, and POU5F1 has been previously observed[10,17,18]. It was therefore considered important to examine the possibility that the tumor described in the present report exhibited EWSR1 rearrangement; however, no “break-apart” signal was observed. Lack of EWSR1 rearrangement was also reported for the gastric parachordoma described by Spivach et al[8]. The recurrent cytogenetic changes present in gastric myoepithelial tumors remain to be characterized.
| Patient | Age (yr) | Gender | Tumor size (cm) | Diagnosis | Follow-up finding (mo) | Immunohistochemical findings |
| Spivach et al[8], 2007 | 65 | Female | 5.5 | Parachordoma | NED1, 36 | CK+2, EMA+, S100+, VIM+, BU-, CD117-, CD10-, GFAP-, calponin-, calretinin-, P63 |
| The present case | 61 | Female | 1.3 | Low graders myoepithelial carcinoma | NED, 10 | AE1/AE3+, CK+, EMA+, S100+, VIM+, synaptophysin+, DOG1-, desmin-, actin M851-, HMB-45-, melan-A(weak), CD117-, CD10(spotty), GFAP-, calretinin-, CD56 |
Discrimination between benign myoepithelioma and malignant myoepithelial carcinoma is essential. As compared with necrosis, mitotic index, and infiltrative pattern, the most reliable parameter for discrimination is cytological atypia[3]. The presence of non-negligible cytological pleomorphism and an infiltrative growth pattern, despite the small tumor size, prompted the diagnosis of low-grade myoepithelial carcinoma rather than myoepithelioma. Age of onset appears to influence prognosis for patients with myoepithelial carcinoma in that this cancer is more prevalent and aggressive in children[4]. Genetic events are proposed to account for the observation that EWSR1 rearrangement, which is more common in children and young adults with these tumors, portends more aggressive tumor behavior[10]. These findings support the detection of EWSR1 rearrangement in the diagnosis of these tumors and as an assessment of prognosis for patients with these tumors.
In conclusion, a case of a rare low-grade myoepithelial carcinoma is reported and the morphological, immunohistochemical, ultrastructural, and molecular features of this tumor are described. The rarity, diverse morphologies, and variable immunoprofiles of visceral myoepithelial tumors are potential causes for diagnostic pitfalls. Multidisciplinary approaches are therefore required for precise diagnosis of these tumors. Myoepithelial tumor should be considered in the differential diagnosis of gastric biphasic tumors.
A 61-year-old female with a history of hypertension and gastroesophageal reflux disease presented with abdominal pain.
A 1.1 cm submucosal lesion noted at gastric high body revealed by panendoscope.
Gastrointestinal stromal tumor or other gastric neoplasms.
Routine metabolic panel and complete blood counts were within normal limits.
Computed tomography revealed a localized lesion without regional lymph node enlargement or distant metastasis
A biphasic tumor composed of epithelioid and spindle cells is noted. Both CK and S100 protein are positive. No EWSR1 rearrangement is seen. The diagnosis is a low-grade myoepithelial carcinoma.
The patient received local excision without adjuvant therapy.
A case of gastric parachordoma, which is classified in the spectrum of myoepithelial tumor, has been reported.
Dual-color florescence in situ hybridization is performed to detect EWSR1 rearrangement. Positive cases were defined as those having split red and green signals separated by a distance at least twice the signal diameter in at least 20 of 100 counted tumor cells.
As for gastric tumors, myoepithelial tumor is not listed in the differential diagnoses due to lack of reports in the literature. The authors exclude other possible mimickers cautiously and render the diagnosis of low grade myoepithelial carcinoma.
This case report illustrates a rare low-grade myoepithelial carcinoma of stomach and its morphological, immunohistochemical and molecular characteristics.
P- Reviewer: Huerta-Franco MR S- Editor: Yu J L- Editor: A E- Editor: Zhang DN
| 1. | Fletcher C, Bridge JA, Hogendoorn P, Mertens F. World Health Organization classification of tumours of soft tissue and bone. International Agency for Research on Cancer. 4th ed. Lyon: IARC Press 2013; . |
| 2. | Michal M, Miettinen M. Myoepitheliomas of the skin and soft tissues. Report of 12 cases. Virchows Arch. 1999;434:393-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 101] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 3. | Hornick JL, Fletcher CD. Myoepithelial tumors of soft tissue: a clinicopathologic and immunohistochemical study of 101 cases with evaluation of prognostic parameters. Am J Surg Pathol. 2003;27:1183-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 379] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 4. | Gleason BC, Fletcher CD. Myoepithelial carcinoma of soft tissue in children: an aggressive neoplasm analyzed in a series of 29 cases. Am J Surg Pathol. 2007;31:1813-1824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 180] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 5. | Kilpatrick SE, Hitchcock MG, Kraus MD, Calonje E, Fletcher CD. Mixed tumors and myoepitheliomas of soft tissue: a clinicopathologic study of 19 cases with a unifying concept. Am J Surg Pathol. 1997;21:13-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 195] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 6. | Higashiyama M, Kodama K, Yokouchi H, Takami K, Kabuto T, Tsuji N, Mano M, Ishiguro S, Ueda T, Yoshikawa H. Myoepithelioma of the lung: report of two cases and review of the literature. Lung Cancer. 1998;20:47-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Strickler JG, Hegstrom J, Thomas MJ, Yousem SA. Myoepithelioma of the lung. Arch Pathol Lab Med. 1987;111:1082-1085. [PubMed] |
| 8. | Spivach A, Zanconati F, Tirabosco R, Falconieri G. Parachordoma of the gastric serosa: report of a myxoid mimicry in an unusual location. Int J Surg Pathol. 2007;15:307-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Gao HX, Liu CX, Zou H, Chun CP, Cui X, Chen Y, Zhang W, Qi Y, Wang N, Liang W. Parachordoma/myoepithelioma of the kidney: first report of a myxoid mimicry in an unusual location. Int J Clin Exp Pathol. 2014;7:1258-1265. [PubMed] |
| 10. | Antonescu CR, Zhang L, Chang NE, Pawel BR, Travis W, Katabi N, Edelman M, Rosenberg AE, Nielsen GP, Dal Cin P. EWSR1-POU5F1 fusion in soft tissue myoepithelial tumors. A molecular analysis of sixty-six cases, including soft tissue, bone, and visceral lesions, showing common involvement of the EWSR1 gene. Genes Chromosomes Cancer. 2010;49:1114-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 386] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 11. | Guillou L, Wadden C, Kraus M, Deitos A, Fletcher C. S-100 protein reactivity in synovial sarcomas-A potentially frequent diagnostic pitfall-Immunohistochemical analysis of 100 cases. Appl Immunohistochem. 1996;4:167-175. |
| 12. | Terry J, Saito T, Subramanian S, Ruttan C, Antonescu CR, Goldblum JR, Downs-Kelly E, Corless CL, Rubin BP, van de Rijn M. TLE1 as a diagnostic immunohistochemical marker for synovial sarcoma emerging from gene expression profiling studies. Am J Surg Pathol. 2007;31:240-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 221] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 13. | Miettinen M, Dow N, Lasota J, Sobin LH. A distinctive novel epitheliomesenchymal biphasic tumor of the stomach in young adults (“gastroblastoma”): a series of 3 cases. Am J Surg Pathol. 2009;33:1370-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Shin DH, Lee JH, Kang HJ, Choi KU, Kim JY, Park do Y, Lee CH, Sol MY, Park JH, Kim HY. Novel epitheliomesenchymal biphasic stomach tumour (gastroblastoma) in a 9-year-old: morphological, ultrastructural and immunohistochemical findings. J Clin Pathol. 2010;63:270-274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Wey EA, Britton AJ, Sferra JJ, Kasunic T, Pepe LR, Appelman HD. Gastroblastoma in a 28-year-old man with nodal metastasis: proof of the malignant potential. Arch Pathol Lab Med. 2012;136:961-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 16. | Poizat F, de Chaisemartin C, Bories E, Delpero JR, Xerri L, Flejou JF, Monges G. A distinctive epitheliomesenchymal biphasic tumor in the duodenum: the first case of duodenoblastoma? Virchows Arch. 2012;461:379-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Brandal P, Panagopoulos I, Bjerkehagen B, Gorunova L, Skjeldal S, Micci F, Heim S. Detection of a t(1; 22)(q23; q12) translocation leading to an EWSR1-PBX1 fusion gene in a myoepithelioma. Genes Chromosomes Cancer. 2008;47:558-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 18. | Brandal P, Panagopoulos I, Bjerkehagen B, Heim S. t(19; 22)(q13; q12) Translocation leading to the novel fusion gene EWSR1-ZNF444 in soft tissue myoepithelial carcinoma. Genes Chromosomes Cancer. 2009;48:1051-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |