Published online Apr 14, 2015. doi: 10.3748/wjg.v21.i14.4261
Peer-review started: September 3, 2014
First decision: November 14, 2014
Revised: November 21, 2014
Accepted: January 8, 2015
Article in press: January 8, 2015
Published online: April 14, 2015
Processing time: 224 Days and 13.8 Hours
AIM: To gain a better understanding of biliary tract intraductal papillary mucinous neoplasm (BT-IPMN).
METHODS: From January 2000 to December 2013, 19 cases of BT-IPMN were retrospectively identified from a total of 343 biliary tract tumors resected in our single institution. Demographic characteristics, clinical data, pathology, surgical strategies, and long-term follow-up were analyzed.
RESULTS: The mean age of the 19 BT-IPMN cases was 53.8 years (range: 25-74 years). The most common symptom was abdominal pain (15/19; 78.9%), followed by jaundice (7/19; 36.8%). Cholangitis was associated with most (16/19; 84.2%) of the BT-IPMN cases. Macroscopically visible mucin was detected in all 19 patients, based on original surgical reports. The most common abnormal preoperative imaging findings for BT-IPMN were bile duct dilation (19/19; 100%) and intraluminal masses (10/19; 52.6%). Thirteen (68.4%) cases involved the intrahepatic bile duct and hilum. We performed left hepatectomy in 11/19 (57.9%), right hepatectomy in 2/19 (10.5%), bile duct resection in 4/19 (21.1%), and pancreatoduodenectomy in 1/19 (5.3%) patients. One (5.3%) patient was biopsied and received a choledochojejunostomy because of multiple tumors involving the right extrahepatic and left intrahepatic bile ducts. Histology showed malignancy in 10/19 (52.6%) patients. The overall median survival was 68 mo. The benign cases showed a non-significant trend towards improved survival compared to malignant cases (68 mo vs 48 mo, P = 0.347). The patient without tumor resection died of liver failure 22 mo after palliative surgery.
CONCLUSION: BT-IPMN is a rare biliary entity. Complete resection of the tumor is associated with good survival, even in patients with malignant disease.
Core tip: Our study involved a large number of patients with biliary tract intraductal papillary mucinous neoplasm (BT-IPMN) from a large Chinese institution. We summarized the clinical features, radiologic findings, pathology, surgical strategies, and long-term follow-up of these patients to achieve a better understanding of this rare disease. Our findings indicated that BT-IPMN is a rare biliary entity and complete resection of the tumor is associated with good survival, even in patients with malignant disease.
- Citation: Wang X, Cai YQ, Chen YH, Liu XB. Biliary tract intraductal papillary mucinous neoplasm: Report of 19 cases. World J Gastroenterol 2015; 21(14): 4261-4267
- URL: https://www.wjgnet.com/1007-9327/full/v21/i14/4261.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i14.4261
In the past decade, biliary tract intraductal papillary mucinous neoplasm (BT-IPMN) has been increasingly recognized as a unique type of biliary neoplasm, coinciding with widespread acceptance of the nomenclature of pancreatic intraductal papillary mucinous neoplasm (P-IPMN)[1-3]. As the name suggests, BT-IPMN is known to be a biliary counterpart of P-IPMN, but with its own separate identity[4-9]. BT-IPMN is histologically defined as a mucinous and papillary neoplasm, with a clear origin from the biliary epithelium, with solitary or diffuse intraductal growth[1]. It is a rare neoplasm involving the intra- and extrahepatic biliary tract and is characterized by mucin-secreting papillary and/or cystic lesions. BT-IPMN is recognized as a precursor of invasive carcinoma (tubular adenocarcinoma or mucinous carcinoma) and 40%-80% of resected BT-IPMNs contain invasive components[10-12]. BT-IPMN has a more favorable prognosis compared with conventional cholangiocarcinoma[13,14]. The number of reports of BT-IPMN with strict histopathologic criteria is limited. Moreover, most of the data regarding BT-IPMN are from retrospective studies with small samples. There is still controversy about several aspects of BT-IPMN, and the clinicopathologic characteristics, surgical strategies, and prognosis of BT-IPMN are largely unclear[1,2,7].
Our study involved a large number of patients with BT-IPMN from a large Chinese institution. The purpose of this study was to summarize the demographic and clinical features, radiologic findings, pathology, surgical strategies, and long-term follow-up of patients with BT-IPMN for a better understanding of this rare disease.
From January 2000 to December 2013, 19 patients with BT-IPMN were retrospectively identified in our institution. All diagnoses were established using strict histopathologic criteria for BT-IPMN: a mucinous and papillary neoplasm demonstrating clear origin from the biliary epithelium, with solitary or diffuse intraductal growth[1]. We excluded mucinous cystic neoplasms of the liver (with ovarian or mesenchymal stroma)[15], lesions originating from the periampullary region of the duodenum[16], and lesions without microscopic or macroscopic mucin secretion. All 19 BT-IPMNs were histologically classified into benign (low- or middle-grade dysplasia) and malignant (high-grade dysplasia or invasive carcinoma)[17].
Clinical data were obtained from the electronic medical records or external medical reports. Demographic characteristics, clinical presentation, preoperative evaluation, pathology, surgical therapy, postoperative course, and long-term outcomes were included. Postoperative complications (all events recorded within 30 d after surgery) were included in our prospective complication database. Survival was measured from the date of operation to date of death or of last follow-up. We conducted telephone interviews and/or outpatient interview to follow-up these patients. This study was approved by the Ethics Committee of Sichuan University.
Survival probability was estimated using the Kaplan-Meier method. Statistical analysis was performed using SPSS version 16.0 (SPSS Inc., Chicago, IL, United States). A P < 0.05 was considered statistically significant.
Demographic characteristics of the 19 BT-IPMN patients, with a mean age of 53.8 years (range: 25-74 years), are shown in Table 1. The clinical features of these patients are shown in Table 2. Abdominal pain was the most common presenting symptom, and the majority of patients showed acute or chronic cholangitis.
| Feature | n (%) |
| Age (yr) | |
| ≤ 40 | 3 (15.8) |
| 40-50 | 4 (21.1) |
| 50-60 | 6 (31.6) |
| ≥ 60 | 6 (31.6) |
| Sex | |
| Male | 11 (57.9) |
| Female | 8 (42.1) |
| Feature | n (%) |
| Presenting symptoms | |
| Abdominal pain | 15 (78.9) |
| Jaundice | 7 (36.8) |
| Weight loss | 3 (15.8) |
| None | 1 (5.3) |
| Schistosomiasis1 | 4 (21.1) |
| Presence of cholangitis | 16 (84.2) |
| Repeated episodes cholangitis | 6 (31.6) |
| Location | |
| Intrahepatic and hilum | 13 (68.4) |
| Extrahepatic | 5 (26.3) |
| Multifocal | 1 (5.3) |
| Serum chemistry | |
| Elevated CEA (> 3.4 ng/dL) | 5 (26.3) |
| Elevated CA 19-9 (> 22 U/mL) | 8 (42.1) |
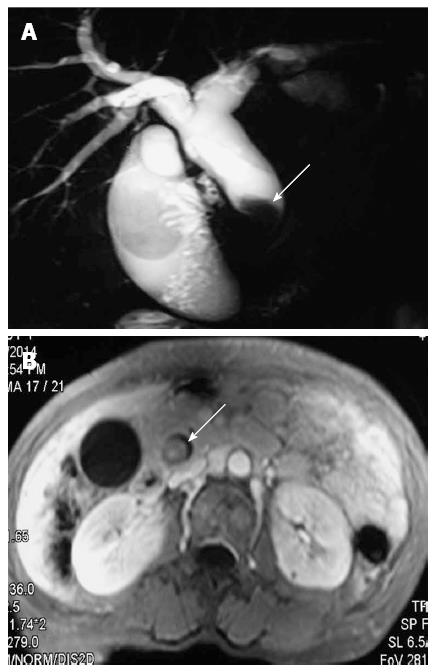
All patients underwent abdominal ultrasonography, and additional imaging examinations were also performed in many patients (Table 3). The bile duct was dilated in all cases, and intraluminal masses were observed in 10/19 (52.6%) cases (Figure 1). Biliary stones were detected in 12/19 (63.2%) patients, primarily located only in the proximal biliary duct (10/12; 83.3%).
| Feature | n (%) |
| Biliary stones (n = 12) | |
| Proximal | 10 (52.6) |
| Proximal and distal | 2 (10.5) |
| Cholecystolithiasis | 0 (0.0) |
| Dilated bile duct (n = 19) | |
| Proximal | 6 (31.6) |
| Proximal and distal | 13 (68.4) |
| Cyst | 10 (52.6) |
| Lesion | 10 (52.6) |
| Liver atrophy | 7 (36.8) |
| Imaging examination | |
| Ultrasonography | 19 (100) |
| Computed tomography | 15 (78.9) |
| Magnetic resonance imaging | 12 (63.2) |
| Intraoperative choledochoscopy | 8 (42.1) |
| Endoscopic retrograde cholangiography | 4 (21.1) |
The majority (11/19; 57.9%) of patients received a left hepatectomy (Table 4). One patient required pancreaticoduodenectomy for tumor clearance and another received biopsy and choledochojejunostomy for multiple tumors of the extrahepatic and right and left intrahepatic bile ducts. No deaths occurred within 30 d after surgery, though 4/19 (21.1%) patients had postoperative complications. Bile leakage occurred with postoperative pneumonia in a 68-year-old patient who underwent local bile duct excision, resulting in a prolonged (65 d) hospitalization and readmission, which was cured through percutaneous drainage and antibiotics. In addition, three patients had postoperative complications that were cured by conservative therapy. Lymphadenectomy was routinely performed, however, no lymph node metastasis was detected in our series.
| Feature | n (%) |
| Left hepatectomy (n = 11) | |
| Lobectomy | 6 (31.6) |
| Segmentectomy | 5 (26.3) |
| Right hepatectomy (n = 2) | |
| Segmentectomy | 2 (10.5) |
| Pancreaticoduodenectomy | 1 (5.3) |
| Bile duct excision | 4 (21.1) |
| Biopsy and choledochojejunostomy | 1 (5.3) |
| Complications (n = 4) | |
| Stress ulcer | 1 (5.3) |
| Intra-abdominal abscess | 1 (5.3) |
| Pneumonia and bile leakage | 1 (5.3) |
| Wound infection | 1 (5.3) |
| Pathology | |
| Benign | 9 (47.4) |
| Malignant | 10 (52.6) |
| Presence of mucin | |
| Macroscopic visible mucin | 19 (100) |
| Microscopic mucin | 19 (100) |
| Lymph node metastasis | 0 (0.0) |
| Death (n = 8) | |
| Benign | 3 (15.8) |
| Malignant | 5 (26.3) |
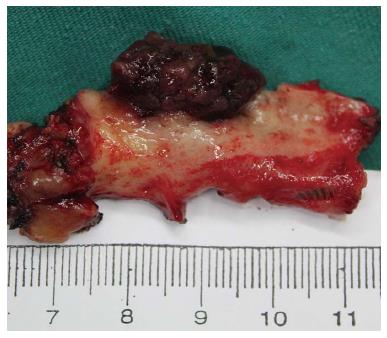
The mean tumor size was 3.5 cm (range: 0.5-12 cm). The gross appearance of BT-IPMN varies with size. Smaller BT-IPMN tumors typically present as an intraluminal mass (Figure 2), though they can appear as cyst-like bile duct dilation. Intraluminal growing intraductal papillary neoplasms (10/19; 52.6%) and visible mucin (19/19; 100%) on the surface of the tumor were typical characteristics of BT-IPMN.
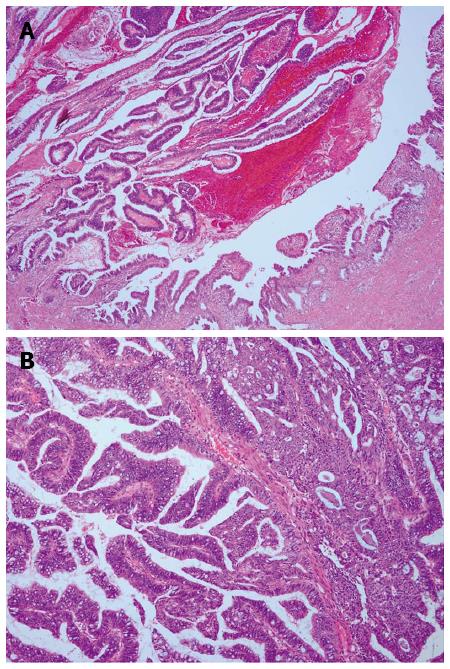
BT-IPMN is known to be classified into four histopathologic subtypes (gastric, intestinal, pancreatobiliary, and oncocytic) based on morphologic appearance and mucin staining properties[15], which are identical to those of P-IPMN. Microscopically, BT-IPMN was mucinous with papillary proliferation of biliary epithelial cells and intraductal growth. Mucin was observed with histopathology (Figure 3) in all 19 patients, and 10/19 (52.6%) cases were malignant (high-grade cytologic atypia), three of which had invasive components.
All patients underwent imaging examinations every 6-12 mo after surgery, with a median follow-up of 73 mo. Margin-negative resection was achieved in 18/19 (94.7%) patients, and palliative surgery (choledochojejunostomy and biopsy) was performed in one patient with malignant multifocal BT-IPMN.
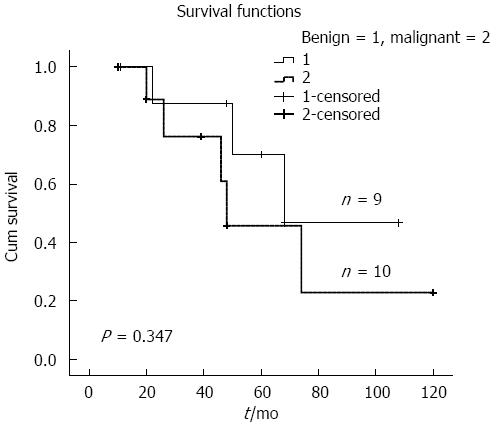
Overall median survival was 68 mo for the entire cohort; benign cases had a somewhat longer survival compared to malignant cases (68 mo vs 48 mo; P = 0.347) (Figure 4). Eight patients with BT-IPMN died; five malignant cases died due to tumors or tumor-related causes, including liver failure in one patient 22 mo after palliative surgery. Death in one benign BT-IPMN case was due to subsequent small cell lung cancer after 26 mo.
Although wide consensus has not yet been reached, BT-IPMN has been increasingly recognized as a unique type of biliary neoplasm and a biliary counterpart of P-IPMN[1,7]. The World Health Organization recognized intraductal papillary neoplasm of the bile duct (IPNB) as a distinct pathological entity in 2010[10]. Ohtsuka et al[16] suggested that IPNB with or without macroscopically visible mucin secretion differed in terms of pathologic features. In our study, BT-IPMN was defined as mucinous papillary neoplasm, demonstrating a clear origin from the biliary epithelium[1], and excluded lesions (such as IPNB) without microscopic or macroscopic mucin secretion. To some extent, BT-IPMN is a presumed subtype of IPNB, which has more similarity to P-IPMN than IPNB itself[16].
BT-IPMN shares some radiologic and clinicopathologic features with P-IPMN, but important differences between them may still exist. The frequency of malignancy is higher in patients with BT-IPMN (64%-89%) than in those with P-IPMN (23%-30%)[1,2,7,18]. Consistent with previous studies, the rate of malignant BT-IPMN in our series was > 50%. There are several reasons for the higher rate of malignancy in patients with BT-IPMN. First, as several recent reports have suggested, the majority of BT-IPMNs are of an intestinal or pancreatobiliary subtype, resembling those of main-duct-type P-IPMN, which is more aggressive than branch-duct P-IPMN[3,7,19]. Second, the biliary tract and the main pancreatic duct have identical embryologic development from the hepatic diverticulum in the foregut mesoderm[2,7].
In the present study, BT-IPMN mostly presented in patients aged 50-70 years, which is consistent with several other studies[1,3,7,10,20]. Although more male BT-IPMNs patients were found in our study, no difference was found in sex distribution based on previous reports[1,7,10]. The most common presenting symptom was abdominal pain, probably due to biliary stones, cholangitis, or high pressure of biliary tract causing mucin hypersecretion, which are associated with BT-IPMN[10,21]. Intraluminal hypersecretion of mucin from the bile duct may intermittently impede bile flow, leading to repeated episodes of cholangitis. Repeated cholangitis was found in approximately 32% of patients with BT-IPMN in our study, as a typical clinical presentation of BT-IPMN. Nearly 63% of BT-IPMNs were associated with biliary stones; most of which were proximal biliary stones. These findings indicate that the process of inflammatory stimulation may play a role in the development of BT-IPMN. BT-IPMNs were predominantly located in the intrahepatic bile duct and hilum, though the primary site of tumor origin does not affect the progress or prognosis of the disease[10,20,21]. Dilated bile ducts, intraluminal lesions, and/or gross cystic dilatation originating from the biliary tract are the most common abnormal preoperative imaging findings in BT-IPMN. Simultaneous proximal and distal bile duct dilation was found in approximately 68% of patients with BT-IPMN in our study. It has diagnostic significance, as with diffused pancreatic duct dilation for P-IPMN. The large amount of mucin discharged into the duct system leads to diffuse duct dilation.
Surgery is the first choice of treatment for patients with BT-IPMN without distant metastasis[22]. Determination of the optimal surgical strategy depends on the site and extent of the lesions. Intraoperative choledochoscopy and surgical margin frozen sectioning are performed to assess tumor location and extension, including superficial spreading along the biliary epithelium[23]. Hepatectomy should be performed for tumors located in the intrahepatic bile duct, whereas pancreatoduodenectomy and bile duct resection are performed for tumors located in the extrahepatic bile duct. Jarnagin et al[24] recommended regional lymphadenectomy for tumors localized in the hilum or distal bile duct. Lymph node metastasis is rare in benign BT-IPMN, and is less common in patients with invasive carcinoma arising from BT-IPMN, compared with conventional cholangiocarcinoma[8]. No patient in our series suffered from lymph node metastases. Portal vein resection is an option for tumors with blood vessel involvement[8]. Theoretically, resection of the entire biliary tract by liver transplantation could be a better option for curative treatment of diffuse BT-IPMN. Palliative surgery was performed in one patient with diffuse BT-IPMN in our study.
Only one patient died 22 mo after palliative surgery, with poorer survival than the overall median survival of 68 mo. Rocha et al[10] found that R0 resection was associated with better median survival than R1 resection. The median survival for the benign group in our study appeared better than for malignant cases, though the lack of significance may have been due to the relatively short follow-up period and small sample size. However, the difference may reflect an intrinsic difference in tumor biology. Complete tumor resection is associated with good survival, even in patients with malignant BT-IPMN.
The small number of patients in the present study prevented us from making strong conclusions. Moreover, a major limitation was the retrospective nature of the study. Diagnostic modalities for BT-IPMN, including imaging and pathology, have varied at different times. Nevertheless, due to the scarcity of patients, we are still justified in speculating on the trends that can be observed in this limited set of data. More multicenter prospective studies are necessary to identify the clinical and pathologic characteristics of BT-IPMN.
In conclusion, BT-IPMN is a rare biliary entity. Complete resection of the tumor is associated with good survival, even in patients with malignant BT-IPMN.
In the past decade, biliary tract intraductal papillary mucinous neoplasm (BT-IPMN) has been increasingly recognized as a unique type of biliary neoplasm, coinciding with widespread acceptance of the nomenclature of pancreatic IPMN. BT-IPMN is a rare neoplasm involving the intra- and extrahepatic biliary tracts and is characterized by mucin-secreting papillary and/or cystic lesions. However, there is still controversy over several aspects of BT-IPMN.
BT-IPMN is a rare biliary entity. The number of reports of BT-IPMN with strict histopathologic criteria is limited. Most of the data regarding BT-IPMN are from retrospective studies with small samples. There is still controversy surrounding several aspects of BT-IPMN, and clinicopathologic characteristics, surgical strategies, and prognosis are largely unclear.
This study involved a large number of patients with BT-IPMN from a large Chinese institution. The authors summarized the clinical features, radiologic findings, pathology, surgical strategies, and long-term follow-up of patients with BT-IPMN, and achieved a better understanding of this rare disease. The findings indicate that BT-IPMN is indeed a rare biliary entity and complete resection of the tumor is associated with good survival, even in patients with malignant disease.
BT-IPMN is a rare biliary entity. Complete resection of the tumor is associated with good survival, even in patients with malignant disease.
BT-IPMN is histologically defined as a mucinous and papillary neoplasm demonstrating a clear origin from the biliary epithelium, with solitary or diffuse intraductal growth.
This is a very interesting paper. The data are clearly presented and extensively discussed on the basis of the recent relevant international literature.
P- Reviewer: Frider B, Ooi LL S- Editor: Ma YJ L- Editor: AmEditor E- Editor: Zhang DN
| 1. | Barton JG, Barrett DA, Maricevich MA, Schnelldorfer T, Wood CM, Smyrk TC, Baron TH, Sarr MG, Donohue JH, Farnell MB. Intraductal papillary mucinous neoplasm of the biliary tract: a real disease? HPB (Oxford). 2009;11:684-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 2. | Sclabas GM, Barton JG, Smyrk TC, Barrett DA, Khan S, Kendrick ML, Reid-Lombardo KM, Donohue JH, Nagorney DM, Que FG. Frequency of subtypes of biliary intraductal papillary mucinous neoplasm and their MUC1, MUC2, and DPC4 expression patterns differ from pancreatic intraductal papillary mucinous neoplasm. J Am Coll Surg. 2012;214:27-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Zen Y, Fujii T, Itatsu K, Nakamura K, Minato H, Kasashima S, Kurumaya H, Katayanagi K, Kawashima A, Masuda S. Biliary papillary tumors share pathological features with intraductal papillary mucinous neoplasm of the pancreas. Hepatology. 2006;44:1333-1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 283] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 4. | Kim HJ, Kim MH, Lee SK, Yoo KS, Park ET, Lim BC, Park HJ, Myung SJ, Seo DW, Min YI. Mucin-hypersecreting bile duct tumor characterized by a striking homology with an intraductal papillary mucinous tumor (IPMT) of the pancreas. Endoscopy. 2000;32:389-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 93] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Oshikiri T, Kashimura N, Katanuma A, Maguchi H, Shinohara T, Shimizu M, Kondo S, Katoh H. Mucin-secreting bile duct adenoma--clinicopathological resemblance to intraductal papillary mucinous tumor of the pancreas. Dig Surg. 2002;19:324-327. [PubMed] |
| 6. | Yamashita Y, Fukuzawa K, Taketomi A, Aishima S, Yoshizumi T, Uchiyama H, Tsujita E, Harimoto N, Harada N, Wakasugi K. Mucin-hypersecreting bile duct neoplasm characterized by clinicopathological resemblance to intraductal papillary mucinous neoplasm (IPMN) of the pancreas. World J Surg Oncol. 2007;5:98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Minagawa N, Sato N, Mori Y, Tamura T, Higure A, Yamaguchi K. A comparison between intraductal papillary neoplasms of the biliary tract (BT-IPMNs) and intraductal papillary mucinous neoplasms of the pancreas (P-IPMNs) reveals distinct clinical manifestations and outcomes. Eur J Surg Oncol. 2013;39:554-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Yeh TS, Tseng JH, Chiu CT, Liu NJ, Chen TC, Jan YY, Chen MF. Cholangiographic spectrum of intraductal papillary mucinous neoplasm of the bile ducts. Ann Surg. 2006;244:248-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Shibahara H, Tamada S, Goto M, Oda K, Nagino M, Nagasaka T, Batra SK, Hollingsworth MA, Imai K, Nimura Y. Pathologic features of mucin-producing bile duct tumors: two histopathologic categories as counterparts of pancreatic intraductal papillary-mucinous neoplasms. Am J Surg Pathol. 2004;28:327-338. [PubMed] |
| 10. | Rocha FG, Lee H, Katabi N, DeMatteo RP, Fong Y, D’Angelica MI, Allen PJ, Klimstra DS, Jarnagin WR. Intraductal papillary neoplasm of the bile duct: a biliary equivalent to intraductal papillary mucinous neoplasm of the pancreas? Hepatology. 2012;56:1352-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 183] [Article Influence: 14.1] [Reference Citation Analysis (2)] |
| 11. | Jung G, Park KM, Lee SS, Yu E, Hong SM, Kim J. Long-term clinical outcome of the surgically resected intraductal papillary neoplasm of the bile duct. J Hepatol. 2012;57:787-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 12. | Yeh TS, Tseng JH, Chen TC, Liu NJ, Chiu CT, Jan YY, Chen MF. Characterization of intrahepatic cholangiocarcinoma of the intraductal growth-type and its precursor lesions. Hepatology. 2005;42:657-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Jonas S, Thelen A, Benckert C, Biskup W, Neumann U, Rudolph B, Lopez-Häänninen E, Neuhaus P. Extended liver resection for intrahepatic cholangiocarcinoma: A comparison of the prognostic accuracy of the fifth and sixth editions of the TNM classification. Ann Surg. 2009;249:303-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 14. | Nanashima A, Sumida Y, Abo T, Nagasaki T, Takeshita H, Fukuoka H, Sawai T, Tanaka K, Yasutake T, Nagayasu T. Patient outcome and prognostic factors in intrahepatic cholangiocarcinoma after hepatectomy. Hepatogastroenterology. 2007;54:2337-2342. [PubMed] |
| 15. | Zen Y, Pedica F, Patcha VR, Capelli P, Zamboni G, Casaril A, Quaglia A, Nakanuma Y, Heaton N, Portmann B. Mucinous cystic neoplasms of the liver: a clinicopathological study and comparison with intraductal papillary neoplasms of the bile duct. Mod Pathol. 2011;24:1079-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 16. | Ohtsuka M, Kimura F, Shimizu H, Yoshidome H, Kato A, Yoshitomi H, Furukawa K, Takeuchi D, Takayashiki T, Suda K. Similarities and differences between intraductal papillary tumors of the bile duct with and without macroscopically visible mucin secretion. Am J Surg Pathol. 2011;35:512-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 17. | Tanaka M, Fernández-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, Kimura W, Levy P, Pitman MB, Schmidt CM. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1714] [Cited by in RCA: 1614] [Article Influence: 124.2] [Reference Citation Analysis (0)] |
| 18. | Schnelldorfer T, Sarr MG, Nagorney DM, Zhang L, Smyrk TC, Qin R, Chari ST, Farnell MB. Experience with 208 resections for intraductal papillary mucinous neoplasm of the pancreas. Arch Surg. 2008;143:639-46; discussion 646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 177] [Article Influence: 10.4] [Reference Citation Analysis (1)] |
| 19. | Nakanuma Y. A novel approach to biliary tract pathology based on similarities to pancreatic counterparts: is the biliary tract an incomplete pancreas? Pathol Int. 2010;60:419-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 20. | Kim KM, Lee JK, Shin JU, Lee KH, Lee KT, Sung JY, Jang KT, Heo JS, Choi SH, Choi DW. Clinicopathologic features of intraductal papillary neoplasm of the bile duct according to histologic subtype. Am J Gastroenterol. 2012;107:118-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (1)] |
| 21. | Wan XS, Xu YY, Qian JY, Yang XB, Wang AQ, He L, Zhao HT, Sang XT. Intraductal papillary neoplasm of the bile duct. World J Gastroenterol. 2013;19:8595-8604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 108] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 22. | Kim JK, Hwang HK, Park JS, Cho SI, Yoon DS, Chi HS. Left hemihepatectomy and caudate lobectomy and complete extrahepatic bile duct resection using transduodenal approach for hilar cholangiocarcinoma arsing from biliary papillomatosis. J Surg Oncol. 2008;98:139-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Ohtsuka M, Shimizu H, Kato A, Yoshitomi H, Furukawa K, Tsuyuguchi T, Sakai Y, Yokosuka O, Miyazaki M. Intraductal papillary neoplasms of the bile duct. Int J Hepatol. 2014;2014:459091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 99] [Article Influence: 9.0] [Reference Citation Analysis (0)] |