Published online Apr 14, 2015. doi: 10.3748/wjg.v21.i14.4365
Peer-review started: July 20, 2014
First decision: August 15, 2014
Revised: September 10, 2014
Accepted: October 15, 2014
Article in press: October 15, 2014
Published online: April 14, 2015
Processing time: 268 Days and 21.9 Hours
AIM: To investigate the correlation between Kirsten rat sarcoma viral oncogene homolog (KRAS) status and the therapeutic effects of anti-epidermal growth factor receptor (EGFR) monoclonal antibodies (MoAbs) in metastatic colorectal cancer (mCRC).
METHODS: Randomized controlled trials (RCTs) were identified and the association between KRAS mutation and clinical outcome in mCRC patients treated with anti-EGFR MoAbs was investigated. Ten RCTs were included in this meta-analysis. Progression-free survival and overall survival were used to assess the strength of the relationship between KRAS mutation and clinical outcome.
RESULTS: In first-line treatment, survival benefit was confined to patients with wild-type KRAS. Chemotherapy regimens and angiogenesis inhibitor treatment influenced the results of the analysis. Wild-type KRAS mCRC patients did not seem to benefit from oxaliplatin-based chemotherapy (PFS: HR = 0.88, 95%CI: 0.70-1.10; OS: HR = 0.93, 95%CI: 0.82-1.04). Clinical benefit in mCRC patients was limited to therapeutic regimens which included anti-EGFR MoAbs and fluorouracil-based therapy (PFS: HR = 0.77, 95%CI: 0.69-0.86; OS: HR = 0.85, 95%CI: 0.75-0.95). When anti-EGFR MoAbs were used as second- or further-line treatment, clinical benefit was still confined to patients with wild-type KRAS.
CONCLUSION: KRAS status is a potential predictive marker of clinical benefit due to anti-EGFR MoAb therapy in mCRC patients.
Core tip: In this study, we evaluated the correlation between Kirsten rat sarcoma viral oncogene homolog (KRAS) status and the therapeutic effects of anti-epidermal growth factor receptor (EGFR) monoclonal antibodies (MoAbs) in patients with metastatic colorectal cancer. We focused on the relationship between KRAS status and the curative effect of anti-EGFR MoAbs in patients with metastatic colorectal cancer, and conducted a systematic meta-analysis of chemotherapy regimens, line of treatment and bevacizumab treatment. This analysis provides the first evidence that patients with wild-type KRAS metastatic colorectal cancer may not benefit from anti-EGFR MoAbs and oxaliplatin-based therapy as first-line treatment. Clinical benefit was confined to therapeutic regimens which included anti-EGFR MoAbs and fluorouracil-based therapy.
- Citation: Song QB, Wang Q, Hu WG. Anti-epidermal growth factor receptor monoclonal antibodies in metastatic colorectal cancer: A meta-analysis. World J Gastroenterol 2015; 21(14): 4365-4372
- URL: https://www.wjgnet.com/1007-9327/full/v21/i14/4365.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i14.4365
Colorectal cancer is one of the most common human malignant diseases and a leading cause of cancer-related death worldwide, accounting for approximately of all cancer incidence and mortality[1]. Over the last decade, the availability of combination chemotherapy and targeted agents has improved the median survival of patients with metastatic colorectal cancer (mCRC)[2,3]. Two biological agents, the monoclonal antibodies (MoAbs), cetuximab and panitumumab, which target the epidermal growth factor receptor (EGFR) have been approved by the food and drug administration (FDA) for mCRC. Kirsten rat sarcoma viral oncogene homolog (KRAS) mutations have emerged as major predictive markers of resistance to anti-EGFR MoAbs. These observations have been shown to be beneficial in small single-arm data and some retrospective analyses of large phase III studies[4-7]. The results of previous reviews demonstrated that mCRC patients with mutant KRAS did not benefit from treatment with anti-EGFR MoAbs either alone or in combination with standard chemotherapy. However, these reviews included data from retrospective and non-randomized studies. Following the completion of several large phase III clinical trials, the role of KRAS mutation in mCRC should be redefined. We aimed to provide a comprehensive evaluation of the relationship between KRAS status and the therapeutic effects of anti-EGFR MoAbs in mCRC patients. Analyses were conducted on chemotherapy regimens, line of treatment and bevacizumab treatment.
Systematic computerized searches of PubMed (up to December 14, 2013) were performed. The search was further augmented by checking the clinical trial registry (http://www.clinicaltrials.gov) for additional studies. The following search terms were used: “metastatic rectal cancer”, “metastatic colon cancer”, “metastatic colorectal cancer”, “mCRC”, “KRAS”, “cetuximab’’, “panitumumab”, “monoclonal antibodies”, “MoAb”. The search was limited to human studies. All eligible studies were retrieved, and their bibliographies were examined for other relevant publications. The results of a randomized controlled trial are often published in a series of articles, thus when the same data were used in several publications, the most recent, largest or complete study of these publications was included in this meta-analysis.
The included studies met the following criteria: (1) Randomized controlled trials published as articles which compared anti-EGFR MoAbs plus chemotherapy or best supportive care (BSC) with chemotherapy or BSC alone in patients with mCRC; (2) Studies evaluating the relationship between KRAS mutation status and response to anti-EGFR MoAbs in mCRC patients; (3) Provide adequate data on progression-free survival (PFS) and overall survival (OS); and (4) Studies with full text articles.
Information was carefully extracted from all eligible studies. The following data were collected from each study: first author’s name, year of publication, number of patients screened, study treatment protocols, response criteria, number of patients by KRAS mutation status, line of treatment, PFS and OS. The clinical endpoints were extracted separately according to KRAS status. Data extraction was performed independently by two of the authors. Disagreement was resolved by discussion between the two authors. If the two authors could not reach a consensus, another author was consulted and a final decision was made by voting.
The primary endpoints were PFS and OS. The association between KRAS status and PFS or OS was expressed as the hazard ratio (HR). Heterogeneity was assessed by the Q-test[8,9]. If significant heterogeneity was found (P < 0.10, I2 > 40%), the random-effects model instead of the fixed-effects model was used for further analysis. To investigate the possible sources of heterogeneity, we conducted subgroup analyses based on the following aspects: the use of bevacizumab, chemotherapy regimen, and the selection of fluorouracil or capecitabine. Egger’s linear regression test[10] was used to assess publication bias, which was adjusted using the trim-and-fill method. All the statistical tests used in this meta-analysis were performed with RevMan 5.4 and STATA version 10.0 (Stata Corporation, College Station, TX, United States).
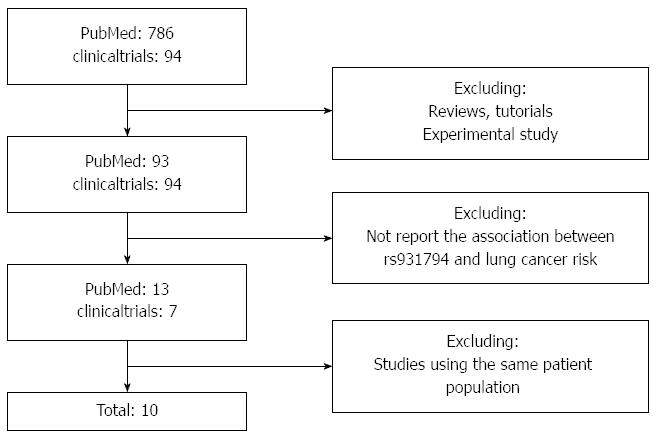
After exclusion of duplicate and irrelevant studies (Figure 1), 10 studies[7,11-19] were identified according to the inclusion criteria of the meta-analysis. Tables 1 and 2 show the primary characteristics of the 10 studies which included patients treated with anti-EGFR MoAbs, all of which were randomized controlled trials. All 10 studies included a total of 8943 patients, and sample sizes ranged from 337 to 1630. KRAS status was available in 7614 patients, 4451 patients had wild-type KRAS and 3163 patients had mutant KRAS. Of the 10 studies, MoAbs were administered as first-line treatment with chemotherapy in 7 studies and MoAbs were administered as second-line or further-line treatment with or without chemotherapy in 3 studies. Hecht et al[14] used two different chemotherapy regimens (FOLFIRI or FOLFOX) combined with cetuximab, and we divided this study into two parts in order to conduct an analysis of chemotherapy regimen. Response Evaluation Criteria in Solid Tumor (RECIST) criteria or WHO criteria were used to classify tumor response in all studies.
| Ref. | Year | Patients assessed | KRAS wt | KRAS mut | Response criteria |
| 1st line treatment | |||||
| Maughan et al[11] | 2011 | 1630 | 729 | 565 | RECIST |
| Van Cutsem et al[12] | 2011 | 1198 | 666 | 397 | WHO |
| Bokemeyer et al[7] | 2011 | 337 | 179 | 136 | WHO |
| Douillard et al[13] | 2010 | 1183 | 656 | 440 | RECIST |
| Hecht et al[14] | 2009 | 823 | 404 | 260 | RECIST |
| Hecht et al[14] | 2009 | 230 | 115 | 86 | RECIST |
| Tol et al[15] | 2009 | 755 | 314 | 206 | RECIST |
| Tveit et al[16] | 2012 | 566 | 303 | 195 | RECIST |
| 2nd line treatment | |||||
| Peeters et al[17] | 2010 | 1186 | 597 | 486 | RECIST |
| Harbison et al[18] | 2012 | 572 | 245 | 208 | RECIST |
| Amado et al[19] | 2008 | 463 | 243 | 184 | RECIST |
| Ref. | Study treatment protocol |
| 1st line treatment | |
| Maughan et al[11] | Ox 85 mg/m2 FA 350 mg/m2 5FU 400 mg/m2 bolus + 2400 mg/m2 infusion ± cetuximab |
| Ox 130 mg/m2 Cap 2000 mg/m2± cetuximab | |
| Van Cutsem et al[12] | Iri 180 mg/m2 FA 400 mg/m2 5FU 400 mg/m2 bolus + 2400 mg/m2 infusion ± cetuximab |
| Bokemeyer et al[7] | Ox 85 mg/m2 FA 400 mg/m2 5FU 400 mg/m2 bolus + 1200 mg/m2 infusion ± cetuximab |
| Douillard et al[13] | Ox 85 mg/m2 FA 400 mg/m2 5FU 400 mg/m2 bolus + 1200 mg/m2 infusion ± panitumumab |
| Hecht et al[14] | Ox/FA/5FU/Bev |
| Hecht et al[14] | Iri/FA/5FU/Bev |
| Tol et al[15] | Ox/Cap/Bev |
| Tveit et al[16] | Ox 85 mg/m2 FA 120 mg/m2 5FU 1000 mg/m2 bolus ± cetuximab |
| 2nd line treatment | |
| Peeters et al[17] | Iri 180 mg/m2 FA 400 mg/m2 5FU 400 mg/m2 bolus + 2400 mg/m2 infusion ± panitumumab |
| Harbison et al[18] | BSC ± cetuximab |
| Amado et al[19] | BSC ± panitumumab |
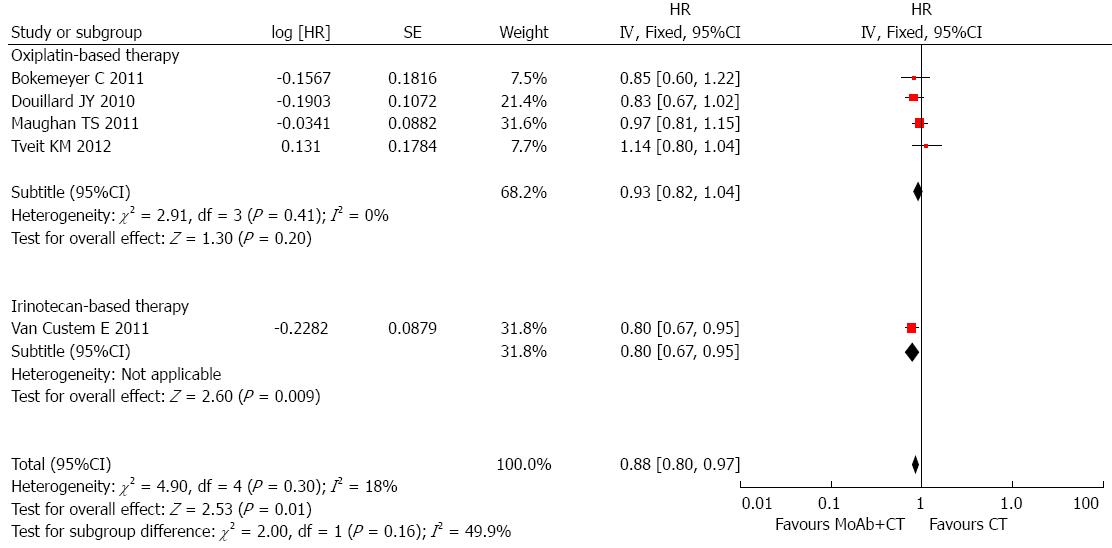
Patients with wild-type KRAS: In total, seven studies supplied adequate information on OS in patients with known KRAS status. The HR summarized survival in the arm treated with cetuximab combined with chemotherapy compared with the arm treated with chemotherapy alone. An HR of more than 1 indicated worse survival in patients treated with chemotherapy alone. There was no evidence of improved OS in patients with wild-type KRAS treated with MoAbs (HR = 1.00, 95%CI: 0.82-1.21), and significant intergroup heterogeneity was observed (I2 = 73%, P = 0.002). In these seven studies, two studies used bevacizumab in both arms. Subgroup analysis was carried out, the pooled HR of trials using bevacizumab was 1.79 (95%CI: 1.26-2.54) and the pooled HR of trials using chemotherapy was 0.88 (95%CI: 0.80-0.97). We excluded trials using bevacizumab and conducted different subgroup analyses to determine heterogeneity. In the trials using oxaliplatin-based chemotherapy, the pooled HR was 0.93 (95%CI: 0.82-1.04), and the P value of Egger’s test was 0.831. Only one study used irinotecan-based chemotherapy, and the HR was 0.80 (95%CI: 0.67-0.95; Figure 2). The relationship between chemotherapy regimen and clinical benefit in mCRC patients with wild-type KRAS treated with anti-EGFR MoAbs needs to be explored in further studies. However, patients with wild-type KRAS did not seem to benefit from anti-EGFR MoAbs and oxaliplatin-based chemotherapy as first-line treatment (HR = 0.93, 95%CI: 0.82-1.04, P = 0.20; Figure 2). Timothy S Maughan’s study supplied sufficient information on the HR of patients treated with fluorouracil and those treated with capecitabine. Therefore, we divided this study into two different studies and conducted another subgroup analysis. The subgroup analysis indicated that fluorouracil and anti-EGFR MoAbs benefited patients in terms of longer OS (HR = 0.85, 95%CI: 0.75-0.95; I2 = 10%, P = 0.34). However, only one trial used capecitabine with anti-EGFR MoAbs and no OS benefit was observed (HR = 0.97, 95%CI: 0.81-1.15).
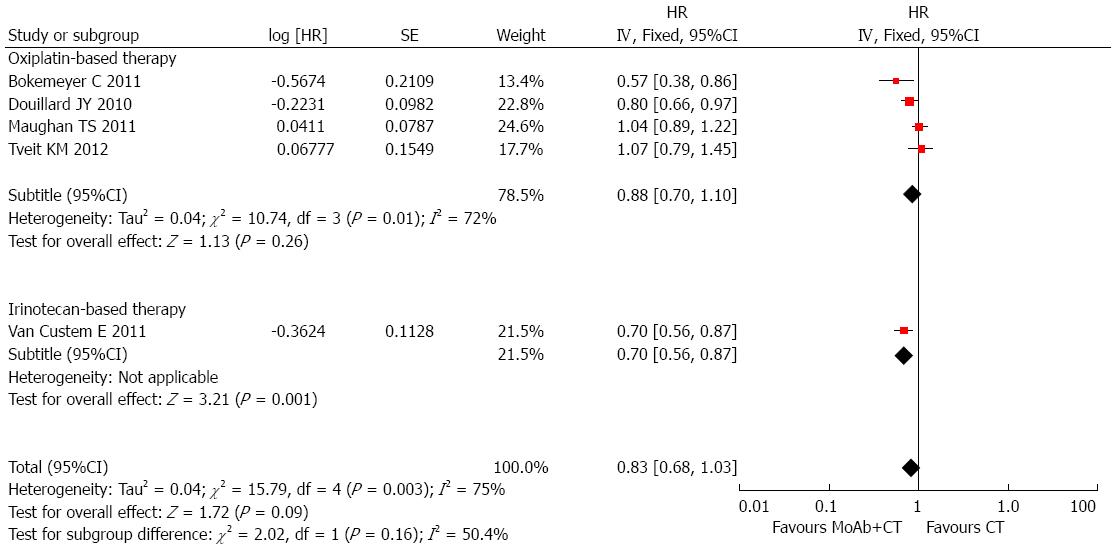
Information on PFS in wild-type KRAS patients was available in 7 studies, and included 6722 patients. KRAS status was detected in 5651 patients and 3366 of these patients had wild-type KRAS. Anti-EGFR MoAbs had no benefit on PFS in trials of first-line treatment (HR = 0.96, 95%CI: 0.79-1.16), with significant intertrial heterogeneity (I2 = 77%, P < 0.0001), and the P value of Egger’s test was 0.824. In the trials using bevacizumab, the pooled HR was 1.27 (95%CI: 1.06-1.51). There was no significant heterogeneity or publication bias between these trials (I2 = 0%, P = 0.59; P value of Egger’s test was 0.571). The pooled HR of trials using conventional chemotherapy was 0.83 (95%CI: 0.68-1.03), and there was evidence of differences in the effect of MoAbs between trials (I2 = 75%, P = 0.0003). No publication bias was found using Egger’s test (P = 0.387). A subgroup analysis was performed to explore the sources of heterogeneity between studies using conventional chemotherapy. In the trials using oxaliplatin-based therapy, the pooled HR was 0.88 (95%CI: 0.70-1.10; I2 = 73%, P = 0.002). Only one trial used irinotecan-based chemotherapy, and the HR was 0.70 (95%CI: 0.56-0.87; Figure 3). In the subgroup analysis based on fluorouracil use, an improvement in PFS with MoAbs was observed, the pooled HR was 0.77 (95%CI: 0.69-0.86), with no publication bias (P = 0.786), however, significant heterogeneity was found (I2 = 48%, P = 0.10). This heterogeneity may have been due to a different fluorouracil dosage form. In Tveit KM’s trial, fluorouracil was given orally and was given intravenously in the other studies. Clinical benefit in mCRC patients with wild-type KRAS was confined to therapeutic regimens which included anti-EGFR MoAbs and fluorouracil based therapy.
Patients with mutant KRAS: For patients with mutant KRAS, there was no survival advantage when anti-EGFR MoAbs were administered as first-line treatment. The pooled HR of PFS was 1.18 (95%CI: 1.06-1.31), with no heterogeneity (I2 = 37%, P = 0.15). The HR of OS was 1.10 (95%CI: 0.98-1.22). There was no significant heterogeneity between these trials. It is clear that the therapeutic effect of anti-EGFR MoAbs was dependent on KRAS status.
Patients with wild-type KRAS: Three studies supplied adequate information on OS in patients with KRAS mutation, two of these studies used BSC as the control group and BSC + anti-EGFR MoAbs as the experimental group. No significant benefit was observed when anti-EGFR MoAbs were used as second- or further-line treatment (HR = 0.81, 95%CI: 0.65-1.02), and heterogeneity was noted between the trials (I2 = 61%, P = 0.08). No publication bias was observed using Egger’s test (P = 0.773). There was a significant improvement in PFS following second-line or further treatment (HR = 0.52, 95%CI: 0.36-0.75), although heterogeneity was noted between these studies (I2 = 84%, P = 0.002). Egger’s test showed that no publication bias existed (P = 0.155). We excluded Marc Peeters’s study as a different therapeutic regimen was used and conducted a new meta-analysis. The pooled HR was 0.43 (95%CI: 0.36-0.53), with no heterogeneity (I2 = 0%, P = 0.73).
Patients with mutant KRAS: Data on survival and KRAS mutation in mCRC patients were reported in three studies involving 2221 patients. There was no evidence of survival benefit with anti-EGFR MoAbs in second-line or further-line treatment in OS (HR = 0.95, 95%CI: 0.82-1.11; I2 = 0%, P = 0.87, Egger’s test: P = 0.807) or PFS (HR = 0.95, 95%CI: 0.82-1.11; I2 = 12%, P = 0.32, Egger’s test: P = 0.358).
The main downstream signaling pathway of EGFR regulates crucial biological activities such as cell differentiation, cell survival, cell proliferation and cell migration[20]. Mutations of members of the downstream signaling pathways have been shown to result in resistance to anti-EGFR MoAbs. KRAS was the first molecular predictor shown to influence responsiveness to EGFR-targeted treatment with cetuximab and panitumumab. However, these data were mainly from retrospective and non-randomized studies. We focused on the relationship between KRAS status and the curative effect of anti-EGFR MoAbs in mCRC patients, and conducted a systematic meta-analysis of chemotherapy regimens, line of treatment and bevacizumab treatment.
For patients with wild-type KRAS receiving MoAbs as first-line treatment no evidence of improved OS was observed. The subgroup analysis indicated a detrimental effect when anti-angiogenic agents were added to anti-EGFR MoAbs in the first-line treatment of mCRC. In theory, targeting both EGFR and VEGF pathways should increase curative effect[21]. A possible explanation for these findings may be that the combination of different antibodies increased toxicity and adverse effects. Another explanation may be that a negative pharmacokinetic interaction between these two biological agents occurred. After excluding trials using bevacizumab, another subgroup analysis was conducted to determine heterogeneity between trials using chemotherapy. We found that wild-type KRAS mCRC patients did not seem to benefit from anti-EGFR MoAbs and oxaliplatin-based chemotherapy as first-line treatment. Fluorouracil use influenced the OS of patients. Clinical benefit in mCRC patients with wild-type KRAS was confined to therapeutic regimens with anti-EGFR MoAbs and fluorouracil-based therapy. It was uncertain from our results whether combination of anti-EGFR MoAbs and capecitabine was effective in mCRC patients, since only one trial used capecitabine in this analysis. However, there were several studies have suggested that capecitabine might be less effective than 5-FU in first line treatment of mCRC patients[22-24]. The association between the chemotherapy regimen and clinical benefit in wild-type KRAS mCRC patients treated with anti-EGFR MoAbs needs to be explored in further studies due to limited available research.
Information on PFS in wild-type KRAS patients was available in 7 studies, and no benefit in PFS was observed in trials of first-line therapy. The subgroup analyses corresponded with the results from subgroup analyses of OS. After excluding trials using bevacizumab, the pooled HR of trials using chemotherapy was 0.83 (95%CI: 0.68-1.03), and significant heterogeneity was observed. Oxaliplatin-based chemotherapy with anti-EGFR MoAbs failed to prolong PFS in patients with wild-type KRAS. However, significant benefit in terms of PFS was observed in trials using MoAbs and fluorouracil. The subgroup analyses failed to explain the sources of heterogeneity. This may be due to different treatment protocols, such as different fluorouracil dosage forms. In Tveit KM’s trial fluorouracil was given orally and was given intravenously in the other studies.
In trials using anti-EGFR MoAbs as second- or further-line treatment, no OS benefit was observed between the control group and experimental group in patients with wild-type KRAS. However, there was significant benefit in PFS. Heterogeneity disappeared after excluding Marc Peeters’s study. The reason for the heterogeneity between these trials was due to different therapeutic regimens. Marc Peeters’s study was the only study to use chemotherapy combined with anti-EGFR MoAbs as the therapeutic regimen, the other two studies used BSC combined with anti-EGFR MoAbs.
From this meta-analysis, it is clear that the clinical benefit of anti-EGFR MoAbs is dependent on KRAS status. For patients with mutant KRAS, there was no survival advantage when anti-EGFR MoAbs were used in any line of treatment.
Our results are in line with those of other studies. Zhang et al[25] evaluated the predictive value of KRAS mutation status and showed a clinical benefit in PFS with anti-EGFR MoAbs in patients with wild-type KRAS, and there was no significant difference in OS between cetuximab-based chemotherapy and chemotherapy alone. However, only 4 RCTs were included in their analysis. Most recently, Vale and colleagues[26] included 10 studies to examine anti-EGFR MoAbs treatment and found that there were clear benefits of anti-EGFR MoAbs in any line of treatment, when used with infusional 5FU-based regimens. In the present meta-analysis, 10 studies were included in the final analysis. We compiled years of research from several RCTs, and updated the conclusion regarding the relationship between KRAS status and the therapeutic effects of anti-EGFR MoAbs in mCRC patients. In this article, analyses of the therapeutic effects of anti-EGFR MoAbs in untreated patients and those who received prior chemotherapy were performed separately. This provided the first evidence that patients with wild-type KRAS did not benefit from anti-EGFR MoAbs with oxaliplatin-based therapy as first-line treatment. Clinical benefit in wild-type KRAS patients was confined to therapeutic regimens which included anti-EGFR MoAbs and fluorouracil-based therapy.
Several limitations of our meta-analysis need to be considered when interpreting these findings. Firstly, our results were based on unadjusted estimates, and a more precise analysis should be conducted with more detailed data based on adjusted estimates for other prognostic factors such as sex, age, tumor location and other biomarkers. Furthermore, although the initial trials were conducted prospectively, tissue specimens were only available for a small percentage of patients which may have resulted in selection bias. Secondly, chemotherapy regimens which were combined with anti-EGFR MoAbs may have influenced the therapeutic effect. The chemotherapy regimen that will provide most benefit to patients when combined with anti-EGFR MoAbs needs to be explored in large randomized trials. In this meta-analysis, oxaliplatin-based chemotherapy did not benefit patients with wild-type KRAS when combined with anti-EGFR MoAbs as first-line treatment. In addition, the number of trials of second- or further-line treatment was too small to drawn an accurate conclusion. This was due to poor recruitment and influenced the findings in our meta-analysis. Finally, although most mutations in KRAS were found at codons 12 and 13 of exon 2, more than 3000 point mutations have been detected in KRAS and different point mutations have different effects on KRAS activity. The recognition of additional point mutations in KRAS and other key members of the EGFR downstream signal pathway may alter the conclusions drawn today.
In conclusion, this meta-analysis provided evidence that KRAS status is a predictive marker for the clinical benefit of anti-EGFR MoAbs in mCRC patients. Only patients with wild-type KRAS will benefit from first-line or further-line treatment with anti-EGFR MoAbs, and patients with mutant KRAS will not benefit. Line of treatment, chemotherapy regimen and combination with bevacizumab influence the clinical therapeutic effects of anti-EGFR MoAbs in these patients. This meta-analysis also provided the first evidence that wild-type KRAS mCRC patients do not benefit from anti-EGFR MoAbs with oxaliplatin-based therapy as first-line treatment. Clinical benefit was confined to therapeutic regimens which included anti-EGFR MoAbs and fluorouracil-based therapy.
Colorectal cancer is one of the most common human malignant diseases and the leading cause of cancer-related death worldwide. Over the last decade, the availability of combination chemotherapy and targeted agents has improved the median survival of patients with metastatic colorectal cancer. Monoclonal antibodies which target the epidermal growth factor receptor have been approved by the FDA for metastatic colorectal cancer. KRAS mutations have emerged as major predictive markers of resistance to anti-EGFR MoAbs. However, these results were obtained from retrospective and non-randomized studies.
A meta-analysis was used to evaluate the relationship between KRAS status and the therapeutic effects of anti-EGFR MoAbs in mCRC patients.
In first-line treatment, survival benefit was confined to patients with wild-type KRAS. Chemotherapy regimens and angiogenesis inhibitor treatment influenced the results of the analysis. Wild-type KRAS mCRC patients did not seem to benefit from oxaliplatin-based chemotherapy (PFS: HR = 0.88, 95%CI: 0.70-1.10; OS: HR = 0.93, 95%CI: 0.82-1.04). Clinical benefit in mCRC patients was limited to therapeutic regimens wihich included anti-EGFR MoAbs and fluorouracil-based therapy (PFS: HR = 0.77, 95%CI: 0.69-0.86; OS: HR = 0.85, 95%CI: 0.75-0.95). When anti-EGFR monoclonal antibodies were used as second- or further-line treatment, clinical benefit was still confined to patients with wild-type KRAS. This meta-analysis provides the first evidence that wild-type KRAS mCRC patients did not benefit from anti-EGFR MoAbs with oxaliplatin-based therapy as first-line treatment. Clinical benefit was confined to therapeutic regimens which included anti-EGFR MoAbs and fluorouracil-based therapy.
Although the initial trials were conducted prospectively, tissue specimens were only available for a small percentage of patients which may have resulted in selection bias. Large prospective clinical trials are required to validate this conclusion. Furthermore, it is apparent that mCRC is a heterogeneous disease with numerous activation mutations in oncogenes and inactivation mutations in tumor suppressor genes. The identification of mutations in other members of the EGFR pathway and the synergetic effect of multiple genetic mutations in the oncogenic signaling cascades may increase the predictive ability of biomarkers.
Anti-EGFR MoAbs: Cetuximab and panitumumab are examples of monoclonal antibodies aimed at the epidermal growth factor receptor. However, the former is of the IgG1 type, the latter of the IgG2 type; and the consequences on antibody-dependent cellular cytotoxicity can be quite different. Monoclonal antibodies block the extracellular ligand binding domain. With the binding site blocked, signal molecules can no longer attach there and activate the tyrosine kinase. KRAS: The KRAS gene provides instructions for making a protein called K-Ras which is involved primarily in regulating cell division. As part of a signaling pathway known as the RAS/MAPK pathway, the protein relays signals from outside the cell to the cell nucleus. These signals instruct the cell to grow and divide or to mature and determine cell differentiation, cell survival, cell proliferation and cell migration. Bevacizumab: Bevacizumab is an angiogenesis inhibitor, and slows the growth of new blood vessels. It is licensed to treat various cancers, including colorectal, lung, breast, glioblastoma, kidney and ovarian cancer. It is a recombinant humanized monoclonal antibody which inhibits angiogenesis by inhibiting vascular endothelial growth factor A.
This is an important analysis of KRAS status and the effects of anti-EGFR MoAbs in mCRC patients. The meta-analysis of randomized controlled trials may provide a comprehensive estimation of the relation between KRAS status and the effects of anti-EGFR MoAbs in mCRC patients.
P- Reviewer: Braet F S- Editor: Ma YJ L- Editor: A E- Editor: Liu XM
| 1. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9215] [Cited by in RCA: 9846] [Article Influence: 820.5] [Reference Citation Analysis (4)] |
| 2. | Kelly C, Cassidy J. Chemotherapy in metastatic colorectal cancer. Surg Oncol. 2007;16:65-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Peeters M, Price T, Van Laethem JL. Anti-epidermal growth factor receptor monotherapy in the treatment of metastatic colorectal cancer: where are we today? Oncologist. 2009;14:29-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | Lièvre A, Bachet JB, Le Corre D, Boige V, Landi B, Emile JF, Côté JF, Tomasic G, Penna C, Ducreux M. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66:3992-3995. [PubMed] |
| 5. | Barault L, Veyrie N, Jooste V, Lecorre D, Chapusot C, Ferraz JM, Lièvre A, Cortet M, Bouvier AM, Rat P. Mutations in the RAS-MAPK, PI(3)K (phosphatidylinositol-3-OH kinase) signaling network correlate with poor survival in a population-based series of colon cancers. Int J Cancer. 2008;122:2255-2259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 246] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 6. | Sastre J, Aranda E, Grávalos C, Massutí B, Varella-Garcia M, Rivera F, Soler G, Carrato A, Manzano JL, Díaz-Rubio E. First-line single-agent cetuximab in elderly patients with metastatic colorectal cancer. A phase II clinical and molecular study of the Spanish group for digestive tumor therapy (TTD). Crit Rev Oncol Hematol. 2011;77:78-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Bokemeyer C, Bondarenko I, Hartmann JT, de Braud F, Schuch G, Zubel A, Celik I, Schlichting M, Koralewski P. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: the OPUS study. Ann Oncol. 2011;22:1535-1546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 532] [Cited by in RCA: 586] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 8. | Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21630] [Cited by in RCA: 25439] [Article Influence: 1106.0] [Reference Citation Analysis (0)] |
| 9. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 45902] [Article Influence: 2086.5] [Reference Citation Analysis (3)] |
| 10. | Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634. [PubMed] |
| 11. | Maughan TS, Adams RA, Smith CG, Meade AM, Seymour MT, Wilson RH, Idziaszczyk S, Harris R, Fisher D, Kenny SL. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet. 2011;377:2103-2114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 766] [Cited by in RCA: 761] [Article Influence: 54.4] [Reference Citation Analysis (2)] |
| 12. | Van Cutsem E, Köhne CH, Láng I, Folprecht G, Nowacki MP, Cascinu S, Shchepotin I, Maurel J, Cunningham D, Tejpar S. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29:2011-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1314] [Cited by in RCA: 1452] [Article Influence: 103.7] [Reference Citation Analysis (0)] |
| 13. | Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010;28:4697-4705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1296] [Cited by in RCA: 1386] [Article Influence: 92.4] [Reference Citation Analysis (0)] |
| 14. | Hecht JR, Mitchell E, Chidiac T, Scroggin C, Hagenstad C, Spigel D, Marshall J, Cohn A, McCollum D, Stella P. A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol. 2009;27:672-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 624] [Cited by in RCA: 634] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 15. | Tol J, Koopman M, Cats A, Rodenburg CJ, Creemers GJ, Schrama JG, Erdkamp FL, Vos AH, van Groeningen CJ, Sinnige HA. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med. 2009;360:563-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1000] [Cited by in RCA: 996] [Article Influence: 62.3] [Reference Citation Analysis (0)] |
| 16. | Tveit KM, Guren T, Glimelius B, Pfeiffer P, Sorbye H, Pyrhonen S, Sigurdsson F, Kure E, Ikdahl T, Skovlund E. Phase III trial of cetuximab with continuous or intermittent fluorouracil, leucovorin, and oxaliplatin (Nordic FLOX) versus FLOX alone in first-line treatment of metastatic colorectal cancer: the NORDIC-VII study. J Clin Oncol. 2012;30:1755-1762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 417] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 17. | Peeters M, Price TJ, Cervantes A, Sobrero AF, Ducreux M, Hotko Y, André T, Chan E, Lordick F, Punt CJ. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol. 2010;28:4706-4713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 709] [Cited by in RCA: 754] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 18. | Harbison CT, Horak CE, Ledeine JM, Mukhopadhyay P, Malone DP, O’Callaghan C, Jonker DJ, Karapetis CS, Khambata-Ford S, Gustafson N. Validation of companion diagnostic for detection of mutations in codons 12 and 13 of the KRAS gene in patients with metastatic colorectal cancer: analysis of the NCIC CTG CO.17 trial. Arch Pathol Lab Med. 2013;137:820-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ, Juan T, Sikorski R, Suggs S, Radinsky R. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626-1634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2504] [Cited by in RCA: 2396] [Article Influence: 140.9] [Reference Citation Analysis (0)] |
| 20. | Oda K, Matsuoka Y, Funahashi A, Kitano H. A comprehensive pathway map of epidermal growth factor receptor signaling. Mol Syst Biol. 2005;1:2005.0010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 771] [Cited by in RCA: 750] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 21. | Tabernero J. The role of VEGF and EGFR inhibition: implications for combining anti-VEGF and anti-EGFR agents. Mol Cancer Res. 2007;5:203-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 340] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 22. | Díaz-Rubio E, Tabernero J, Gómez-España A, Massutí B, Sastre J, Chaves M, Abad A, Carrato A, Queralt B, Reina JJ. Phase III study of capecitabine plus oxaliplatin compared with continuous-infusion fluorouracil plus oxaliplatin as first-line therapy in metastatic colorectal cancer: final report of the Spanish Cooperative Group for the Treatment of Digestive Tumors Trial. J Clin Oncol. 2007;25:4224-4230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 200] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 23. | Porschen R, Arkenau HT, Kubicka S, Greil R, Seufferlein T, Freier W, Kretzschmar A, Graeven U, Grothey A, Hinke A. Phase III study of capecitabine plus oxaliplatin compared with fluorouracil and leucovorin plus oxaliplatin in metastatic colorectal cancer: a final report of the AIO Colorectal Study Group. J Clin Oncol. 2007;25:4217-4223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 219] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 24. | Fuchs CS, Marshall J, Barrueco J. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: updated results from the BICC-C study. J Clin Oncol. 2008;26:689-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 180] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 25. | Zhang L, Ma L, Zhou Q. Overall and KRAS-specific results of combined cetuximab treatment and chemotherapy for metastatic colorectal cancer: a meta-analysis. Int J Colorectal Dis. 2011;26:1025-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Vale CL, Tierney JF, Fisher D, Adams RA, Kaplan R, Maughan TS, Parmar MK, Meade AM. Does anti-EGFR therapy improve outcome in advanced colorectal cancer? A systematic review and meta-analysis. Cancer Treat Rev. 2012;38:618-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |