Published online Apr 14, 2015. doi: 10.3748/wjg.v21.i14.4159
Peer-review started: November 9, 2014
First decision: December 11, 2014
Revised: December 30, 2014
Accepted: January 30, 2015
Article in press: January 30, 2015
Published online: April 14, 2015
Processing time: 160 Days and 1.4 Hours
AIM: To compare Institut Georges Lopez (IGL-1) and Celsior preservation solutions for hepatic endothelium relaxation and liver cold ischemia reperfusion injury (IRI).
METHODS: Two experimental models were used. In the first one, acetylcholine-induced endothelium-dependent relaxation (EDR) was measured in isolated ring preparations of rat hepatic arteries preserved or not in IGL-1 or Celsior solutions (24 h at 4 °C). To determine nitric oxide (NO) and cyclooxygenase EDR, hepatic arteries were incubated with L-NG-nitroarginine methyl ester (L-NAME), an inhibitor of endothelium nitric oxide synthase (eNOS), or with L-NAME plus indomethacin, an inhibitor of cyclooxygenase. In the second experiment, rat livers were cold-stored in IGL-1 or Celsior solutions for 24 h at 4 °C and then perfused “ex vivo” for 2 h at 37 °C. Liver injury was assessed by transaminase measurements, liver function by bile production and bromosulfophthalein clearance, oxidative stress by malondialdehyde levels and catalase activity and alterations in cell signaling pathways by pAkt, pAMPK, eNOS and MAPKs proteins level.
RESULTS: After cold storage for 24 h with either Celsior or IGL-1, EDR was only slightly altered. In freshly isolated arteries, EDR was exclusively mediated by NO. However, cold-stored arteries showed NO- and COX-dependent relaxation. The decrease in NO-dependent relaxation after cold storage was significantly more marked with Celsior. The second study indicated that IGL-1 solution obtained better liver preservation and protection against IRI than Celsior. Liver injury was reduced, function was improved and there was less oxidative stress. IGL-1 solution activated Akt and AMPK, which was concomitant with increased eNOS expression and nitrite/nitrate levels. Furthermore, MAPKs kinases were regulated in livers preserved with IGL-1 solution since reductions in p-p38, p-ERK and p-JNK protein levels were observed.
CONCLUSION: IGL-1 solution preserved NO-dependent relaxation better than Celsior storage solution and enhanced liver graft preservation.
Core tip: Vascular endothelium dysfunction plays an important role in various pathophysiological conditions. Protection of the vascular endothelium is a critical factor in organ preservation. This evaluation of the endothelium-dependent relaxation of rat hepatic artery after preservation in Celsior and Institut Georges Lopez (IGL-1) solutions provides the first head-to-head comparison of these two storage solutions. Our results show that cold storage of the hepatic artery with IGL-1 preserves nitric oxide-dependent endothelium-mediated relaxation better than cold storage with Celsior solution. In addition, we provide evidence that IGL-1 is more efficient than Celsior for liver preservation using an isolated perfused rat liver model.
- Citation: Tabka D, Bejaoui M, Javellaud J, Roselló-Catafau J, Achard JM, Abdennebi HB. Effects of Institut Georges Lopez-1 and Celsior preservation solutions on liver graft injury. World J Gastroenterol 2015; 21(14): 4159-4168
- URL: https://www.wjgnet.com/1007-9327/full/v21/i14/4159.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i14.4159
During transplantation, all organs are exposed to ischemia reperfusion injury (IRI). These lesions are related to hypothermia and hypoxia during ex vivo preservation of the graft and to the rewarming associated with reoxygenation during reperfusion[1-3]. Throughout preservation, tissues are exposed to cold and deprived of oxygen and nutrients, leading to the accumulation of metabolic waste. At the cellular level, the main biochemical changes of cold ischemia are ATP depletion, inhibition of oxidative metabolism, alteration of ionic homeostasis and increased acidosis due to anaerobic glycolysis[4]. These biochemical changes result in the establishment of a number of processes that will be amplified during the reperfusion phase.
Providing optimal organ preservation remains an important way to ensure the quality of the transplanted organ. The University of Wisconsin (UW) solution is the standard liquid for the procurement of abdominal organs and their static cold storage[4-6]. Celsior preservation solution, originally used for cardioplegia[7-9], has demonstrated its safety and effectiveness for cold preservation of the lungs[10], liver[11], kidneys[12] and pancreas[13,14] in different clinical studies and is now widely used in European countries. It is a colloid-free extracellular-type solution containing high molecular weight impermeants (lactobionic acid and mannitol), free radical scavengers (reduced glutathione) and an energy precursor (glutamic acid). Histidine is added in order to buffer intracellular acidosis and contributes to preventing calcium overload. Studies suggest that the benefits of this solution are due to its low viscosity, which results in better flushing of the biliary tree and of the hepatic vasculature during graft procurement[11,15].
Institut Georges Lopez-1 (IGL-1) preservation solution was designed following the same specifications as the UW solution; however, it is characterized by the inversion of K+ and Na+ concentrations and the replacement of hydroxyethyl starch by a high molecular weight polyethylene glycol of 35 kDa (PEG35)[16]. It has demonstrated its effectiveness for cold preservation of kidney[17], intestine[18], liver[19] and pancreas[20] in experimental and clinical settings. The benefits of IGL-1 are due, in part, to its capacity to increase nitric oxide (NO) levels, thus protecting the liver against IRI and mitigating the alterations to hepatic microcirculation[21]. In an experimental model of rat liver transplantation, it was recently observed that IGL-1 reduced endoplasmic reticulum stress and apoptosis[22]. Therefore, the use of IGL-1 solution may offer certain advantages for attenuating injury associated with liver transplantation.
Although several comparative studies of preservation solutions have evaluated the benefits and disadvantages of each solution in animal experimental models and randomized controlled trials[6,12,23], no direct head-to-head comparison of IGL-I and Celsior has been reported to date.
The purpose of this study was therefore to compare the protective effects of Celsior and IGL-1 solutions against IRI and to study their effects on endothelium-dependent relaxation (EDR).
Male Sprague-Dawley rats weighing 250-300 g were used. They were acclimatized to laboratory conditions (23 °C, 12 h/12 h light/dark, 50% humidity, ad libitum access to food and water) for 2 wk prior to experimentation. All experiments were in accordance with guidelines for the ethical care of experimental animals of the European Community and were approved by the Regional Ethics Committee for Animal Experimentation of Limousin (CREEAL) (authorization No. 33). Two studies were conducted with different experimental models: hepatic artery rings in the first and isolated perfused rat liver (IPRL) in the second.
The tissue preparation procedure was performed as previously reported[24]. Briefly, rats were anesthetized with isoflurane inhalation and then underwent laparotomy. The hepatic artery was exposed and cleaned of adhering fat and connective tissues. After sacrificing the rat, the hepatic artery was cut and stored in Celsior (n = 11) or IGL-1 (n = 11) storage solutions at 4 °C for 24 h (composition in Table 1) and then suspended in the organ bath. Hepatic artery rings from the control group (n = 12) were suspended immediately in the organ bath without cold storage.
| Components | IGL-1 | Celsior |
| K+ (mmol/L) | 25 | 15 |
| Na+ (mmol/L) | 125 | 100 |
| Mg2+ (mmol/L) | 5 | 13 |
| Ca2+ (mmol/L) | - | 0.25 |
| Cl- (mmol/L) | - | 40 |
| SO42- (mmol/L) | 5 | - |
| Diphosphate (mmol/L) | 25 | - |
| Histidine (mmol/L) | - | 30 |
| Raffinose (mmol/L) | 30 | - |
| Lactobionic acid (mmol/L) | 100 | 80 |
| Mannitol (mmol/L) | - | 60 |
| Polyethylene glycol-35 (g/L) | 1 | - |
| Reduced glutathione (mmol/L) | 3 | 3 |
| Allopurinol (mmol/L) | 1 | - |
| Adenosine (mmol/L) | 5 | - |
| Glutamic acid (mmol/L) | - | 20 |
| pH (room T°) | 7.3 ± 0.1 | 7.3 ± 0.1 |
Vasomotor responses were assessed as previously described[24]. The hepatic arteries were cut in rings of 2-3 mm in length and were then maintained between two stainless steel wires (100 μm) connected to an isometric force transducer (Powerlab, AD Instruments). The rings were placed in an organ bath (9 mL, 37 °C) filled with gassed (95% O2 - 5% CO2) Krebs Bicarbonate Buffer (KBB, pH 7.4) (VWR, France). Tissues were stretched to their optimum tension of 0.75 g and equilibrated and then washed with fresh KBB at 20 min intervals for 60 min. Care was taken to avoid rubbing the endothelial surface of the vessels with the intact endothelium.
To determine EDR, hepatic artery rings were maximally pre-contracted with phenylephrine (Phe 10-6 mol/L, Sigma) before cumulative application of acetylcholine (ACh 10-9 to 3 × 10-6 mol/L, Sigma). Relaxation activity was evaluated as the effective concentration of ACh producing 50% relaxation of pre-contracted rings (EC50).
To study the respective contribution of endothelial nitric oxide synthase (eNOS) and cyclooxygenase (COX) to vessel relaxation, ACh-induced relaxation curves were constructed with artery rings pretreated either with L-NG-nitroarginine methyl ester (L-NAME 10-4 mol/L, Sigma), an inhibitor of eNOS, or with the combination of L-NAME and indomethacin (10-4 mol/L, Sigma), a cyclooxygenase inhibitor.
The rats were anesthetized with intraperitoneal administration of urethane (1.5 g/kg). The surgical procedure was performed as previously reported[21]. Briefly, after laparotomy, a catheter was inserted into the common bile duct for bile collection and the aorta, vena cava and portal vein were dissected. Organs were washed out with Celsior (n = 6) or IGL-1 (n = 6) storage solution and then excised and trimmed of adhering tissue. Livers were stored in a small container with 50 mL of either solution for 24 h at 4 °C. Livers of the control group (n = 6) were flushed with Ringer’s lactate and immediately perfused ex vivo without cold ischemic storage.
Before ex vivo reperfusion, all the livers were exposed to 20 min ischemia at room temperature (in order to simulate the rewarming period during surgical implantation in vivo) and then flushed with 10 mL of Ringer’s lactate solution. Effluent liquid was sampled for measurement of cumulative transaminases after prolonged ischemia. Livers were then connected via the portal vein to a recirculating perfusion system for 120 min at 37 °C. The reperfusion liquid (140 mL) was KBB enriched with 5% albumin (Sigma). The buffer was continuously gassed with a 95% O2 and 5% CO2 gas mixture and subsequently passed through a heat exchanger (37 °C) and bubble trap prior to entering the liver. At the end of the normothermic reperfusion, the effluent fluid and tissue specimens were collected for examination.
The extent of hepatic injury after cold preservation and reperfusion periods was assessed in terms of activities of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in perfusate. Enzyme activities were determined at 340 nm with an UV-visible spectrometer using a commercialized kit (bioMérieux, France).
Hepatic clearance was performed as previously reported[21]. Briefly, 1 mg of bromosulfophthalein (BSP, Sigma) was added to the perfusate after 30 min of normothermic perfusion. BSP clearance in bile was measured at 580 nm and expressed as percentage of perfusate content.
Bile production was monitored during reperfusion via cannulation of the bile duct and collection of bile. Bile output was reported as μL/g of liver.
Lipid peroxidation was used as an indirect indicator of the oxidative injury induced by reactive oxygen species. It was assessed by determination of malondialdehyde (MDA) with the thiobarbiturate reaction.
Catalase activity was measured by the amount of hydrogen peroxide split by catalase in 5, 10, 15, 20, 25 and 30 s at 25 °C. The reaction was started by addition of the liver supernatant and assayed at 240 nm using a UV-visible spectrometer.
Nitrate and nitrite levels in the liver tissue were determined by the Greiss reaction using a colorimetric assay kit (Cayman Chemical, United States).
Frozen tissue was homogenized as previously described[25]. Equal amounts of protein were suspended in Laemmli buffer and loaded on SDS-PAGE gel, then transferred to PVDF membranes. Membranes were immunoblotted using the following antibodies: eNOS (Transduction Laboratories, Lexington, KY, United States), β-actin (Sigma Chemical, St. Louis, MO, United States), total and phosphorylated protein kinase B (AKT), mitogen-activated protein kinase (MAPK) p38, extracellular signal-regulated kinases (ERK) and c-Jun N-terminal kinase (JNK), total and phosphorylated AMP activated protein kinase (AMPK) (Cell Signaling, Beverly, MA, United States). Proteins were visualized on X-ray film via chemiluminescence (Bio-Rad Laboratories, Hercules, CA, United States) and quantified by scanning densitometry.
Results were expressed as mean ± SEM and were evaluated by one-way ANOVA followed by the Newman-Keuls test for multiple comparisons (Graph Pad Prism software version 4 for Windows). Statistical significance was defined as a P < 0.05.
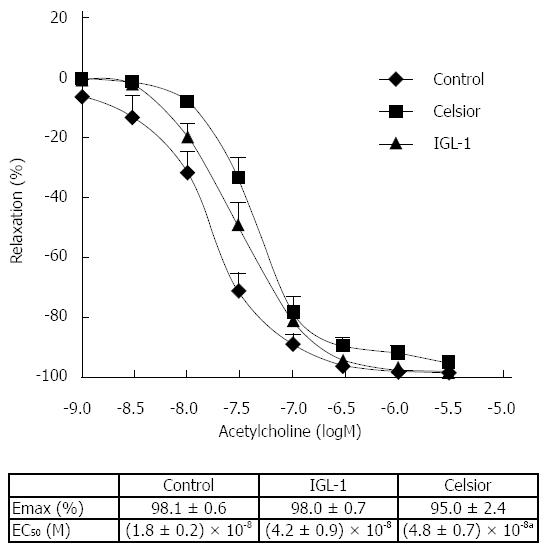
EDR of hepatic artery rings stored in Celsior and IGL-1 liquids was compared with that of freshly isolated arteries (Figure 1). No statistical differences between the experimental groups were found for ACh relaxation (Emax). However, the curves of both cold-stored groups were shifted to the right with regard to the control group and the relaxation activity was significantly decreased in the Celsior group [EC50 = (4.8 ± 0.7) × 10-8vs (1.8 ± 0.2) × 10-8 for control and Celsior groups respectively, P < 0.05].
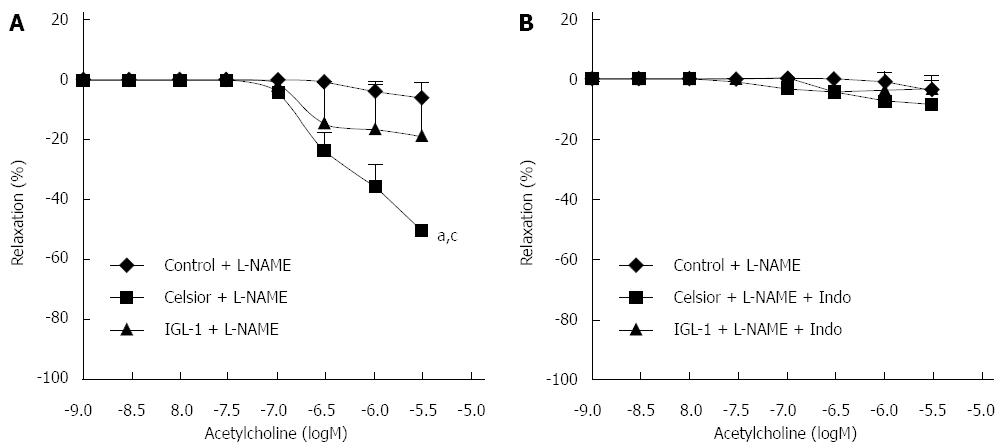
The artery rings were incubated with L-NAME in order to evaluate the role of eNOS pathway in vessel relaxation after cold storage. As shown in Figure 2A, EDR was almost completely inhibited in the control group after incubation with L-NAME, evidencing that, in freshly isolated hepatic arteries, the endothelium-dependent relaxation was virtually exclusively NO-dependent. A small relaxation was still observed in the IGL-1 group, with no significant difference compared to controls. However, hepatic artery rings preserved in Celsior solution showed greater relaxation than in the other groups (P < 0.05). Moreover, the incubation of cold-stored hepatic arteries in the presence of both L-NAME and indomethacin (Figure 2B) completely abolished the relaxation induced by ACh.
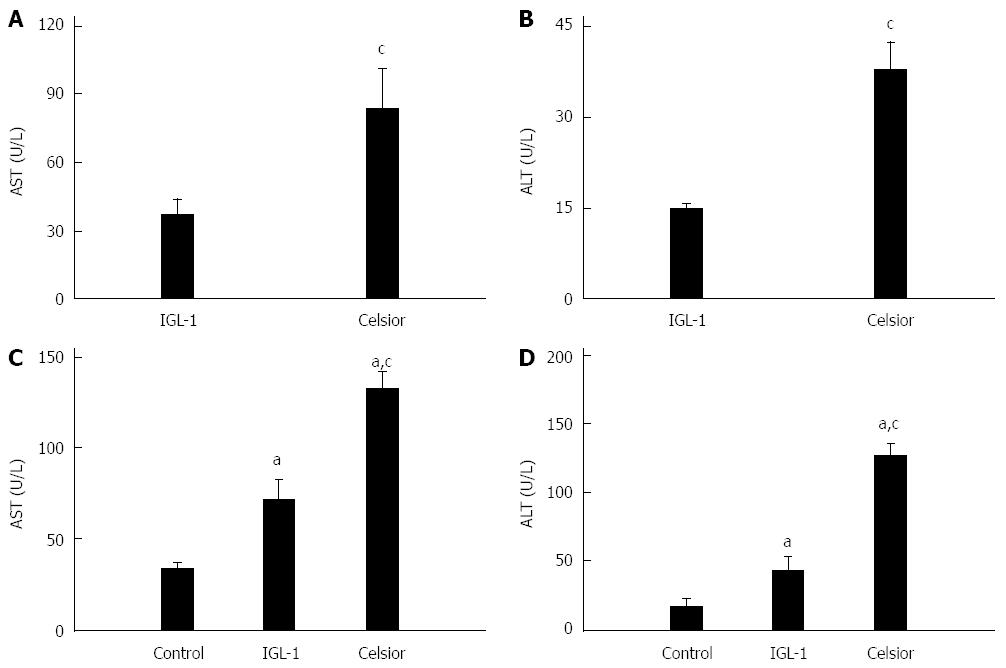
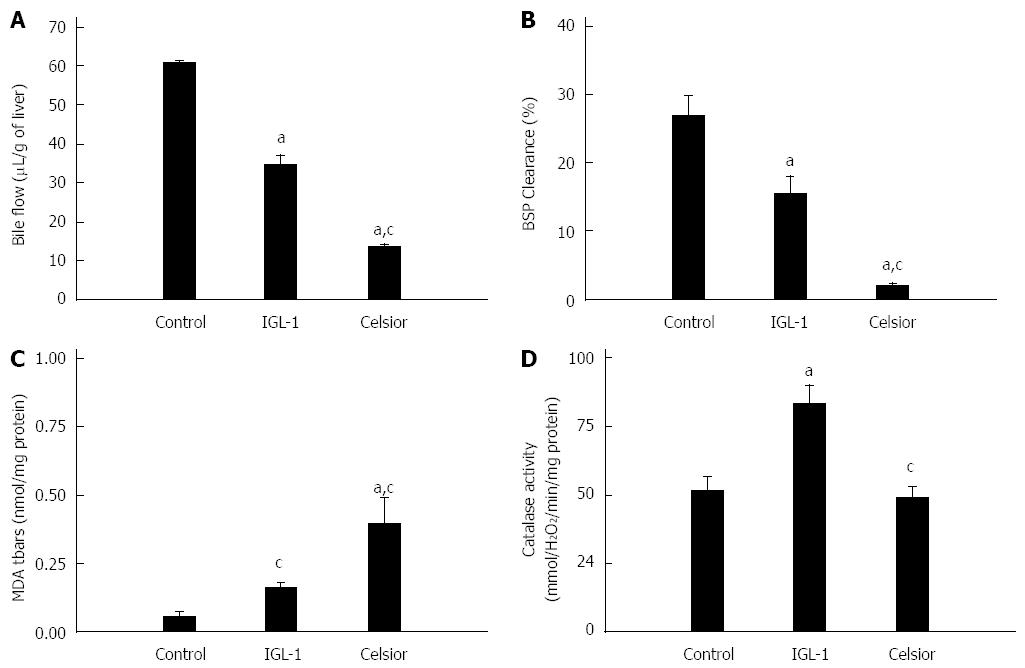
The livers preserved in the IGL-1 released significantly lower levels of AST and ALT than those preserved in the Celsior solution after 24 h of cold ischemia (Figure 3A and B) and after 2 h of normothermic reperfusion (Figure 3C and D). Liver function was assessed by the measurement of bile production and BSP clearance. As indicated in Figure 4A and B, both bile flow and BSP clearance were significantly higher in the IGL-1 group than in the Celsior group.
In order to evaluate the effect of preservation solutions on oxidative stress, we measured MDA levels (Figure 4C) and catalase activity (Figure 4D). We found a significant reduction in MDA in liver preserved in IGL-1 solution when compared to Celsior. Livers preserved in IGL-1 solution also showed higher catalase activity after normothermic perfusion than those preserved in Celsior solution.
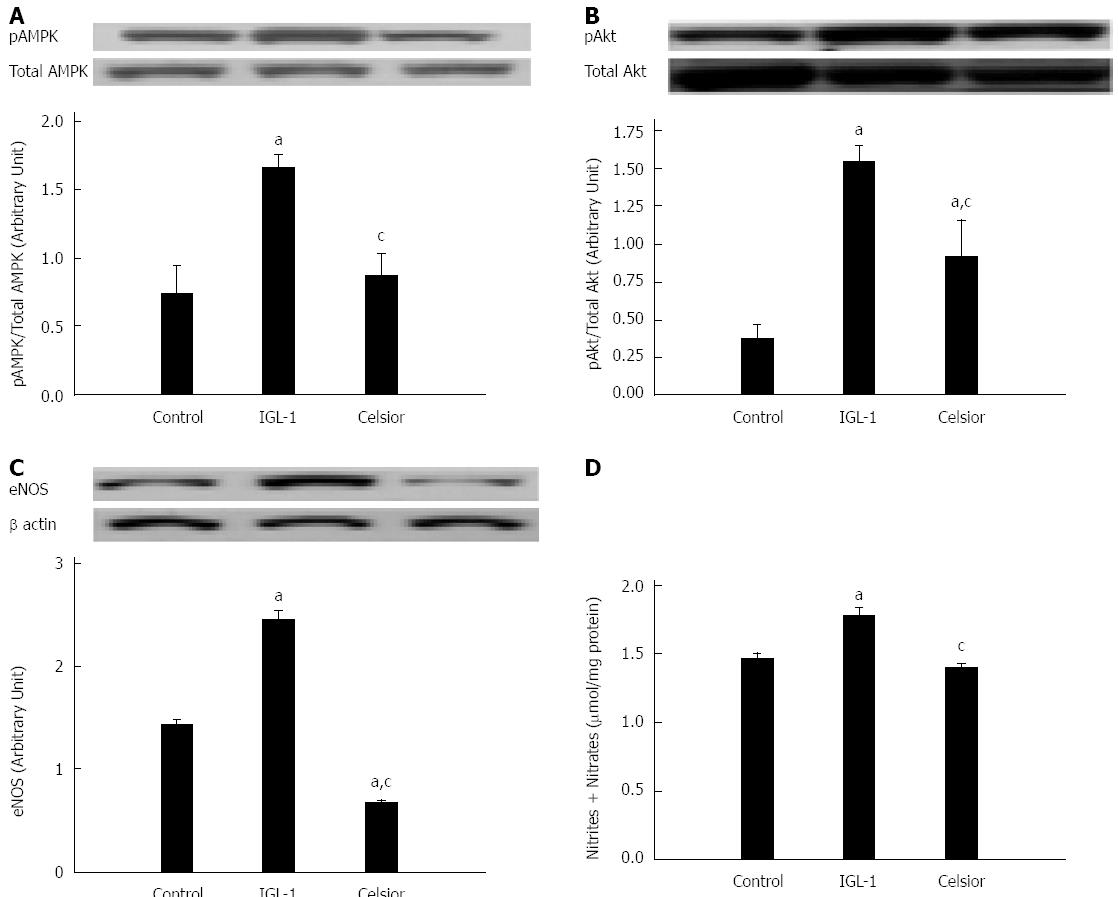
The role of NO after cold storage was evaluated by the phosphorylation status of AMPK (Figure 5A) and AKT (Figure 5B) and their target protein eNOS (Figure 5C) after cold preservation and reperfusion. The use of IGL-1 solution significantly increased the phosphorylation of AMPK and AKT. This was concomitant with eNOS activation and increased NO production, as evidenced by the higher concentrations of nitrites and nitrates in tissues (Figure 5D).
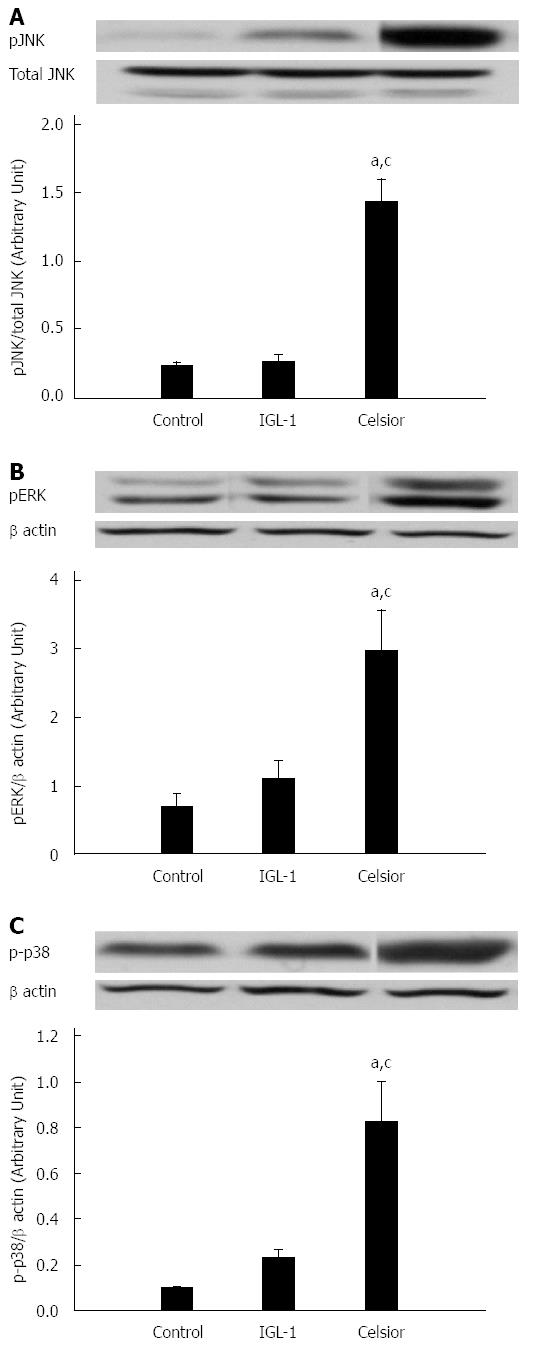
We also examined whether the MAPKs kinase signaling pathway was activated after liver cold preservation and reperfusion. As shown in Figure 6, we found that the levels of phosphorylated JNK, ERK and p38 MAPK were significantly higher in the Celsior group than in the control and IGL-1 groups. No statistical differences were observed between IGL-1 and control groups.
Vascular endothelium dysfunction plays an important role in various pathophysiological conditions. Protection of the vascular endothelium is a critical factor in organ preservation[26-28]. In the present study, we aimed to evaluate the EDR of rat hepatic artery after preservation in Celsior and IGL-1 solutions. The EDR mechanism is based on three effectors: NO, prostanoids derived from COX and EDHF, whose exact nature has not been fully elucidated[29]. Current data show that the endothelium is significantly damaged in stored tissues. Jeng et al[30] showed that cold preservation of human hepatic artery in UW solution, even for a short period, attenuated the EDR and maintained the hypoxia-induced contraction, suggesting an increased risk of vasospasm and thrombosis. A more recent study comparing eight preservation solutions, not including IGL-1, evidenced altered endothelial structure and function after exposure to a combination of warm and cold ischemia[31]. Our results indicate that, after cold storage for 24 h with either Celsior or IGL-1, EDR was only slightly altered. Emax was not affected and the concentration-response curves of cold-stored hepatic arterial segments with both preservation solutions were shifted only slightly to the right. The small increase in the EC50 was only significant for Celsior preservation in comparison with the freshly isolated control arteries. Interestingly, the analysis of the effect of NO and COX inhibition revealed substantial differences. Whereas in freshly isolated arteries EDR was virtually abolished after incubation with L-NAME, indicating that the relaxation was exclusively NO-dependent, cold-stored arteries were still relaxed following NO inhibition. This remaining relaxation was completely blunted with indomethacin pre-incubation, suggesting that cold storage was responsible for the decrease in NO- dependent relaxation and for the recruitment of a compensatory induction of COX-dependent relaxation. The molecular mechanisms involved in the reciprocal regulation of COX and NOS enzymes are complex and still not fully understood[32]. It has been reported that the increased production of the vasodilating prostaglandin I2 (PGI2) by COX is caused by the NOS inhibitor L-NAME, indicating that low concentrations of NO are necessary to induce the production of prostacyclin. Gambone et al[33] have proposed that this interaction between PGI2 and NO may represent a compensatory mechanism allowing an increase in one of these agents when the concentration of the other one decreases. Our data emphasize reduced NO production rather than elevated prostacyclin production in hepatic arteries undergoing 24 h of cold storage. This compensatory mechanism likely explains why the final result of acetylcholine stimulation relaxation (Emax) is similar in freshly isolated and cold-stored hepatic artery rings.
As regards the comparison of the two preservation solutions, our most salient finding is that, whereas the prostacyclin-mediated relaxation accounted for 50% of the maximal relaxation in arteries stored with Celsior, it accounted for significantly less (20%) after storage with IGL-1. In other words, 50% of NO-dependent relaxation was lost after storage with Celsior, whereas 80% of the NO-dependent relaxation of hepatic arteries was preserved after storage with IGL-1.
The future success of liver transplantation will depend on achieving marked improvements in graft quality and preservation techniques[6,34]. Static cold storage is the most commonly applied method for liver grafts. Preservation solutions play a crucial role in maintaining graft viability and in improving outcomes after transplantation[35]. In the second part of this study, we compared the effects of Celsior and IGL-1 solutions on liver cold preservation and demonstrated that IGL-1 solution was more efficient in preventing cold IRI. This effective protection was concomitant with the prevention of oxidative stress and the subsequent improvement in the hepatic function, reflected by bile production and BSP clearance.
It is well known that sinusoidal endothelial cells cannot tolerate prolonged cold ischemia[27]. Endothelial dysfunction is commonly associated with a decrease in eNOS-derived NO bioavailability, leading to the initiation of liver reperfusion injury[36]. It is now agreed that eNOS activation protects against cold ischemia by the subsequent release of NO[28]. One intracellular route that mediates the activation of eNOS in ischemic liver is the Akt-eNOS pathway[37,38]. Moreover, AMPK, an enzyme that senses the cellular energy balance and regulates downstream signaling pathways, has been shown to activate eNOS and increase NO release in rat liver after cold preservation[39]. AMPK activation is known to limit endothelial dysfunction after cold preservation and protect against IRI[40,41]. Our results show a significant increase in Akt and AMPK phosphorylation, concomitant with improvements in eNOS and nitrite and nitrate levels in livers stored in IGL-1 solution when compared to Celsior solution. This may demonstrate the ability of IGL-1 solution to prevent endothelial dysfunction through the activation of eNOS by both AMPK and AKT.
The renewed interest in organ preservation has resulted in the recognition of new mechanisms involved in graft cytoprotection and viability. In the last part of this study, we investigated the repercussion of cold preservation of livers with Celsior and IGL-1 solutions on MAPKs. The MAPK superfamily represents a group of proteins (pP38, pJNK and pERK) that are known to be important mediators of cold ischemia-related events. It has been reported that MAPKs are activated after cold preservation and contribute to liver apoptosis and necrosis[42,43]. The use of IGL-1 seems to minimize the harmful effects of cold storage, as shown by the lower levels of phosphorylated p38, JNK and ERK when compared to Celsior group.
In conclusion, this study shows that cold storage of hepatic artery with IGL-1 preserves NO-dependent endothelium-mediated relaxation better than storage with Celsior. In addition, we provide evidence that IGL-1 is more efficient than Celsior for liver preservation.
Organ preservation solutions maintain graft viability until the transplantation of the organ. Providing optimal organ preservation remains an important way to ensure the quality of the transplanted organ.
Celsior preservation solution, which was originally used for cardioplegia, has proved its safety and effectiveness for cold preservation of the lungs, liver, kidneys and pancreas in different clinical studies and is now widely used in European countries. Institut Georges Lopez (IGL-1) preservation solution has proved its effectiveness for cold preservation of kidney, intestine, liver and pancreas in clinical settings. Its benefits are due, in part, to its capacity to increase the levels of nitric oxide (NO), thus protecting the liver against I/R injury and mitigating the alterations to hepatic microcirculation. Vascular endothelium dysfunction plays an important role in various pathophysiological conditions. Protection of the vascular endothelium is a critical factor in organ preservation. In this study, the authors demonstrate that IGL-1 solution preserved NO-dependent relaxation better than Celsior storage solution and enhanced liver graft preservation.
Several comparative studies of preservation solutions have aimed to evaluate the benefits and disadvantages of each of these solutions on animal experiments models and randomized controlled trials. This is the first study to provide a head-to-head comparison between IGL-1 and Celsior. This study demonstrates that IGL-1 solution is more effective for preserving endothelium function after cold preservation.
This study highlights the importance of the storage solution in the preservation of endothelium function and graft quality. Moreover, it may help researchers to develop more effective preservation solutions and may guide clinicians’ choice of solution when risk factors are present.
This is a nice paper providing new insights into mechanisms of action of IGL-1 and Celsior preservation solutions in liver cold ischemia reperfusion injury. The authors have shown that a decrease in NO dependent relaxation after cold storage was less pronounced with IGl-1 solution and that IGL-1 offered better protection against IRI in comparison to Celsior.
P- Reviewer: Rydzewski A S- Editor: Qi Y L- Editor: Roemmele A E- Editor: Ma S
| 1. | Henry SD, Nachber E, Tulipan J, Stone J, Bae C, Reznik L, Kato T, Samstein B, Emond JC, Guarrera JV. Hypothermic machine preservation reduces molecular markers of ischemia/reperfusion injury in human liver transplantation. Am J Transplant. 2012;12:2477-2486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 122] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 2. | Zaouali MA, Boncompagni E, Reiter RJ, Bejaoui M, Freitas I, Pantazi E, Folch-Puy E, Abdennebi HB, Garcia-Gil FA, Roselló-Catafau J. AMPK involvement in endoplasmic reticulum stress and autophagy modulation after fatty liver graft preservation: a role for melatonin and trimetazidine cocktail. J Pineal Res. 2013;55:65-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 3. | Brunner SM, Junger H, Ruemmele P, Schnitzbauer AA, Doenecke A, Kirchner GI, Farkas SA, Loss M, Scherer MN, Schlitt HJ. Bile duct damage after cold storage of deceased donor livers predicts biliary complications after liver transplantation. J Hepatol. 2013;58:1133-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 4. | Belzer FO, Southard JH. Principles of solid-organ preservation by cold storage. Transplantation. 1988;45:673-676. [PubMed] |
| 5. | O'Callaghan JM, Knight SR, Morgan RD, Morris PJ. Preservation solutions for static cold storage of kidney allografts: a systematic review and meta-analysis. Am J Transplant. 2012;12:896-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 6. | Parsons RF, Guarrera JV. Preservation solutions for static cold storage of abdominal allografts: which is best? Curr Opin Organ Transplant. 2014;19:100-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Menasché P, Termignon JL, Pradier F, Grousset C, Mouas C, Alberici G, Weiss M, Piwnica A, Bloch G. Experimental evaluation of Celsior, a new heart preservation solution. Eur J Cardiothorac Surg. 1994;8:207-213. [PubMed] |
| 8. | Karam G, Compagnon P, Hourmant M, Despins P, Duveau D, Noury D, Boudjema K. A single solution for multiple organ procurement and preservation. Transpl Int. 2005;18:657-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | George TJ, Arnaoutakis GJ, Baumgartner WA, Shah AS, Conte JV. Organ storage with University of Wisconsin solution is associated with improved outcomes after orthotopic heart transplantation. J Heart Lung Transplant. 2011;30:1033-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | De Santo LS, Romano G, Amarelli C, Della Corte A, Onorati F, Torella M, De Feo M, Nappi GA, Cotrufo M. Pilot study on prevention of lung injury during surgery for type A acute aortic dissection: no evident improvements with celsior flushing through the pulmonary artery. Int J Artif Organs. 2003;26:1032-1038. [PubMed] |
| 11. | Boudjema K, Grandadam S, Compagnon P, Salamé E, Wolf P, Ducerf C, Le Treut P, Soubrane O, Cherqui D, Mouchel C. Efficacy and safety of Celsior preservation fluid in liver transplantation: one-year follow up of a prospective, multicenter, non-randomized study. Clin Transplant. 2012;26:199-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Tillou X, Collon S, Surga N, Jaureguy M, Viart L, Mazouz H, Gigante M. Comparison of UW and Celsior: long-term results in kidney transplantation. Ann Transplant. 2013;18:146-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Uhlmann D, Armann B, Ludwig S, Escher E, Pietsch UC, Tannapfel A, Teupser D, Hauss J, Witzigmann H. Comparison of Celsior and UW solution in experimental pancreas preservation. J Surg Res. 2002;105:173-180. [PubMed] |
| 14. | Boggi U, Vistoli F, Del Chiaro M, Signori S, Croce C, Pietrabissa A, Berchiolli R, Marchetti P, Del Prato S, Mosca F. Pancreas preservation with University of Wisconsin and Celsior solutions: a single-center, prospective, randomized pilot study. Transplantation. 2004;77:1186-1190. [PubMed] |
| 15. | van As AB, Lotz Z, Tyler M, Kahn D. Impact of Celsior solution on hepatocellular, reperfusion and endothelial cell injury after liver transplantation. Dig Liver Dis. 2001;33:181-186. [PubMed] |
| 16. | Ben Abdennebi H, Steghens JP, Hadj-Aïssa A, Barbieux A, Ramella-Virieux S, Gharib C, Boillot O. A preservation solution with polyethylene glycol and calcium: a possible multiorgan liquid. Transpl Int. 2002;15:348-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 17. | Codas R, Petruzzo P, Morelon E, Lefrançois N, Danjou F, Berthillot C, Contu P, Espa M, Martin X, Badet L. IGL-1 solution in kidney transplantation: first multi-center study. Clin Transplant. 2009;23:337-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Yandza T, Tauc M, Canioni D, Rogel-Gaillard C, Bernard G, Bernard A, Gugenheim J. Effect of polyethylene glycol in pig intestinal allotransplantation without immunosuppression. J Surg Res. 2012;176:621-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Dondéro F, Paugam-Burtz C, Danjou F, Stocco J, Durand F, Belghiti J. A randomized study comparing IGL-1 to the University of Wisconsin preservation solution in liver transplantation. Ann Transplant. 2010;15:7-14. [PubMed] |
| 20. | Niclauss N, Wojtusciszyn A, Morel P, Demuylder-Mischler S, Brault C, Parnaud G, Ris F, Bosco D, Badet L, Benhamou PY. Comparative impact on islet isolation and transplant outcome of the preservation solutions Institut Georges Lopez-1, University of Wisconsin, and Celsior. Transplantation. 2012;93:703-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Ben Mosbah I, Roselló-Catafau J, Franco-Gou R, Abdennebi HB, Saidane D, Ramella-Virieux S, Boillot O, Peralta C. Preservation of steatotic livers in IGL-1 solution. Liver Transpl. 2006;12:1215-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 22. | Mosbah IB, Zaouali MA, Martel C, Bjaoui M, Abdennebi HB, Hotter G, Brenner C, Roselló-Catafau J. IGL-1 solution reduces endoplasmic reticulum stress and apoptosis in rat liver transplantation. Cell Death Dis. 2012;3:e279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Lema Zuluaga GL, Serna Agudelo RE, Zuleta Tobón JJ. Preservation solutions for liver transplantation in adults: celsior versus custodiol: a systematic review and meta-analysis with an indirect comparison of randomized trials. Transplant Proc. 2013;45:25-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 24. | Nasser M, Clere N, Botelle L, Javellaud J, Oudart N, Faure S, Achard JM. Opposite effects of angiotensins receptors type 2 and type 4 on streptozotocin induced diabetes vascular alterations in mice. Cardiovasc Diabetol. 2014;13:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Bejaoui M, Zaouali MA, Folch-Puy E, Pantazi E, Bardag-Gorce F, Carbonell T, Oliva J, Rimola A, Abdennebi HB, Roselló-Catafau J. Bortezomib enhances fatty liver preservation in Institut George Lopez-1 solution through adenosine monophosphate activated protein kinase and Akt/mTOR pathways. J Pharm Pharmacol. 2014;66:62-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Janssen H, Janssen PH, Broelsch CE. Value of energy substrates in HTK and UW to protect human liver endothelial cells against ischemia and reperfusion injury. Eur Surg Res. 2004;36:26-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Stolz DB, Ross MA, Ikeda A, Tomiyama K, Kaizu T, Geller DA, Murase N. Sinusoidal endothelial cell repopulation following ischemia/reperfusion injury in rat liver transplantation. Hepatology. 2007;46:1464-1475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 28. | Russo L, Gracia-Sancho J, García-Calderó H, Marrone G, García-Pagán JC, García-Cardeña G, Bosch J. Addition of simvastatin to cold storage solution prevents endothelial dysfunction in explanted rat livers. Hepatology. 2012;55:921-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 29. | Knes JM, Hansen TN, Gilligan B, Woo H, Mangino M, Haworth RA, Southard JH. Loss of endothelium-dependent relaxation in abdominal aorta preserved in a co-storage system. Transpl Int. 2005;17:699-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 30. | Jeng LB, Lin PJ, Yao PC, Chen MF, Tsai KT, Chang CH. Impaired endothelium-dependent relaxation of human hepatic arteries after preservation with the University of Wisconsin solution. Arch Surg. 1997;132:7-12. [PubMed] |
| 31. | Wilson CH, Stansby G, Haswell M, Cunningham AC, Talbot D. Evaluation of eight preservation solutions for endothelial in situ preservation. Transplantation. 2004;78:1008-1013. [PubMed] |
| 32. | Cuzzocrea S, Salvemini D. Molecular mechanisms involved in the reciprocal regulation of cyclooxygenase and nitric oxide synthase enzymes. Kidney Int. 2007;71:290-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 96] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 33. | Gambone LM, Murray PA, Flavahan NA. Synergistic interaction between endothelium-derived NO and prostacyclin in pulmonary artery: potential role for K+ATP channels. Br J Pharmacol. 1997;121:271-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Akhtar MZ, Henderson T, Sutherland A, Vogel T, Friend PJ. Novel approaches to preventing ischemia-reperfusion injury during liver transplantation. Transplant Proc. 2013;45:2083-2092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 35. | Ploeg RJ, van Bockel JH, Langendijk PT, Groenewegen M, van der Woude FJ, Persijn GG, Thorogood J, Hermans J. Effect of preservation solution on results of cadaveric kidney transplantation. The European Multicentre Study Group. Lancet. 1992;340:129-137. [PubMed] |
| 36. | Peralta C, Jiménez-Castro MB, Gracia-Sancho J. Hepatic ischemia and reperfusion injury: effects on the liver sinusoidal milieu. J Hepatol. 2013;59:1094-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 471] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 37. | Zaouali MA, Padrissa-Altés S, Ben Mosbah I, Ben Abdennebi H, Boillot O, Rimola A, Saidane-Mosbahi D, Roselló-Catafau J. Insulin like growth factor-1 increases fatty liver preservation in IGL-1 solution. World J Gastroenterol. 2010;16:5693-5700. [PubMed] |
| 38. | Grossini E, Pollesello P, Bellofatto K, Sigaudo L, Farruggio S, Origlia V, Mombello C, Mary DA, Valente G, Vacca G. Protective effects elicited by levosimendan against liver ischemia/reperfusion injury in anesthetized rats. Liver Transpl. 2014;20:361-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 39. | Ben Mosbah I, Roselló-Catafau J, Alfany-Fernandez I, Rimola A, Parellada PP, Mitjavila MT, Lojek A, Ben Abdennebi H, Boillot O, Rodés J. Addition of carvedilol to University Wisconsin solution improves rat steatotic and nonsteatotic liver preservation. Liver Transpl. 2010;16:163-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 40. | Bouma HR, Ketelaar ME, Yard BA, Ploeg RJ, Henning RH. AMP-activated protein kinase as a target for preconditioning in transplantation medicine. Transplantation. 2010;90:353-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 41. | Padrissa-Altés S, Zaouali MA, Bartrons R, Roselló-Catafau J. Ubiquitin-proteasome system inhibitors and AMPK regulation in hepatic cold ischaemia and reperfusion injury: possible mechanisms. Clin Sci (Lond). 2012;123:93-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 42. | Amersi F, Shen XD, Anselmo D, Melinek J, Iyer S, Southard DJ, Katori M, Volk HD, Busuttil RW, Buelow R. Ex vivo exposure to carbon monoxide prevents hepatic ischemia/reperfusion injury through p38 MAP kinase pathway. Hepatology. 2002;35:815-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 187] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 43. | Kaizu T, Ikeda A, Nakao A, Tsung A, Toyokawa H, Ueki S, Geller DA, Murase N. Protection of transplant-induced hepatic ischemia/reperfusion injury with carbon monoxide via MEK/ERK1/2 pathway downregulation. Am J Physiol Gastrointest Liver Physiol. 2008;294:G236-G244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |