Published online Apr 14, 2015. doi: 10.3748/wjg.v21.i14.4275
Peer-review started: August 20, 2014
First decision: November 4, 2014
Revised: November 17, 2014
Accepted: December 5, 2014
Article in press: December 8, 2014
Published online: April 14, 2015
Processing time: 238 Days and 2.2 Hours
AIM: To prepare the specific magnetic resonance (MR) probes for detection of hepatocellular carcinoma (HCC) using one-pot method.
METHODS: The carboxylated dextran-coated nanoparticles were conjugated with anti-α-fetoprotein (anti-AFP) or anti-glypican 3 (anti-GPC3) antibodies through 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride/N-hydroxysuccinimide (EDC/NHS)-mediated reaction to synthesize the probes. The physical and chemical properties of the probes were determined by transmission electron microscopy (TEM) and dynamic light scattering, and the relaxivity was compared to uncombined ultrasmall superparamagnetic iron oxide nanoparticles (USPIONs) using a 1.5T clinical MR scanner. The binding efficiency of the antibodies to nanoparticles was measured with an ultraviolet-visible spectrophotometer. In addition, the probes were incubated with targetable cells in vitro.
RESULTS: The superparamagnetic MR probes (anti-GPC3-USPION probe and anti-AFP-USPION probe) were synthesized using one-pot method. Their mean hydrodynamic diameter was 47 nm with a broader slight size distribution. The coupling efficiency of carboxylated dextran-coated ultrasmall superparamagnetic iron oxide (USPIO) with anti-GPC3 or anti-AFP antibody was 15.9% and 88.8%, respectively. Each of the USPIO nanoparticles may bind 3 GPC3 antibodies or 12 AFP antibodies. The statistical analysis showed no significance (P > 0.05) in shortening the T1 and T2 values when comparing the USPIO-AFP or USPIO-GPC3 to USPIO. Analysis of TEM images revealed that anti-GPC3-USPION probes and anti-AFP-USPION probes could specifically enter into the HepG2 cell by combining with the GPC3 receptors or AFP receptors, whereas the HepG2 cell sample incubated with USPIONs showed no or few nanoparticles in the cytoplasm.
CONCLUSION: The synthesized probes using one-pot method can be used for in vitro experimental study and have potential clinical application in MR imaging for detection of hepatocellular carcinomas.
Core tip: The preparation process of magnetic resonance probes should be as simple as possible in order to have mass production. We developed a method named one-pot method by modifying the traditional methods to prepare an anti-glypican 3-ultrasmall superparamagnetic iron oxide nanoparticle (USPION) probe and an anti-α-fetoprotein-USPION probe and determined their physical and chemical properties and bioactivity. The results showed that this method is simple and convenient to synthesize the magnetic resonance molecular probes. The synthesized probes entered into the specific cells in vitro.
- Citation: Li YW, Chen ZG, Zhao ZS, Li HL, Wang JC, Zhang ZM. Preparation of magnetic resonance probes using one-pot method for detection of hepatocellular carcinoma. World J Gastroenterol 2015; 21(14): 4275-4283
- URL: https://www.wjgnet.com/1007-9327/full/v21/i14/4275.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i14.4275
Hepatocellular carcinoma is the fifth most common malignant tumor, more frequently seen in China. The results of treatment for hepatocellular carcinoma are poor, because it is most often diagnosed in late stages. Although there have been significant advances in various methods, early detection of hepatocellular carcinoma still needs further research.
With the advancement of the molecular imaging technology, the molecular magnetic resonance (MR) imaging is becoming immense interest because of its capability to yield highly detailed anatomic and molecular information in vivo. However, effective application of this technology relies greatly on the specific nanoprobes. Many specific probes have been reported in diagnosis of malignant tumors[1-3], such as breast cancer, pancreatic cancer, and ovarian tumors. However, little is known about the MR molecular probes specific for hepatic cell carcinoma, although there have been many studies using other ligands to synthesis nanoprobes for detection of HCC[4-6].
An MR molecular probe consists of two elements, magnetic nanoparticles and targetable markers such as antibodies or ligands. Ultrasmall superparamagnetic iron oxide (USPIO) was applied more commonly as a magnetic nanoparticle in life sciences, especially the nanoparticles with a diameter less than 100 nm and narrow distribution in size and high magnetism, which can significantly decrease T1 and T2/T2* values of a tissue. In addition, these nanoparticles have strongly magnetic susceptibility. The contrast between USPIO nanoparticles (USPIONs)-containing tissues and other USPIONs free tissues increased, especially in T2*-weighted imaging[7]. The USPIO nanoparticle has a magnetic core formed by Fe3+ and Fe2+ oxide crystal, which has ultrasuperparamagnetic behavior, and an external coat to produce biocompatibility in vivo and provide a place for conjugation with the antibodies or ligands. Because the specificity of MR nanoprobes was determined by the antibodies or ligands, we should make efforts in selecting antibodies for design of the probes. The antibodies should have high specificity, selectivity and stability.
Today many investigators[8-11] are prone to conjugate USPIONs with monoclonal antibodies or polypeptides to prepare the MR nanoprobes. These probes are only synthesized in laboratory through one-step or two-step method and cannot be produced largely. We developed a method named “one-pot method” by modifying the two-step method, to conjugate USPIONs with glypican 3 (GPC3) antibodies or anti-α-fetoprotein (AFP) antibodies because AFP is the most utilized surveillance biomarker for hepatocellular carcinoma and GPC3 is a more sensitive and specific biomarker for hepatocellular carcinoma which can be used to detect early-stage disease as recent studies have shown[12,13], and formed the MR probes specific for hepatic cell carcinoma. The average core size, size distribution, morphology and magnetic properties were measured by transmission electron microscopy (TEM), dynamic light scattering, and 1.5T MR scanning. The binding efficiency of the antibodies to nanoparticles was measured with an ultraviolet-visible spectrophotometer. In addition, the probes were incubated with targetable cells in vitro. The results showed that it is simple and convenient to synthesize the MR molecular probes using the one-spot method. Although the coupling difference with various antibodies (anti-AFP antibody or GPC3 antibody) was observed, the synthesized probes can be used as a contrast agent for clinical MR imaging.
All reagents used for synthesis were purchased from commercial sources. These were sodium periodate and Dextran 10000 from Sinopharm Chemical Reagent Co., LTD. (China); ferric chloride hexahydrate from Shantou Xilong Chemical Co., LTD. (China); FeNH4SO4·6H2O from Fuchen Chemical Reagent Company (Tianjin, China); NaOH from Beijing Chemical Works (China); GPC3 monoclonal antibody from Htpharma Technology Development (Beijing) Co., Ltd; AFP monoclonal antibody from Baitai Biotechnology (Beijing) Co., Ltd.; and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) and N-hydroxysuccinimide (NHS) from Thermo Company.
USPIONs coated by dextran with carboxylate groups were synthesized at Wangleyu lab, Department of Chemistry, Beijing University of Chemical Technology. Aqueous coprecipitation of magnetite was used. Briefly, 0.3 g of dextran 10000 was dissolved in 10 mL deionized water, and then 0.4 g of sodium periodate was added to a final concentration of 100 mmol. The hydroxyl groups on dextran were partially oxidized to carboxylate groups at 80 °C for 20 min. After purification by dialysis to remove excess sodium periodate, oxidized dextran resolution was mixed with 1 mmol ferric chloride hexahydrate and 2 mmol FeNH4SO4·6H2O in an aqueous solution with an excess of concentrated sodium hydroxide (15 mmol). The mixture in a reaction boiler was heated to 160 °C for 10 h and then cooled down to room temperature. To make the products precipitate, 0.1 M/L HCl was added to the mixture. After centrifugation at 5000 rpm for 5 min, the supernatant was decanted. The process was repeated three times, and then the final black products were resuspended in deionized water for further use.
EDC (1 mg) and sulfo-NHS (2 mg) were dissolved in 0.5 mL phosphate-buffered saline (PBS, pH = 7.4), and the pH value was adjusted to 5.0 by titrating the mixture solution with 0.2 N HCl. Carboxylated dextran-coated USPIO (1 mL, 5 mg/mL) was added to the solution and allowed to react for 2 h. After adding 0.5 mL of 1 mg/mL anti-AFP or 200 μg/mL anti-GPC3 antibody (0.5 mL PBS was added to the control), the mixture was stirred and allowed to react for 3 h. The pH value of the mixture solution was then adjusted to 7.0 by titrating with 0.2 N NaOH. After reaction for further 30 min, the solution was centrifuged at 5000 rpm/min for 5 min and the supernatant was discarded to obtain the products. The formed USPIO-antibody probes were resuspended in PBS at 4 °C for further application.
The prepared MR molecular probes were divided into two parts, one for measurement of the coupling ability of carboxylated dextran-coated USPIO with antibody and the other for measurement of relaxivity.
The average core size, size distribution, and morphology were examined using a transmission electron microscope (Hitach 7600, Japan) at a voltage of 100 kV in the magnification range from × 40000 to × 600000. Samples were drop-cast onto a 200-mesh copper grid and were air-dried at room temperature before being loaded into the microscope. To examine the hydrodynamic diameters of the magnetic molecular probes, dynamic light scattering measurements were performed using a Malvern laser granulometer (Zetasizer Nano ZS90, Malvern, United Kingdom) at 25 °C. Their magnetic properties and the magnetic saturation were determined with a Superconducting Quantum Interference Device (SQUID).
Determination of iron content in antibody-USPIO: The flame atom absorbing law was used to measure the iron content in antibody-USPIO. Samples were prepared by acid digestion. Antibody-USPIO sample (40 μL) was aspirated exactly by means of micropipette aspiration. When the sample adsorbed on the tip of micropipette was wiped out, the micropipette was dipped 3-4 mm undersurface of the fluid in a centrifuge tube containing 1.2 mL dilute solution, and then the sample was discharged slowly. Forty microliters of dilute solution was absorbed and then discharged. This process was repeated two times. The prepared sample was measured by atomic absorption spectroscopy (BHS 100, Bohui Innovation Technology Co. Ltd, Beijing).
Determination of antibody content in antibody-USPIO: The free antibody content in supernatant fluid was determined after centrifugation, and then the content of antibody coupled with USPIO was calculated. The supernatant fluid (1.7 mL) was dialyzed with 0.2 M PBS (pH 7.0) to displace the solution disturbing the accuracy of measurement. During the dialyzing process using 2 L dialyzing fluid at 2-8 °C for 24 h, dialyzing fluid was changed 4 times per 2 h. After the last time the dialysis lasted overnight. The prepared samples were mixed with AB solution of the BCA Protein Assay Reagent, in which A solution was mixed with B solution at a rate of 50:1 and at a proportion of 20:1 for incubation at 37 °C in a water bath for 30 min. The incubated samples were taken out and then were measured with an ultraviolet-visible spectrophotometer (DU800, BECKMANCOULER Company).
Preparation of 1% agar solution: Agar (1 g) was added to 100 mL deionized water and was heated up to 80 °C. After the agar was dissolved completely, the solution was cooled down to 50 °C. The resulting 1% agar solution was used to carry the antibody-USPIO.
Preparation of samples for MR scanning: The concentration of 5 mg/mL iron of the anti-AFP-USPIO, anti-GPC3-USPIO or USPIO was diluted to 0.25, 0.125, 0.0625, 0.031 or 0.016 mg/mL. These test samples were centrifuged and all the supernatant fluids were discarded. Each of the precipitated samples was added to a Pendoff test containing 2 mL of 1% agar solution. The mixture was shook well and then cooled down to room temperature.
MR scanning: The test tubes and control sample (2 mL of 1% agar solution) were imaged using a 1.5T MR scanner (Excite, GE Medical Systems, Milwaukee, WI, USA) with a standard circularly polarized quadrature knee coil. To avoid susceptibility artifacts from the surrounding air in the scans, all tubes were placed in a water-containing plastic container at room temperature.
To measure the T1 relaxation times, axial spin echo (SE) sequences were obtained with a fixed echo time (TE) of 9 ms and multiple repetition time (TR) values of 1200, 900, 600 and 300 ms, whereas axial SE images with a fixed TR of 2000 ms and increasing TEs of 12, 24, 36, and 48 ms were obtained for measurements of T2 relaxation times. All sequences were acquired with a field of view of 160 mm × 160 mm, a matrix of 192 × 160 pixels, a slice thickness of 5.0 mm, a gap of 1.0 mm and one acquisition.
The data acquired using the above mentioned methods were transferred into the Function tool on the workstation. T1 maps were calculated from four SE images with a fixed TE of 9 ms and variable TR values of 1200, 900, 600 and 300 ms using a nonlinear function least-square curve fitting on a pixel-by-pixel basis. T2 maps were calculated accordingly from four SE images with a fixed TR of 2000 and TE values of 12, 24, 36 and 48 ms. Then the T1 and T2 relaxation times of test tubes and control samples were derived by ROI measurements of the test samples on these T1 and T2 maps. T1 and T2 maps were calculated assuming monoexponential signal decay. Only data points with signal intensities significantly above the noise level were analyzed.
HepG2 cells are human hepatocellular carcinoma cells expressing GPC3 receptors or AFP receptors, confirmed by flow cytometry using monoclonal anti-human glypican 3 antibody or anti-AFP antibody. Cells in 6-well plates, (each well containing 5 mL medium and 1 × 106 HepG2 cells) were incubated with anti-AFP-USPIO, anti-GPC3-USPIO or USPIO for 4 h in a humidified 5% CO2 atmosphere at 37 °C, respectively. The iron concentrations of the probes used were 500 or 125 μg/mL. Afterwards, the adherent cells were washed three times with PBS (0.1 mol/L, pH 7.4), trypsinized, and centrifuged for sedimentation (10 min, 250 g, 20 °C) in order to remove unbounded particles. The cells were then fixed in electron microscopic specimen stationary liquid and analyzed using a transmission electron microscope (Hitachi 7600, Japan) at a voltage of 80 kV in the magnification range from × 40000 to × 600000.
Six regions of interest in each sample were measured. T1 and T2 values are presented as mean ± SE. To compare differences in quantitative data between different samples, a two-tailed paired t-test was used. P < 0.05 was considered statistically significant. All analyses were processed using SPSS 11.5 software (sequence license 30001359390).
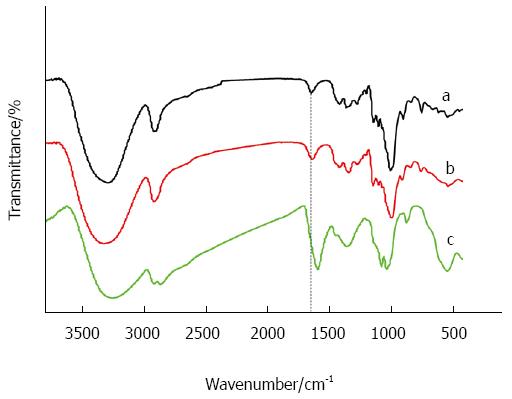
The hydroxyl groups on dextran were partially oxidized to carboxylate groups by using sodium periodate. From Figure 1 we can see the peak of carboxylate groups. The carboxylated dextran was then coated on the surface of USPIONs. Through covalent conjugation of carboxylate groups with antibodies the magnetic molecular probes were prepared.
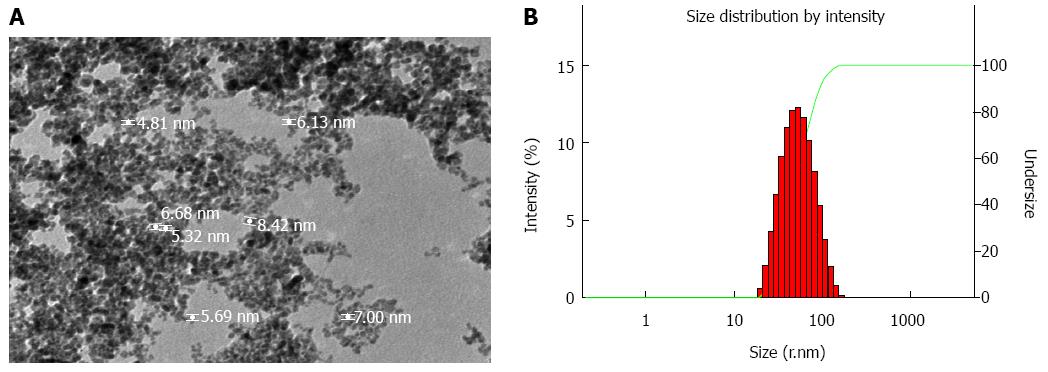
As shown in Figure 2A, the magnetic molecular probe showed a core/shell spherical structure with a core diameter of 5-8 nm. The nanoparticles displayed homogeneous size and good dispersity in solution. Dynamic light scattering demonstrated a broader slightly size distribution and the mean hydrodynamic diameter of the magnetic molecular probes was 47 nm (Figure 2B).
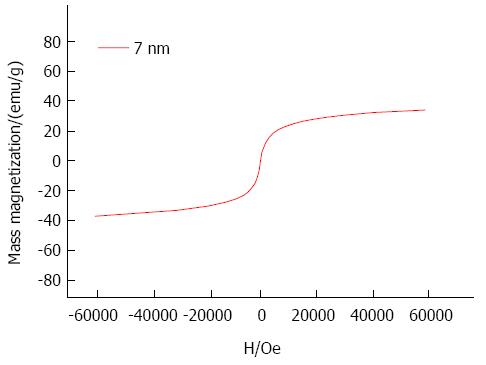
The superparamagnetic behavior of the nanoparticles was checked by magnetization measurement SQUID). The hysteresis curve (Figure 3) indicated superparamagnetic characteristics at room temperature, meaning that the thermal energy can overcome the anisotropy energy barrier of a single particle, and the net magnetization of the particle assemblies in the absence of an external field is zero. The nanoparticles showed a saturation magnetization of 35.5 emu/g at 0.6T with a coercivity of zero.
The iron content in antibody-USPIO was 1.20 mg measured by the flame atom absorbing law. The free antibody content in supernatant fluid (1.7 mL) was determined using an ultraviolet-visible spectrophotometer. The results showed the concentration of anti-GPC3 was 12 μg/mL and the anti-AFP 33 μg/mL. Therefore the contents of bound anti-GPC3 and bound anti-AFP were 79.6 μg/5 mg and 443.9 μg/5 mg, and the coupling efficiency was 15.9% and 88.8%, respectively. Each of the USPIO nanoparticles may bind three GPC3 antibodies or 12 AFP antibodies.
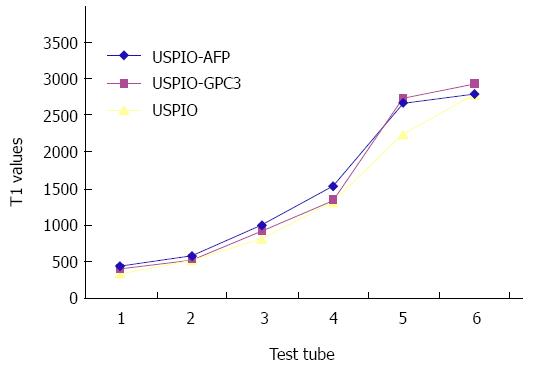
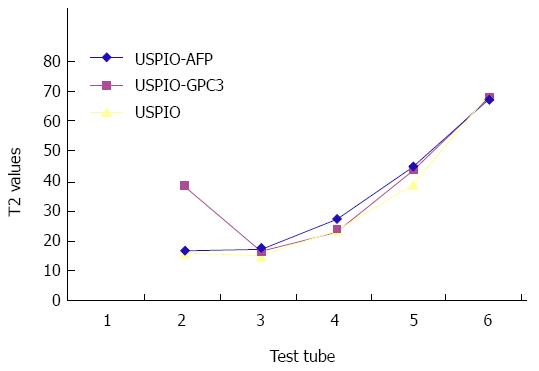
From Tables 1 and 2 we can see that all the USPIO-AFP, USPIO-GPC3 and USPIO could shorten T1 and T2 values of the agar solution, especially T2 value. The higher the concentration, the smaller the values. Although the statistical analysis showed no significance (P > 0.05) in shortening the T1 and T2 values among the USPIO-AFP, USPIO-GPC3 and USPIO, the degree of T1 and T2 value decrease was higher by pure USPIO than by USPIO-AFP or USPIO-GPC3. Moreover, the slight difference existed between USPIO-AFP and USPIO-GPC3 in shortening the T1 and T2 values, and USPIO-AFP with more antibodies affected magnetization more than USPIO-GPC3 with fewer antibodies (Figures 4 and 5).
| 1 | 2 | 3 | 4 | 5 | Control | |
| (0.25 mg) | (0.125 mg) | (0.0625 mg) | (0.031 mg) | (0.016 mg) | ||
| USPIO-AFP | 427.72 ± 98.67 | 570.35 ± 110.75 | 994.36 ± 164.68 | 1535.9 ± 254.45 | 2658.2 ± 284.99 | 2783.4 |
| USPIO-GPC3 | 392.50 ± 93.25 | 526.85 ± 109.83 | 923.55 ± 156.32 | 1341.8 ± 255.78 | 2718.1 ± 283.32 | 2916.1 |
| USPIO | 331.08 ± 86.73 | 523.08 ± 121.47 | 805.85 ± 145.60 | 1306.3 ± 202.93 | 2240.9 ± 268.20 | 2776.4 |
| 1 | 2 | 3 | 4 | 5 | Control | |
| (0.25 mg) | (0.125 mg) | (0.0625 mg) | (0.031 mg) | (0.016 mg) | ||
| USPIO-AFP | 15.18 ± 0.67 | 15.92 ± 1.21 | 25.93 ± 1.13 | 43.48 ± 1.60 | 66.20 | |
| USPIO-GPC3 | 36.64 ± 0.42 | 14.98 ± 0.97 | 21.48 ± 1.20 | 42.18 ± 1.52 | 66.50 | |
| USPIO | 14.83 ± 0.37 | 13.44 ± 0.85 | 22.17 ± 1.02 | 37.3 ± 1.37 | 67.67 |
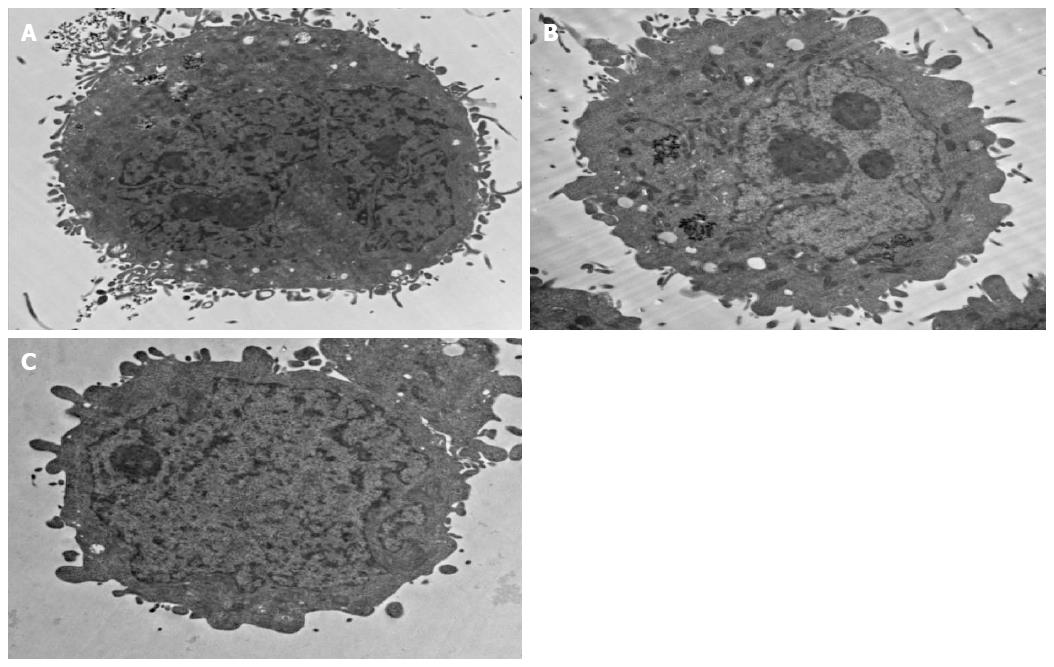
Figure 6A and 6B shows a TEM picture of a HepG2 cell sample incubated with anti-GPC3-USPION probes or anti-AFP-USPION probes for 4 h, respectively. Incorporation of the probes into intracellular organelles follows the endocytosis pathway. First, the probes interact with the GPC3 receptors or AFP receptors expressed on the surface of the HepG2 cell via antigen-antibody combinations. Then they accumulate into membrane invaginations and are enclosed into the cytoplasm. Analysis of TEM images revealed that densely packed nanoparticles were in the endosomes. But the content of the probes incorporated intracellularly was different between HepG2 cells incubated with anti-GPC3-USPION probes and HepG2 cells with anti-AFP-USPION probes, and a higher content of anti-GPC3-USPION probes was observed in our experiments.
In contrast, the TEM picture of a HepG2 cell sample incubated with USPIONs showed no or little nanoparticles in the cytoplasm (Figure 6C).
This study applied condensation coupling of carboxyl nanoparticles with amines on the antibodies to produce MRI biological probes. It is well known that there were two methods, single step method and two-step method, to couple carboxylate particles with amine-containing molecules such as antibody through an aqueous, carbodiimide-mediated process using EDC and/or sulfo-NHS[14]. The single-step method used EDC alone as coupling agent and is only for molecules having one or more amines without any carboxylates, whereas the two-step method is appropriate for molecules that have both amines and carboxylates, in which EDC and sulfo-NHS are all used. Firstly, the carboxylate particles are activated with the water-soluble EDC to create an intermediate ester under acidic conditions (pH 3.5-4.5), which is reactive directly with amines on molecules to form an unstable intermediate ester. With the addition of sulfo-NHS, another intermediate, the sulfo-NHS ester is produced. This intermediate is more stable and has high solubility, so that it reacts with the attacking amine groups quickly. After the formation of the intermediate ester the reaction medium was adjusted to mildly alkaline pH conditions (e.g., pH 8.5), facilitating the covalent conjugation of carboxylate nanoparticles with antibodies. Because carbodiimide-mediated antibody polymerization will occur due to the presence of both amines and carboxylates on antibodies, the excess EDC in the solution must be removed before adding antibodies. This will prevent the decrease of the amount of antibodies coupled to carboxylate nanoparticles as well as the biologic function of the antibody[15]. This method has a good quality control, but the fussy operation and strict control per step limit mass production. In this study, we modified the two-step method to synthesize an MR nanoprobe based on the design conception, in which we focused on coupling effect and actual application effect, as well as the preparation process as simple as possible. It is known as “one-pot method”, in which both activation and coupling processes were completed in entirely aqueous conditions without removing excess EDC and/or NHS before adding antibodies.
The synthesized probe must have some characteristics such as optimal size and the physical and chemical characteristics of the coating materials to escape the phagocytosis by the mononuclear phagocytic system and retain their biological activity of the targeted molecules in the bloodstream. The MR probes can cross the capillary into tissues and have a chance to combine the lesions specifically through the specific molecules such as monoclonal antibodies or a ligand on the surface of the probes. Many studies have shown that particle size has an important role in the blood clearance of stealth probes. The optimal size (hydrodynamic diameter) range is 8-50 nm[16-18]. The probe with a hydrodynamic diameter less than 5.5 nm can be removed quickly from the body via the renal system. When probes with a hydrodynamic diameter larger than 50 nm were injected into the body, they were also removed from the bloodstream rapidly, typically a matter of minutes, and usually accumulate in the liver and spleen.
A disadvantage of the molecular MR imaging is low sensitivity. If the magnetic strength of the USPIOs decreased greatly in the design and preparation of probes, the lesion will not be detected by MRI even though it was targeted with the probes. Leuschner et al[19] compared the probe combined 60 mg SPIONs to 3.7 mg LHRH and SPIONs. They demonstrated that the saturation magnetization and the Zeta potential of SPIONs were higher than those of LHRH-SPION. The Zeta potential of SPIONs was positively charged (28.5 ± 1.93 mV), whereas LHRH-SPION was almost neutral (-2.2 ± 0.85 mV). Although the magnetization values of LHRH-SPION decreased by 5%-6% compared to SPIONs due to binding LHRH in LHRH-SPION samples, they assumed that the decrease was acceptable. Chen et al[20] and Huang et al[21] came to the same conclusion. However, Zhang et al[22] reported that there was no difference between SPION and SPION-antibody. Our experimental results also showed no significance in shortening the T1 and T2 values among the USPIO-AFP, USPIO-GPC3 and USPIO.
From the above experiments, it was seen that the content of antibodies connected with nanoparticles was related to the kinds of antibodies used for conjugation, although their concentrations used for coupling were different. Our results were the same with others. Some laboratories[23] reported that 2-3 antibodies per nanoparticle was coupled, however, some investigators[19] reported up to 12 antibodies per nanoparticle, according to different tumor marker requirement. The synthesized anti-GPC3 USPIO probes and anti-AFP USPIO probes were respectively transported into cells through combination of ligands or antibodies with specific receptors on the surface of cells, but the contents of the two probes were different. This phenomenon may be due to the different intensity of GPC3- or AFP- receptors expressed on the surface of HepG2 cells. However, HepG2 cells incubated with USPIONs showed few nanoparticles in cytoplasm. This may be due to nonspecific endocytosis, which will be further studied.
The present preparation method had several limitations. First, as described above, we used random covalent conjugation to couple the antibody with carboxylate nanoparticles. The antigen binding sites with antibody were inevitably connected to nanoparticles[24,25], which will decrease the function of the antibody. In addition, the immunogenicity of the synthesized probes was not determined.
In conclusion, it is simple and convenient to synthesize the MR molecular probes using one-pot method. The coupling efficiency of antibodies with nanoparticles was enough compared to results reported by other investigators. The amount of antibodies coupled with the probes can be controlled according to the needs and does not change the magnetization significantly. The synthesized probes can enter targetable cells in in vitro cell studies. Therefore, the probes synthesized using one-pot method can be used as an MR contrast agent for animal experimental study and have a potential clinical application in MR imaging for early detection of hepatic cell carcinoma.
Hepatocellular carcinoma is the fifth most common malignant tumor, more frequently seen in China. The results of treatment for hepatocellular carcinoma are poor because it is often diagnosed in late stages. Although there have been significant advances in various methods, early detection of hepatocellular carcinoma still needs further research.
With the advancement of the molecular imaging technology, the molecular MR imaging is becoming immense interest because of its highly detailed anatomic and molecular resolution in vivo. Many specific probes have been reported in diagnosis of malignant tumors, such as breast cancer, pancreatic cancer, and ovarian tumors. However, little is known about the MR molecular probes specific for hepatic cell carcinoma.
This study synthesized the magnetic resonance (MR) molecular probes specific for hepatic cell carcinoma using one-pot method. The results suggest that the synthesized anti-glypican 3 (anti-GPC3)-USPION (ultrasmall superparamagnetic iron oxide nanoparticle) probes or anti-α-fetoprotein (anti-α-AFP)-USPION probes interact with the GPC3 receptors or AFP receptors expressed on the surface of the HepG2 cell and entered into the cytoplasm. This will be expected to make a breakthrough in the early diagnosis of liver cancer.
The synthesized probes can be used as an MR contrast agent for animal experimental study and have potential clinical application in MR imaging for early detection of hepatic cell carcinoma.
This is a good study in which the authors introduced a new method to prepare the MR probes for detection of hepatocellular carcinoma. The results showed that the method is simple and can be used to synthesize the MR molecular probes largely. The synthesized probes can specially enter targetable cells in in vitro cell studies.
P- Reviewer: Shen J S- Editor: Yu J L- Editor: Wang TQ E- Editor: Liu XM
| 1. | Yan C, Wu Y, Feng J, Chen W, Liu X, Hao P, Yang R, Zhang J, Lin B, Xu Y. Anti-αvβ3 antibody guided three-step pretargeting approach using magnetoliposomes for molecular magnetic resonance imaging of breast cancer angiogenesis. Int J Nanomedicine. 2013;8:245-255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Zuo HD, Yao WW, Chen TW, Zhu J, Zhang JJ, Pu Y, Liu G, Zhang XM. The effect of superparamagnetic iron oxide with iRGD peptide on the labeling of pancreatic cancer cells in vitro: a preliminary study. Biomed Res Int. 2014;2014:852352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Shahbazi-Gahrouei D, Abdolahi M. Superparamagnetic iron oxide-C595: Potential MR imaging contrast agents for ovarian cancer detection. J Med Phys. 2013;38:198-204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Jia FG, Zhang XD, Xu YK, Meng Z. [Synthesis of Gal-BSA-SPIO and magnetic resonance imaging of ASG receptors in rabbits bearing liver VX2 tumor and human liver]. Nanfang Yike Daxue Xuebao. 2009;29:191-194. [PubMed] |
| 5. | Lee CM, Jeong HJ, Kim EM, Kim DW, Lim ST, Kim HT, Park IK, Jeong YY, Kim JW, Sohn MH. Superparamagnetic iron oxide nanoparticles as a dual imaging probe for targeting hepatocytes in vivo. Magn Reson Med. 2009;62:1440-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | Huo T, Du X, Zhang S, Liu X, Li X. Gd-EDDA/HYNIC-RGD as an MR molecular probe imaging integrin alphanubeta3 receptor-expressed tumor-MR molecular imaging of angiogenesis. Eur J Radiol. 2010;73:420-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Qiao R, Yang C, Gao M. Superparamagnetic iron oxide nanoparticles: from preparations to in vivo MRI applications. J. Mater Chem. 2009;19:6274-6293. [RCA] [DOI] [Full Text] [Cited by in Crossref: 530] [Cited by in RCA: 534] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 8. | You XG, Tu R, Peng ML, Bai YJ, Tan M, Li HJ, Guan J, Wen LJ. Molecular magnetic resonance probe targeting VEGF165: preparation and in vitro and in vivo evaluation. Contrast Media Mol Imaging. 2014;9:349-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Guo Q, Liu Y, Xu K, Ren K, Sun W. Mouse lymphatic endothelial cell targeted probes: anti-LYVE-1 antibody-based magnetic nanoparticles. Int J Nanomedicine. 2013;8:2273-2284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Shahbazi-Gahrouei D, Abdolahi M. Detection of MUC1-expressing ovarian cancer by C595 monoclonal antibody-conjugated SPIONs using MR imaging. ScientificWorldJournal. 2013;2013:609151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Li Y, Chen Z, Li F, Wang J, Zhang Z. Preparation and in vitro studies of MRI-specific superparamagnetic iron oxide antiGPC3 probe for hepatocellular carcinoma. Int J Nanomedicine. 2012;7:4593-4611. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Kandil D, Leiman G, Allegretta M, Trotman W, Pantanowitz L, Goulart R, Evans M. Glypican-3 immunocytochemistry in liver fine-needle aspirates : a novel stain to assist in the differentiation of benign and malignant liver lesions. Cancer. 2007;111:316-322. [PubMed] |
| 13. | Ligato S, Mandich D, Cartun RW. Utility of glypican-3 in differentiating hepatocellular carcinoma from other primary and metastatic lesions in FNA of the liver: an immunocytochemical study. Mod Pathol. 2008;21:626-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Hermanson GT. Polymeric microspheres and nanoparticles. New York: Academic Press 2008; vol.14, p. 588. |
| 15. | Bonfield TL, John N, Barna BP, Kavuru MS, Thomassen MJ, Yen-Lieberman B. Multiplexed particle-based anti-granulocyte macrophage colony stimulating factor assay used as pulmonary diagnostic test. Clin Diagn Lab Immunol. 2005;12:821-824. [PubMed] |
| 16. | Nowak J, Wiekhorst F, Trahms L, Odenbach S. The influence of hydrodynamic diameter and core composition on the magnetoviscous effect of biocompatible ferrofluids. J Phys Condens Matter. 2014;26:176004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Antonelli A, Sfara C, Battistelli S, Canonico B, Arcangeletti M, Manuali E, Salamida S, Papa S, Magnani M. New strategies to prolong the in vivo life span of iron-based contrast agents for MRI. PLoS One. 2013;8:e78542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Dong C, Irudayaraj J. Hydrodynamic size-dependent cellular uptake of aqueous QDs probed by fluorescence correlation spectroscopy. J Phys Chem B. 2012;116:12125-12132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Leuschner C, Kumar CS, Hansel W, Soboyejo W, Zhou J, Hormes J. LHRH-conjugated magnetic iron oxide nanoparticles for detection of breast cancer metastases. Breast Cancer Res Treat. 2006;99:163-176. [PubMed] |
| 20. | Chen DH, Liao MH. Preparation and characterization of YADH- bound magnetic nanoparticles. J Mol Cat B: Enzym. 2002;16:283-291. [RCA] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 95] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 21. | Huang SH, Liao MH, Chen DH. Direct binding and characterization of lipase onto magnetic nanoparticles. Biotechnol Prog. 2003;19:1095-1100. [PubMed] |
| 22. | Zhang K, Li JD, Zhang RP, Xin L, Li J. The preliminary study of molecular imaging of colorectal cancer cells with superparamagnetic iron oxide-based MR targeting probe containing vascular endothelial growth factor in vitro. J Chin Radiol. 2010;44:84-89. |
| 23. | Funovics MA, Kapeller B, Hoeller C, Su HS, Kunstfeld R, Puig S, Macfelda K. MR imaging of the her2/neu and 9.2.27 tumor antigens using immunospecific contrast agents. Magn Reson Imaging. 2004;22:843-850. [PubMed] |
| 24. | Masuda T, Miyoshi E. Cancer biomarkers for hepatocellular carcinomas: from traditional markers to recent topics. Clin Chem Lab Med. 2011;49:959-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Lin PC, Chen SH, Wang KY, Chen ML, Adak AK, Hwu JR, Chen YJ, Lin CC. Fabrication of oriented antibody-conjugated magnetic nanoprobes and their immunoaffinity application. Anal Chem. 2009;81:8774-8782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |