Published online Apr 7, 2015. doi: 10.3748/wjg.v21.i13.4089
Peer-review started: August 14, 2014
First decision: August 27, 2014
Revised: September 10, 2014
Accepted: October 14, 2014
Article in press: October 15, 2014
Published online: April 7, 2015
Processing time: 236 Days and 0.3 Hours
Primary lymphoepithelioma-like carcinoma in the liver is extremely rare. A few cases of lymphoepithelioma-like cholangiocarcinoma have been reported, but few radiologic features were described. We reviewed 23 cases of lymphoepithelioma-like cholangiocarcinoma reported between 1996 and 2014 and describe a rare case of a 35-year-old woman in our hospital who was diagnosed with lymphoepithelioma-like cholangiocarcinoma of the liver and was a hepatitis B carrier. The tumor (1.6 cm) in our patient appeared to be hypoechoic in sonographic images and hypodense in computed tomography (CT) images. In addition, it was homogeneous hypointense in T1-weighted magnetic resonance (MR) images (MRI) and hyperintense in T2-weighted MRI. Dynamic gadolinium-enhanced MRI showed typical image pattern of hepatocellular carcinoma (HCC). The patient underwent a laparoscopic left hepatic lobectomy, and the resected tumor consisted of well-differentiated glandular cells with extensive lymphocytic infiltration that were immunoreactive to CK (AE1/AE3), CD3, and CD20. In addition, the tumor was positive for Epstein-Barr virus-encoded RNA in situ hybridization. Finally, lymphoepithelioma-like cholangiocarcinoma was diagnosed. In previous studies, the incidence is highest among middle-aged people. Most tumors appeared to be hypodense with either hypovascular or hypervascular patterns in CT images. This case report is the first study to address sonography, CT, and MRI observations and delineate pathologic correlations. We suggest that the imaging pattern of lymphoepithelioma-like cholangiocarcinoma, either the typical cholangiocarcinoma pattern or a mimic of HCC, should be considered in the differential lists for HCC.
Core tip: We report the first case of lymphoepithelioma-like cholangiocarcinoma observed using sonography, computed tomography, and magnetic resonance images and delineate the pathologic correlations. According to a review of previous studies, lymphoepithelioma-like cholangiocarcinoma may affect more middle-aged woman. We suggest that the imaging pattern of lymphoepithelioma-like cholangiocarcinoma, either a typical cholangiocarcinoma pattern or a mimic of hepatocellular carcinoma, should be considered in the differential lists for hepatocellular carcinoma.
- Citation: Liao TC, Liu CA, Chiu NC, Yeh YC, Chiou YY. Lymphoepithelioma-like cholangiocarcinoma: A mimic of hepatocellular carcinoma on imaging features. World J Gastroenterol 2015; 21(13): 4089-4095
- URL: https://www.wjgnet.com/1007-9327/full/v21/i13/4089.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i13.4089
Lymphoepithelioma-like carcinoma (LELC) is a tumor with morphologic features similar to those of undifferentiated nasopharyngeal carcinoma that occurs outside the nasopharynx and is associated with Epstein-Barr virus (EBV) infection. In the liver, this type of tumor is extremely rare, and only 23 cases of lymphoepithelioma-like cholangiocarcinoma have been described in reports focused on histologic and immunohistochemical analyses[1-12]. A recent study that reported seven female cases of EBV-associated lymphoepithelioma-like cholangiocarcinoma demonstrated molecular genetic pathology, including frequent DNA hypermethylation[9]. However, few radiologic features have been described. Therefore, we report a case of lymphoepithelioma-like cholangiocarcinoma in a young patient and review the literature on imaging features. According to our review of relevant research, our case report is the first to describe observations made using imaging procedures such as sonography, computed tomography (CT), magnetic resonance imaging (MRI).
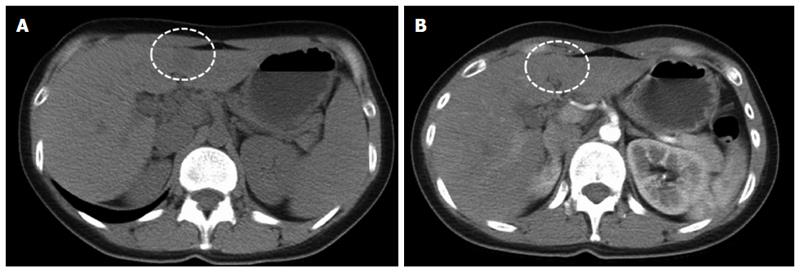
This report involves the case of a 35-year-old woman who was a chronic hepatitis B carrier. She had no other systemic diseases, such as hypertension or diabetes mellitus. In 2013, a small hypoechoic hepatic nodule was discovered during regular annual sonographic examination, and an abdominal CT at a local hospital revealed a small hypodense nodule with mild enhancement at the medial segment of the left hepatic lobe (Figure 1). She then visited our center for a second opinion.
During hospitalization, the patient did not complain of abdominal problems, and physical examinations and laboratory tests, including that of her serum alpha-fetoprotein level (1.3), did not indicate any abnormalities. The patient did not meet the criteria for a diagnosis of hepatocellular carcinoma (HCC) according to the American Association for the Study of Liver Diseases Practice Guidelines[13]. Because the CT scan was not dynamic contrast enhanced and the acquisition time was too early to be differentiated as a small HCC or other hepatic tumor. We then evaluated other tumor markers, including carbohydrate antigen (CA19-9; 33 μ/mL) and carcinoembryonic antigen (CEA; 1.4 ng/mL), which were within normal limits. A repeat sonographic examination and dynamic abdominal MRI were scheduled to investigate the possibility of other hepatic neoplasms.
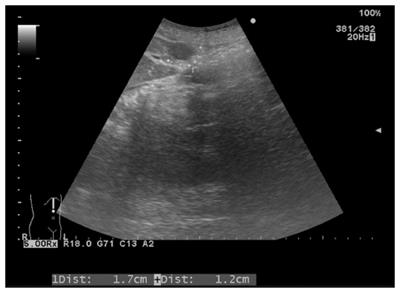
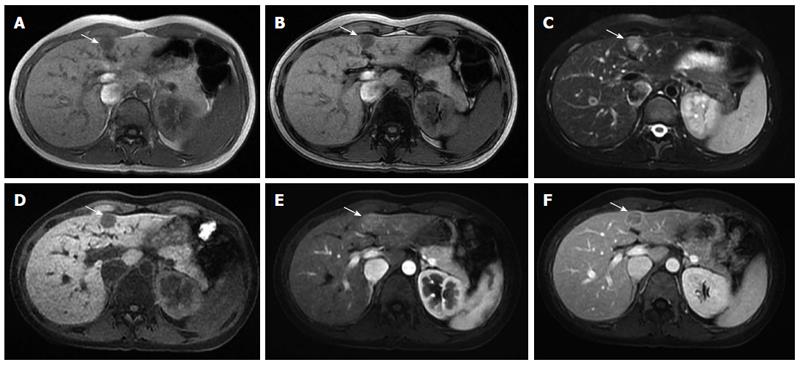
Sonographic examination revealed a small (1.7 cm × 1.2 cm) well-defined and homogeneous hypoechoic nodule protruding into the liver surface at the lateral segment (Figure 2). The MR image depicted a well-defined 1.7-cm nodule at S4b of the liver (Figure 3). The nodule appeared to be homogeneous hypointense on in-phase and out of phase T1-weighted images without decreased signal intensity, meaning no intracellular fat (Figure 3A and B), and hyperintense on axial T2-weighted images (Figure 3C). No calcification, hemorrhaging, or fat components were observed in the lesion. In addition, hepatolithiasis and intrahepatic bile duct dilatation were absent. The main portal vein and its major branches were patent. Dynamic gadolinium-enhanced MRI indicated hypointense on pre-contrast T1-weighted image (Figure 3D), early arterial enhancement in the arterial phase (Figure 3E), washout in portal venous phase and delayed fibrous capsule enhancement (Figure 3F). These are typical imaging characteristics of HCC.
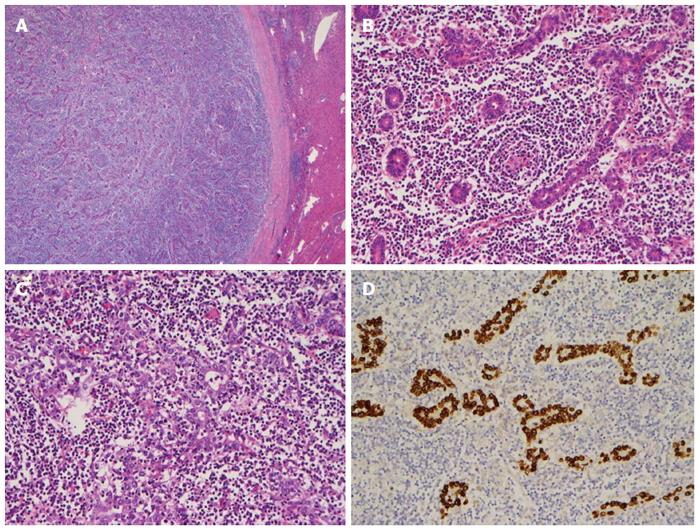
Tentatively diagnosed with HCC, the patient subsequently underwent a laparoscopic left lobectomy. During surgery, a solitary 1.7 cm whitish tumor was located at the medial segment of the liver, immediately below the falciform ligament. The tumor was well defined and gray-white in color with a thick fibrous capsule and without extrahepatic capsular extension (Figure 4A).
Histologically, the tumor consisted of well-differentiated glandular cells with extensive lymphocytic infiltration and scattered lymphoid follicles (Figure 4B). In a few areas, a syncytial growth pattern was observed in the tumor (Figure 4C). The tumor cells were diffusely positive for CK (AE1/AE3) and EBV-encoded RNA (EBER) in situ hybridization (Figure 4D). CD3 and CD20 stains indicated mixed B- and T-cell infiltration. The adjacent non-tumorous liver tissue did not reveal significant histopathologic abnormality. The final diagnosis was stage I EBV-related lymphoepithelioma-like cholangiocarcinoma. The patient recovered gradually after surgery and was discharged from our hospital.
After HCC, intrahepatic cholangiocarcinoma is the most common primary hepatic malignant tumor type, and cholangiocarcinoma is the most prevalent in Southeast Asia. However, radiologists encounter difficulty in diagnosing cholangiocarcinoma because of its wide spectrum of radiologic appearances. Intrahepatic cholangiocarcinoma is classified according to morphology into mass-forming, periductal infiltrating, and intraductal growing types[14]. Typically, CT features of mass-forming cholangiocarcinoma include homogeneous attenuation and irregular peripheral enhancements with gradual centripetal enhancement[15-17]. Intrahepatic duct stones with upper stream duct dilatation are also observed. Sonographic examinations indicate a mostly hyperechoic and heterogeneous mass with an ill-defined margin. Tumors > 3 cm in size are usually hyperechoic, whereas tumors < 3 cm are hypo- or isoechoic[18,19]. In MR images, the mass exhibits hypointensity on T1-weighted imaging and an irregular margin with hyperintensity on T2-weighted imaging. Contrast-enhanced T1-weighted images show peripheral and centripetal enhancement[20-22].
Twelve studies containing 23 cases of lymphoepithelioma-like cholangiocarcinoma were reviewed (7 men, 16 women; mean age, 53.2 y; range, 19-79 y) (Table 1). The incidence is highest among middle-aged people. Chronic hepatitis has been identified as a risk factor for cholangiocarcinoma. In present reviewed study, there were 8 patients with hepatitis B and one patient with hepatitis C. The tumor diameters ranged from 1.7 to 10.0 cm (mean size, 4.7 cm). Larger tumors tended to present with abdominal pain or discomfort (8/23 cases), whereas most of small tumors were discovered incidentally (10/23 cases). Regarding the tumor location, no difference was observed between the right hepatic lobe and the left hepatic lobe. All patients underwent surgical resection for tumor treatment and were pathologically approved. Almost all of the patients were positive for EBER in situ hybridization (21/23 cases). In a recent study, 7 cases (all female) of EBV-associated lymphoepithelioma-like cholangiocarcinoma were described with clinical presentations similar to our case[9]. In addition, the results showed that EBV-associated lymphoepithelioma-like cholangiocarcinoma had a favorable overall survival, and was frequently associated with distinctive DNA hypermethylation, which is an important epigenetic mechanism for inactivating tumor suppressor genes involved in tumorigenesis.
| Ref. | Case | Age (yr)/sex | Site/size (cm) | Hepatitis | EBV | Images | Symptom | Treatment |
| Hsu et al[12] | 1 | 47/F | Left lobe/10.0 | Negative | + | Angiography: hypovascular CT: hypovascular | Abdominal fullness | Left lobectomy |
| Chen et al[1] | 2 | 67/F | S8/5.0 | C | + | Sonography: mixed echoic Angiography: hypervascular | RUQ pain | Hepatectomy |
| 3 | 41/M | S2/3.0 | B | + | Angiography: hypervascular | Epigastric pain | Resection | |
| Jeng et al[6] | 4 | 47/F | Left lobe/10.0 | Unknown | + | CT: hypovascular | Abdominal fullness and a firm epigastric mass | Left lobectomy |
| 5 | 42/M | S6 / 3.0 | Unknown | + | CT: heterogeneous density | Incidental finding | Segmentectomy | |
| 6 | 67/F | Lateral segment/3.0 | Unknown | + | CT: hypodense | Incidental finding | Left lobectomy | |
| 7 | 50/M | Left lobe/4.0 | B | + | CT: hypodense | Vague epigastric pain | Left lobectomy | |
| 8 | 50/F | Right lobe/4.0 | B | + | Sonography: hypoechoic | Incidental finding | Atypical hepatectomy | |
| Huang et al[4] | 9 | 60/F | S5/3.0 | B | + | Sonography: hypoechoic CT: hypodense, hypovascular | Incidental finding | Resection |
| Adachi et al[2] | 10 | 64/M | Left lobe/4.0 | Unknown | + | CT: heterogeneous density | Fever | Lateral segmentectomy |
| Ortiz et al[11] | 11 | 19/F | Left lobe/5.5 | Negative | + | CT: Hypovascular | Abdominal fullness | Left lobectomy |
| Szekely et al[10] | 12 | 60/M | Unknown 6.0 | Unknown (non-B, non-C) | - | Sonography: Unknown (no mention) | Incidental finding | Resection |
| Henderson-Jackson et al[3] | 13 | 63/F | Medial segment/3.8 | Unknown | + | CT: hypodense, | Right flank pain and back pain | Resection |
| Right lobe/1.6 | CT: hypodense, | Resection | ||||||
| Kim et al[7] | 14 | 61/M | S6/2.2 | Unknown | + | Sonography: hypoechoic CT: low density w/o enhancement MR: T1 slightly hypointensity, T2 hyperintensity, progressive enhancement in periphery of the lesion | Unknown | Resection |
| Lee et al[8] | 15 | 79/M | Lateral segment/3.7 | B | - | CT: hypervascular | Incidental finding | Left lobectomy |
| Hur et al[5] | 16 | 57/F | S6/2.6 | Non-B Non-C | + | MR: centrifugal enhancement | Incidental finding | Segmentectomy |
| Chan et al[9] | 17 | 53/F | Right lobe/1.6 | B | + | No report (ultrasound, CT, or MR) | Incidental finding | Resection |
| 18 | 40/F | Right lobe/7.5 | B | + | Incidental finding | Resection | ||
| 19 | 57/F | Left lobe/7.1 | Negative | + | Non-painful vague and abdominal mass | Resection | ||
| 20 | 56/F | Left lobe/6.0 | Negative | + | Dyspepsia and reflux symptoms | Resection | ||
| 21 | 59/F | Left lobe/6.0 | B | + | Incidental finding | Resection | ||
| 22 | 45/F | Left lobe/3.0 | Negative | + | Biliary colic | Resection | ||
| 23 | 57/F | Right lobe/3.0 | Negative | + | Incidental finding | Resection | ||
| Current study | 24 | 35/F | Medial segment/1.6 | B | + | Sonography: hypoechoic CT: hypodense with enhancement MR: peripheral and the centripetal enhancement | Incidental finding | Left lobectomy |
The reviewed studies focused on histologic and immunohistochemical findings, and information on imaging features was inadequate. They demonstrated that tumors with hypodense CT images may be either hypovascular or hypervascular. Three small tumors exhibited hypoechoic echogenicity in sonographic examinations. Only two case reports described MRI findings: the typical MRI features (T1 hypointensity, T2 hyperintensity, progressive enhancement pattern) were observed in one case, whereas in the second case, a centrifugal enhancement pattern was observed and the T1 and T2 signals were unknown. In summary, a lymphoepithelioma-like cholangiocarcinoma may present with several typical characteristics of mass-forming cholangiocarcinoma.
Most patients diagnosed with lymphoepithelioma-like cholangiocarcinoma had a medical history of chronic hepatitis. Because some intrahepatic tumors contain both elements of cholangiocarcinoma and HCC in the same nodule, the imaging characteristics may overlap[23]. HCC and metastatic tumors should be considered when typical characteristics of mass-forming cholangiocarcinoma are not observed. Therefore biopsy may be needed for confirmation of the diagnosis before surgery. In addition, 18fluorodeoxyglucose-positron emission tomography (18F-FDG PET) is of value for the diagnosis and staging of cholangiocarcinoma, and demonstrates high accuracy for detecting unsuspected distant metastases[24]. However, to the best of our knowledge, the application of this method for discriminating LELC and other histologic types of cholangiocarcinoma has not been studied.
Our patient had a history of chronic hepatitis without jaundice and a normal serum alpha-fetoprotein level. In addition, a typical pattern for HCC (T1 hypointensity, T2 hyperintense, early arterial enhancement, washout on the portal venous phase, and delayed fibrous capsule enhancement) was observed in MR images; therefore, the preoperative diagnosis was HCC.
In conclusion, these previous studies indicated that lymphoepithelioma-like cholangiocarcinoma is a rare variant of cholangiocarcinoma that affects more middle-aged females. This case report and review article is the first study to describe the findings from ultrasound, CT, and MRI. Various atypical patterns of mass-forming cholangiocarcinoma are based on tumor components. Even though the imaging findings of the liver tumor present a typical pattern of HCC, a lymphoepithelioma-like cholangiocarcinoma still needs to be considered in the differential list in the setting of chronic hepatitis, especially in females with EBV infection. Diagnosing lymphoepithelioma-like cholangiocarcinoma remains a challenge for clinic physicians, surgeons, and radiologists.
A 35-year-old woman had a history of chronic hepatitis without jaundice and a normal serum alpha-fetoprotein level.
A hepatic nodule was incidentally found under surveillance of chronic hepatitis B.
Hepatocellular carcinoma and mass-forming intrahepatic cholangiocarcinoma.
The serum alpha-fetoprotein, carbohydrate antigen 19-9, and carcinoembryonic antigen levels were within normal limits.
The dynamic magnetic resonance imaging (MRI) resembled the typical pattern for hepatocellular carcinoma (T1 hypointensity, T2 hyperintense, early arterial enhancement, washout on the portal venous phase, and delayed fibrous capsule enhancement).
Histologic examination showed well-circumscribed tumor with a thick fibrous capsule; the majority of the tumor was composed of well-differentiated glandular cells with dense lymphocytic infiltration and scattered lymphoid follicles, a poorly differentiated growth pattern. Tumor cells were positive for Epstein-Barr virus-encoded RNA in situ hybridization.
A laparoscopic left lobectomy was performed.
This case report is the first to describe observations of lymphoepithelioma-like cholangiocarcinoma made using sonography, computed tomography (CT), and MRI.
Lymphoepithelioma-like cholangiocarcinoma is a rare tumor with morphologic features similar to those of undifferentiated nasopharyngeal carcinoma that occurs outside the nasopharynx and is associated with Epstein-Barr virus infection.
Mass-forming intrahepatic cholangiocarcinoma has variable enhancement patterns on dynamic CT and MRI, thus the imaging interpretation should be careful. Biopsy may be needed for confirming the diagnosis if the imaging was not a typical pattern.
The authors reported on the rare imaging characteristics of a lymphoepithelioma-like cholangiocarcinoma and it is of potential interest and relevance.
P- Reviewer: Conti B, Lin CW, Ma L, Solinas A S- Editor: Qi Y L- Editor: AmEditor E- Editor: Wang CH
| 1. | Chen TC, Ng KF, Kuo T. Intrahepatic cholangiocarcinoma with lymphoepithelioma-like component. Mod Pathol. 2001;14:527-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Adachi S, Morimoto O, Kobayashi T. Lymphoepithelioma-like cholangiocarcinoma not associated with EBV. Pathol Int. 2008;58:69-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Henderson-Jackson E, Nasir NA, Hakam A, Nasir A, Coppola D. Primary mixed lymphoepithelioma-like carcinoma and intra-hepatic cholangiocarcinoma: a case report and review of literature. Int J Clin Exp Pathol. 2010;3:736-741. [PubMed] |
| 4. | Huang Y, Tsung JS, Lin CW, Cheng TY. Intrahepatic cholangiocarcinoma with lymphoepithelioma-like carcinoma component. Ann Clin Lab Sci. 2004;34:476-480. [PubMed] |
| 5. | Hur YH, Kim HH, Koh YS, Seoung JS, Cho CK. Lymphoepithelioma-like cholangiocarcinoma not associated with Epstein-Barr virus. ANZ J Surg. 2011;81:652-653. [PubMed] |
| 6. | Jeng YM, Chen CL, Hsu HC. Lymphoepithelioma-like cholangiocarcinoma: an Epstein-Barr virus-associated tumor. Am J Surg Pathol. 2001;25:516-520. [PubMed] |
| 7. | Kim YC, Park MS, Chung YE, Kim MJ, Park YN, Kang JH, Kim KA, Kim KW. MRI findings of uncommon non-hepatocyte origin primary liver tumours with pathological correlation. Br J Radiol. 2010;83:1080-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Lee W. Intrahepatic lymphoepithelioma-like cholangiocarcinoma not associated with epstein-barr virus: a case report. Case Rep Oncol. 2011;4:68-73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Chan AW, Tong JH, Sung MY, Lai PB, To KF. Epstein-Barr virus-associated lymphoepithelioma-like cholangiocarcinoma: a rare variant of intrahepatic cholangiocarcinoma with favourable outcome. Histopathology. 2014;65:674-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Szekely E. Lymphoepithelioma-like cholangiocarcinoma (LELC) not associated with Epstein-Barr virus. Am J Surg Pathol. 2001;25:1464-1466. [PubMed] |
| 11. | Ortiz MR, Garijo G, Adrados M, López-Bonet E, Acero D, Bernadó L. Epstein-Barr Virus-Associated Cholangiocarcinoma with Lymphoepithelioma-Like Component. Int J Surg Pathol. 2000;8:347-351. [PubMed] |
| 12. | Hsu HC, Chen CC, Huang GT, Lee PH. Clonal Epstein-Barr virus associated cholangiocarcinoma with lymphoepithelioma-like component. Hum Pathol. 1996;27:848-850. [PubMed] |
| 13. | Bruix J, Sherman M, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6555] [Article Influence: 468.2] [Reference Citation Analysis (1)] |
| 14. | Liver Cancer Study Group of Japan. Classification of primary liver cancer. Tokyo, Japan: Kanehara-Shuppan 1997; . |
| 15. | Ros PR, Buck JL, Goodman ZD, Ros AM, Olmsted WW. Intrahepatic cholangiocarcinoma: radiologic-pathologic correlation. Radiology. 1988;167:689-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 102] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Han JK, Choi BI, Kim AY, An SK, Lee JW, Kim TK, Kim SW. Cholangiocarcinoma: pictorial essay of CT and cholangiographic findings. Radiographics. 2002;22:173-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 119] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 17. | Choi BI, Lee JH, Han MC, Kim SH, Yi JG, Kim CW. Hilar cholangiocarcinoma: comparative study with sonography and CT. Radiology. 1989;172:689-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 79] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Wernecke K, Henke L, Vassallo P, von Bassewitz DB, Diederich S, Peters PE, Edel G. Pathologic explanation for hypoechoic halo seen on sonograms of malignant liver tumors: an in vitro correlative study. AJR Am J Roentgenol. 1992;159:1011-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 35] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Wibulpolprasert B, Dhiensiri T. Peripheral cholangiocarcinoma: sonographic evaluation. J Clin Ultrasound. 1992;20:303-314. [PubMed] |
| 20. | Maetani Y, Itoh K, Watanabe C, Shibata T, Ametani F, Yamabe H, Konishi J. MR imaging of intrahepatic cholangiocarcinoma with pathologic correlation. AJR Am J Roentgenol. 2001;176:1499-1507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 124] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 21. | Manfredi R, Barbaro B, Masselli G, Vecchioli A, Marano P. Magnetic resonance imaging of cholangiocarcinoma. Semin Liver Dis. 2004;24:155-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 113] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 22. | Park HS, Lee JM, Choi JY, Lee MW, Kim HJ, Han JK, Choi BI. Preoperative evaluation of bile duct cancer: MRI combined with MR cholangiopancreatography versus MDCT with direct cholangiography. AJR Am J Roentgenol. 2008;190:396-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 106] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 23. | Fowler KJ, Sheybani A, Parker RA, Doherty S, M Brunt E, Chapman WC, Menias CO. Combined hepatocellular and cholangiocarcinoma (biphenotypic) tumors: imaging features and diagnostic accuracy of contrast-enhanced CT and MRI. AJR Am J Roentgenol. 2013;201:332-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 152] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 24. | Weber A, Schmid RM, Prinz C. Diagnostic approaches for cholangiocarcinoma. World J Gastroenterol. 2008;14:4131-4136. [PubMed] |