Published online Apr 14, 2015. doi: 10.3748/wjg.v21.i14.4385
Peer-review started: October 15, 2014
First decision: November 14, 2014
Revised: November 28, 2014
Accepted: January 30, 2015
Article in press: January 30, 2015
Published online: April 14, 2015
Processing time: 182 Days and 11.8 Hours
An 80-year-old man was under annual surveillance esophagogastroduodenoscopy after endoscopic submucosal dissection (ESD) for early gastric cancer (EGC). Two years after the initial ESD, a 0-IIc type metachronous EGC lesion, 8 mm in size, without an ulcer scar, was found in the gastric antrum. The estimated tumor depth was up to the mucosa, and biopsy revealed well and poorly differentiated adenocarcinoma. ESD was performed for this lesion and en bloc resection with negative margins was achieved. Histopathological examination revealed an adenosquamous carcinoma 8 mm in size invading the deep submucosal layer (1600 μm), with lymphovascular invasion, consistent with the diagnosis of non-curative resection. Additional gastrectomy was recommended for this patient; however, two months after the ESD, preoperative computed tomography revealed multiple liver metastases, and the patient was considered as an unsuitable candidate for surgical resection. Systemic chemotherapy was therefore started; however, the patient died of gastric cancer 27 mo after the second ESD. Early gastric adenosquamous carcinoma localized to the mucosa and submucosa is extremely rare and its clinical behavior is not well known. The present report is very significant in that it underscores the distinct possibility of gastric adenosquamous carcinoma being very aggressive and fatal even when detected at an early cancer.
Core tip: Herein is described a rare case of gastric adenosquamous carcinoma which resulted in liver metastasis and gastric cancer-related death, even though the tumor was detected at an early cancer by periodic surveillance esophagogastroduodenoscopy after gastric endoscopic submucosal dissection. Early gastric adenosquamous carcinoma is extremely rare and its clinical behavior is not well known. The present study is very significant in that it underscores the distinct possibility of gastric adenosquamous carcinoma being very aggressive and fatal even when detected at an early cancer.
- Citation: Shirahige A, Suzuki H, Oda I, Sekiguchi M, Mori G, Abe S, Nonaka S, Yoshinaga S, Sekine S, Kushima R, Saito Y, Fukagawa T, Katai H. Fatal submucosal invasive gastric adenosquamous carcinoma detected at surveillance after gastric endoscopic submucosal dissection. World J Gastroenterol 2015; 21(14): 4385-4390
- URL: https://www.wjgnet.com/1007-9327/full/v21/i14/4385.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i14.4385
Adenosquamous carcinoma is an exceedingly rare tumor, accounting for only 0.14%-1.9% of all cases of gastric cancer[1-6]. It is a very aggressive cancer, and in the majority of cases, these tumors are already at an advanced stage at diagnosis, with lymph node metastasis and liver metastasis. This type of cancer is associated with a very poor prognosis, with the 5-year survival rate of 0-22%[1,3,6-8]. There are few reports on early gastric adenosquamous carcinoma localized to the mucosa and submucosa; therefore, the clinical behavior of this type of cancer is still not well known[6,9,10]. We document a rare case of gastric adenosquamous carcinoma which resulted in liver metastasis and gastric cancer-related death, even though the tumor was detected at an early cancer by periodic surveillance esophagogastroduodenoscopy (EGD) two years after curative endoscopic submucosal dissection (ESD) for early gastric cancer (EGC).
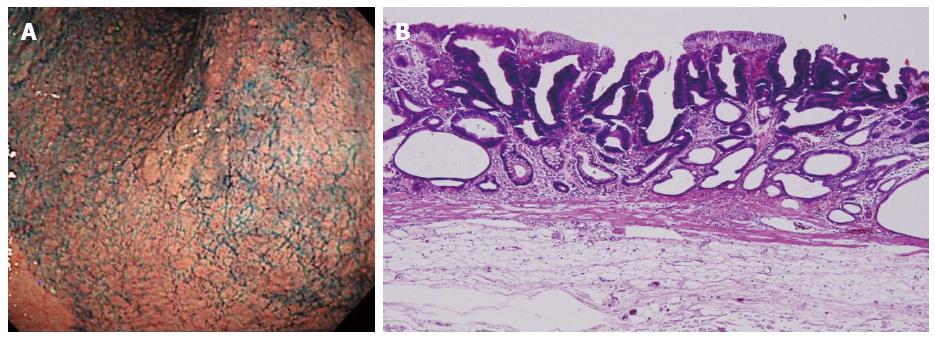
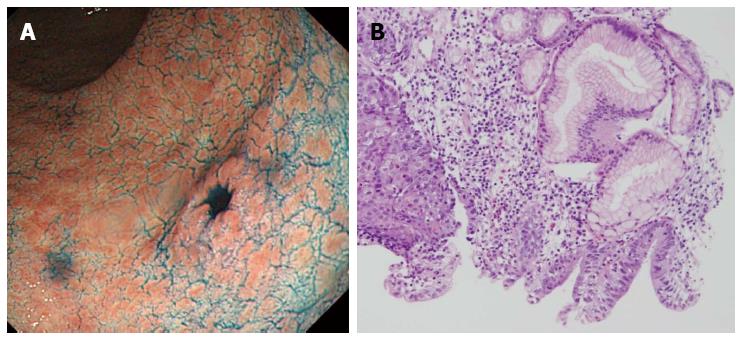
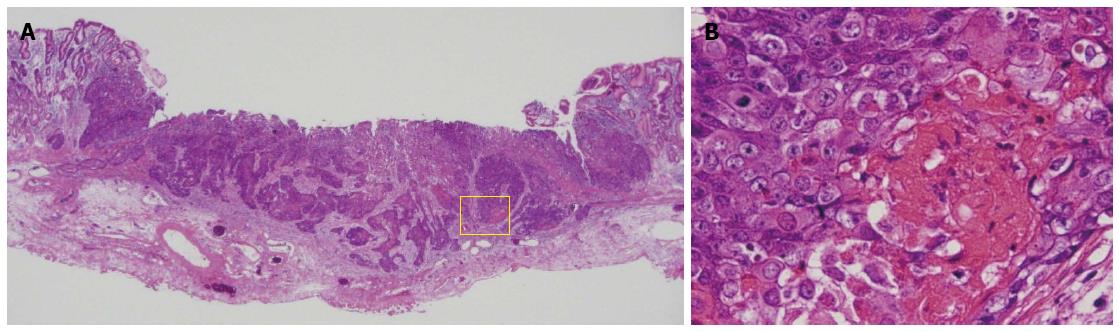
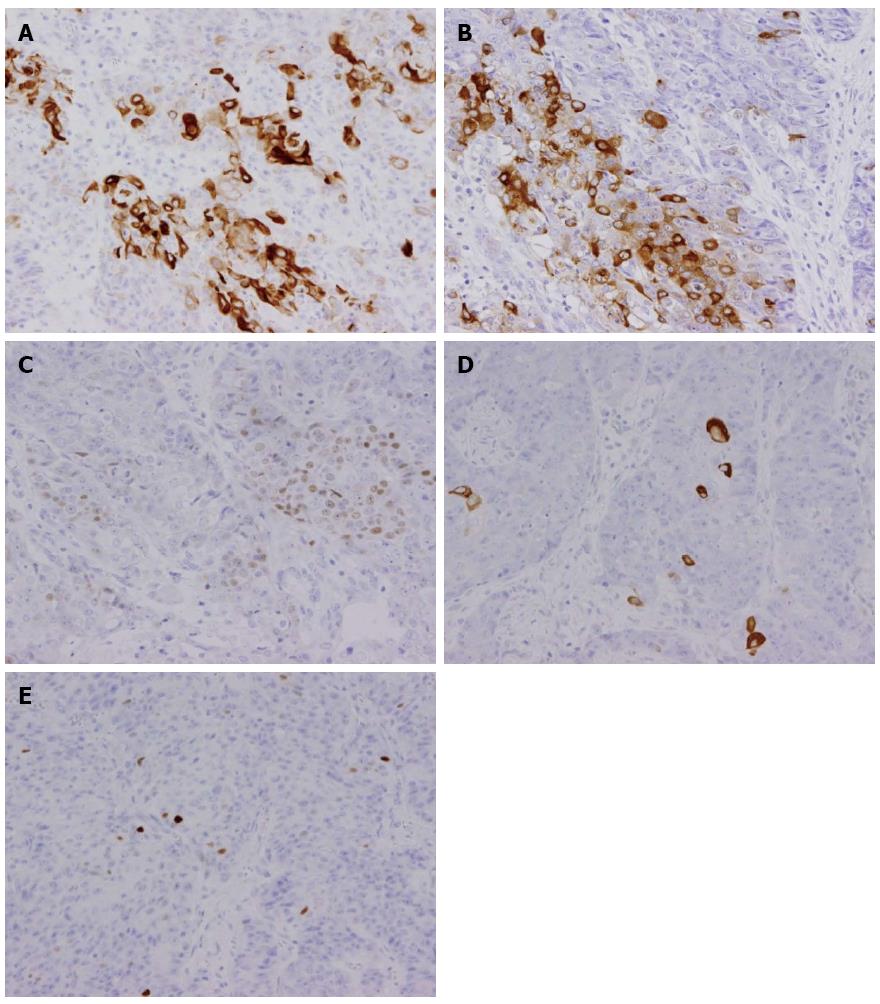
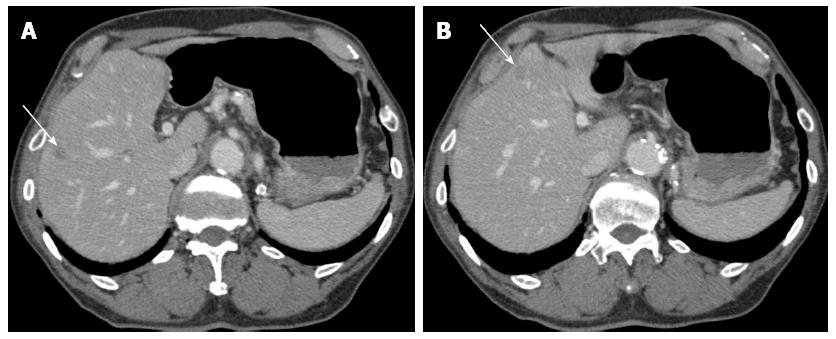
An 80-year-old man was admitted to our hospital in June 2005 for endoscopic treatment of EGC. We performed ESD for the initial EGC lesion, a 0-IIa type well differentiated adenocarcinoma limited to the mucosa measuring 10 mm in diameter, with no ulcer scar, on the lesser curvature of the middle gastric body (Figure 1). Histopathological findings revealed a well differentiated mucosal adenocarcinoma measuring 10 mm in diameter without lymphovascular involvement or ulcer scar, and tumor-free margins. This lesion fulfilled the absolute pathological criteria and was diagnosed as a curative resection[11]. After the initial ESD, EGD was performed annually, and revealed no evidence of tumor recurrence; the serum levels of tumor markers such as carcinoembryonic antigen (CEA) also remained within normal range. However, in November 2007, 0-IIc type metachronous gastric cancer (MGC), 8 mm in diameter, with no ulcer scar, was found on the greater curvature of the gastric antrum (Figure 2). The estimated tumor depth was up to the mucosa and biopsy revealed well and poorly differentiated adenocarcinoma. ESD was performed for this lesion and en bloc resection with negative margins was achieved. Histopathological examination revealed an adenosquamous carcinoma invading the deep submucosal layer (1600 μm), 8 mm in diameter, with lymphovascular invasion, consistent with the diagnosis of non-curative resection (Figure 3). Immunohistochemical staining was focally positive for CK5/6, CEA, CDX2, and a few cells were positive for CK14, P63, findings that were suggestive of adenosquamous differentiation of the tumor (Figure 4). Additional gastrectomy was recommended for the patient. However, two months after the ESD (in January 2008), preoperative computerized tomography (CT) revealed multiple liver metastases (Figure 5), and the patient was considered as an unsuitable candidate for surgical resection. Systemic chemotherapy was therefore started; however, the patient died of gastric cancer 27 mo after the second ESD.
Rolleston et al[12] first reported gastric adenosquamous carcinoma in 1905. Gastric adenosquamous carcinoma consists of both adenocarcinomatous and squamous cell carcinomatous components, more than a quarter of the tumor being composed of the squamous cell carcinoma component[13]. Gastric adenosquamous carcinoma is exceedingly rare, accounting for 0.14% to 1.9% of all cases of gastric cancer[1-6]. In the majority of cases of gastric adenosquamous carcinoma, the tumor is already at an advanced stage at diagnosis. The tumor carries a devastating prognosis, with 5-year survival rates ranging from 0 to 22%[1,3,6-8]. Early gastric adenosquamous carcinoma extending to the mucosa and submucosa is extremely rare. Samejima et al[9] first reported early gastric adenosquamous carcinoma in 1974 and there have been only 10 case reports of this type of carcinoma since, including the present case[6,9,10]. Among the 10 cases of early gastric adenosquamous carcinoma, five died of gastric cancer (50%) (four with liver metastases and one with distant lymph node metastasis). As compared to the excellent prognosis of the common type of early gastric adenocarcinoma, with 5-year survival rates of about 90%[14], the prognosis of early gastric adenosquamous carcinoma is much poorer. In addition, among the five fatal cases, including the present case, 3 were found to have liver metastasis or distant lymph node metastasis by the time of surgery; the present case had multiple liver metastases on a CT obtained two months after the ESD. Therefore, the present case report is very significant in that it underscores the distinct possibility of gastric adenosquamous carcinoma being very aggressive and fatal even when detected at an early cancer.
The histogenesis of adenosquamous carcinoma is not clear, although there are five hypotheses[1,4,6-8,10,15-17]: (1) squamous metaplasia of an adenocarcinoma; (2) cancerization of metaplastic non-neoplastic squamous cells; (3) cancerization of ectopic squamous epithelium; (4) differentiation of multipotential undifferentiated cancer cells toward both squamous and glandular cells; and (5) collision of concurrent adenocarcinoma and squamous cell carcinoma. Most of the squamous cell carcinomatous components were located at an invasive site, in contrast to the adenocarcinomatous components being located in the mucosal region adjacent to the squamous cell carcinoma component. Therefore, many authors now believe that the squamous neoplastic component results from metaplastic change of the adenocarcinoma component. Our present case also showed pathological findings that were consistent with this hypothesis.
The tumor in the present patient was detected as an MGC during periodic EGD two years after curative ESD for an EGC lesion fulfilling the absolute indications for ESD[11]. Higher incidence of MGCs (in the range of 1.8% to 12.8%) after endoscopic treatment for EGC has been reported previously[18-22]. Among the 1537 EGC patients who underwent curative ESD for absolute indications or for expanded indications from 1999 to 2006 at our hospital, a total of 348 MGC lesions were detected in 244 patients (15.9%), consistent with previous reports. Unfortunately, we encountered seven cases of MGC with gastric cancer-related death, even though radical resection can be performed in most cases. Among these, in four cases, the advanced MGC was detected over 5 years after the initial ESD, because periodic surveillance examinations were not performed over 5 years. On the other hand, in the remaining three patients including the present case, the MGCs were of a higher malignancy grade at the time of their detection, despite the patient receiving periodic follow-up examinations. Thus, it is necessary to bear in mind the possibility of development of such fatal MGCs during periodic follow-up examinations after curative gastric ESD.
In conclusion, we have described a rare case of submucosal invasive gastric adenosquamous carcinoma that resulted in gastric cancer-related death, even though this tumor was detected at an early cancer during periodic surveillance examinations after curative gastric ESD.
An 80-year-old man was under annual surveillance esophagogastroduodenoscopy (EGD) after endoscopic submucosal dissection (ESD) for early gastric cancer (EGC).
0-IIc type metachronous gastric cancer.
Common type of early gastric cancer.
The serum levels of tumor markers such as carcinoembryonic antigen were within normal range.
EGD revealed small gastric cancer clinically estimated as intramucosal lesion.
Histopathological examination of ESD resected specimen revealed an adenosquamous carcinoma invading the deep submucosal layer with lymphovascular invasion.
The patient received systemic chemotherapy for multiple liver metastases detected after the ESD.
Early gastric adenosquamous carcinoma localized to the mucosa and submucosa is extremely rare and its clinical behavior is not well known.
Gastric adenosquamous carcinoma consists of both adenocarcinomatous and squamous cell carcinomatous components, more than a quarter of the tumor being composed of the squamous cell carcinoma component.
Herein is described a rare case of gastric adenosquamous carcinoma which resulted in liver metastasis and gastric cancer-related death, even though the tumor was detected at an early cancer by periodic surveillance EGD after gastric ESD.
This case report reveals the distinct possibility of gastric adenosquamous carcinoma being very aggressive and fatal even when detected at an early cancer.
P- Reviewer: Kiriyama S S- Editor: Yu J L- Editor: Logan S E- Editor: Ma S
| 1. | Straus R, Heschel S, Fortmann DJ. Primary adenosquamous carcinoma of the stomach. A case report and review. Cancer. 1969;24:985-995. [PubMed] |
| 2. | Aoki Y, Tabuse K, Wada M, Katsumi M, Uda H. Primary adenosquamous carcinoma of the stomach: experience of 11 cases and its clinical analysis. Gastroenterol Jpn. 1978;13:140-145. [PubMed] |
| 3. | Nakamura K, Ueyama T, Yao T, Xuan ZX, Ambe K, Adachi Y, Yakeishi Y, Matsukuma A, Enjoji M. Pathology and prognosis of gastric carcinoma. Findings in 10,000 patients who underwent primary gastrectomy. Cancer. 1992;70:1030-1037. [PubMed] |
| 4. | Toyota N, Minagi S, Takeuchi T, Sadamitsu N. Adenosquamous carcinoma of the stomach associated with separate early gastric cancer (type IIc). J Gastroenterol. 1996;31:105-108. [PubMed] |
| 5. | Kim YS, Heo WS, Chae KH, Gang YS, Jung JH, Kim SH, Seong JK, Lee BS, Jeong HY, Song KS. [Clinicopathological features and differences of p53 and Ki-67 expression in adenosquamous and squamous cell carcinomas of the stomach]. Korean J Gastroenterol. 2006;47:425-431. [PubMed] |
| 6. | Maeda M, Taniguchi H, Sekiguchi S, Katai H, Kushima R. Adenosquamous cell carcinoma of the stomach - A clinicopathologic analysis of 23 cases. Stomach and Intestine (abstract in English). 2010;45:1959-1966. |
| 7. | Altshuler JH, Shaka JA. Squamous cell carcinoma of the stomach. Review of the literature and report of a case. Cancer. 1966;19:831-838. [PubMed] |
| 8. | Mori M, Iwashita A, Enjoji M. Adenosquamous carcinoma of the stomach. A clinicopathologic analysis of 28 cases. Cancer. 1986;57:333-339. [PubMed] |
| 9. | Samejima Y, Mizuno T, Sasakawa M, Takemura H, Watanabe A, Nagamatsu M. A case report of early gastric adenocarcinoma and brief review of its literature. Stomach and Intestine (abstract in English). 1974;9:783-788. |
| 10. | Yoshida K, Manabe T, Tsunoda T, Kimoto M, Tadaoka Y, Shimizu M. Early gastric cancer of adenosquamous carcinoma type: report of a case and review of literature. Jpn J Clin Oncol. 1996;26:252-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1723] [Cited by in RCA: 1895] [Article Influence: 135.4] [Reference Citation Analysis (0)] |
| 12. | Rolleston HD, Trevor RS. A case of columnar-celled carcioma of stomach showing squamous celled metaplasia. J Pathol Bacteriol. 1905;10:418-422. [RCA] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Japanese Research Society for Gastric Cancer: Japanese Classification of Gastric Carcinoma. 1st ed. Tokyo: Kanehara-Shuppan publisher 1995; 42. |
| 14. | Nashimoto A, Akazawa K, Isobe Y, Miyashiro I, Katai H, Kodera Y, Tsujitani S, Seto Y, Furukawa H, Oda I. Gastric cancer treated in 2002 in Japan: 2009 annual report of the JGCA nationwide registry. Gastric Cancer. 2013;16:1-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 372] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 15. | Sailer S. Diffuse metaplastic gastritis in a patient with prolonged cachexia and macrocystic anemia. Arch Pathol. 1943;35:730-743. |
| 16. | Wood DA. Adenoacanthoma of the pyloric end of the stomach. Arch Pathol. 1943;36:177-189. |
| 17. | Boswell JT, Helwig EB. Squamous cell carcinoma and adenoacanthoma of the stomach. a clinicopathologic study. Cancer. 1965;18:181-192. [PubMed] |
| 18. | Takekoshi T, Baba Y, Ota H, Kato Y, Yanagisawa A, Takagi K, Noguchi Y. Endoscopic resection of early gastric carcinoma: results of a retrospective analysis of 308 cases. Endoscopy. 1994;26:352-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 135] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Kojima T, Parra-Blanco A, Takahashi H, Fujita R. Outcome of endoscopic mucosal resection for early gastric cancer: review of the Japanese literature. Gastrointest Endosc. 1998;48:550-54; discussion 550-54;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 179] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 20. | Nakajima T, Oda I, Gotoda T, Hamanaka H, Eguchi T, Yokoi C, Saito D. Metachronous gastric cancers after endoscopic resection: how effective is annual endoscopic surveillance? Gastric Cancer. 2006;9:93-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 170] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 21. | Kobayashi M, Narisawa R, Sato Y, Takeuchi M, Aoyagi Y. Self-limiting risk of metachronous gastric cancers after endoscopic resection. Dig Endosc. 2010;22:169-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Han JS, Jang JS, Choi SR, Kwon HC, Kim MC, Jeong JS, Kim SJ, Sohn YJ, Lee EJ. A study of metachronous cancer after endoscopic resection of early gastric cancer. Scand J Gastroenterol. 2011;46:1099-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |