Published online Apr 7, 2015. doi: 10.3748/wjg.v21.i13.3944
Peer-review started: September 4, 2014
First decision: October 14, 2014
Revised: October 22, 2014
Accepted: December 5, 2014
Article in press: December 8, 2014
Published online: April 7, 2015
Processing time: 216 Days and 1.8 Hours
AIM: To identify the clinicopathological predictors of lymph node (LN) metastasis and evaluate the outcomes of endoscopic submucosal dissection (ESD) in papillary adenocarcinoma-type early gastric cancers (EGCs).
METHODS: From January 2005 to May 2013, 49 patients who underwent surgical operation and 24 patients who underwent ESD for papillary adenocarcinoma-type EGC were enrolled to identify clinicopathological characteristics and predictive factors of LN metastasis and to evaluate the outcomes of ESD for papillary adenocarcinoma-type EGC.
RESULTS: Most papillary adenocarcinoma-type EGCs were located in the lower third of the stomach and had an elevated macroscopic shape. The overall prevalence of LN metastasis was 18.3% (9/49). The presence of lymphovascular invasion was found to be a predictor of LN metastasis (P = 0.016). According to current indication criteria of ESD, 6 and 11 of the 49 patients had absolute and expanded indications for ESD, respectively. Two patients (11.8%) with expanded indication for ESD had LN metastasis. Of the 24 patients who underwent ESD, 13 (54%) achieved out-of-ESD indication, with 9 of those 13 patients undergoing surgical operation due to non-curative resection.
CONCLUSION: The use of ESD should be carefully considered for papillary adenocarcinoma-type EGC with suspected ESD indication after pre-treatment work-up because of the higher frequency of LN metastasis and additional surgeries.
Core tip: Papillary adenocarcinoma-type early gastric cancers (EGCs) are classified as differentiated-type adenocarcinoma and, therefore, treated with endoscopic submucosal dissection (ESD) according to the same indication criteria as other differentiated-type adenocarcinoma, such as tubular adenocarcinoma. However, the rate of lymph node metastasis under the current ESD indication criteria was somewhat high, and more than half of the patients who underwent ESD as a primary treatment for papillary carcinoma-type EGC ultimately achieved out-of-ESD indication. Therefore, the use of ESD should be more carefully considered for papillary adenocarcinoma-type EGCs with suspected ESD indication after pre-treatment work-up.
- Citation: Lee HJ, Kim GH, Park DY, Lee BE, Jeon HK, Jhi JH, Song GA. Is endoscopic submucosal dissection safe for papillary adenocarcinoma of the stomach? World J Gastroenterol 2015; 21(13): 3944-3952
- URL: https://www.wjgnet.com/1007-9327/full/v21/i13/3944.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i13.3944
Recent developments in endoscopic submucosal dissection (ESD) have enabled en bloc resection of early gastric cancer (EGC) with a negligible risk of lymph node (LN) metastasis irrespective of tumor size or the presence of submucosal fibrosis. ESD has many advantages compared with surgical treatments, such as more accurate histological diagnosis, minimally invasive procedure, high curative resection rate, and low local recurrence rate. Therefore, ESD is widely accepted as a standard treatment strategy for EGC[1-4]. The indications of ESD for EGC have been gradually expanded[1,4]. ESD of absolute and expanded indications is recognized as a safe treatment modality for EGC based on recent studies of the long-term outcomes of ESD[2,4,5].
The World Health Organization classification of gastric carcinomas recognized four main histological patterns based on the predominant histological pattern of the carcinoma, which often co-exists with less dominant elements of other histological patterns: tubular, papillary, mucinous, and poorly cohesive (including signet ring cell type)[6]. Among these entities, papillary adenocarcinoma is a rare histologic variant of gastric adenocarcinoma that is characterized histologically by epithelial projections scaffolded by a central fibrovascular core[7]. Its biological behavior and prognostic significance is still unclear because of its rarity. Currently, papillary adenocarcinoma is classified into differentiated-type adenocarcinoma (based on the Japanese classification of gastric carcinoma)[8] and intestinal type (based on the Lauren classification)[6]. However, papillary adenocarcinoma has been reported to have a higher rate of liver metastasis and LN involvement, as well as a lower overall 5-year survival rate compared with non-papillary gastric carcinomas such as tubular adenocarcinoma[7,9].
Given the more aggressive features of papillary adenocarcinoma, an inevitable question is whether papillary and tubular adenocarcinomas should be treated according to the same ESD indication criteria. It is doubtful that the same ESD indication criteria can be rationally applied to gastric carcinomas with or without considerable papillary adenocarcinoma components[10-12]. To date, many studies have reported on the safety and outcomes of ESD for differentiated-type adenocarcinoma of the stomach[1-4]. However, the number of papillary adenocarcinomas included in these studies was small. To our knowledge, studies investigating the safety and outcomes of ESD for papillary adenocarcinoma-type EGC alone have not been conducted. Therefore, this study aimed to investigate the clinicopathological predictors of LN metastasis and evaluate the outcomes of ESD in papillary adenocarcinoma-type EGCs.
From January 2005 to May 2013, a total of 1,510 patients underwent surgical operation or endoscopic resection for EGC at the Pusan National University Hospital. Of these 1510 patients, 64 were histologically diagnosed with papillary adenocarcinoma-type EGC (Figure 1). All patients underwent abdominal computed tomography (CT) to determine the presence of LN or distant metastases before ESD. Endoscopic ultrasonography was also performed as needed in order to rule out submucosal invasion. We divided the 64 patients into 2 groups: group 1 consisted of 49 patients who underwent surgical operation for papillary adenocarcinoma-type EGC and group 2 consisted of 24 patients who underwent ESD as a primary treatment for papillary adenocarcinoma-type EGC. Group 1 patients were enrolled to identify clinicopathological characteristics and predictive factors of LN metastasis in papillary adenocarcinoma-type EGC. Group 2 patients were enrolled to evaluate the outcomes of ESD for papillary adenocarcinoma-type EGC. All patients provided written informed consent before ESD or surgical operation. The study protocol was reviewed and approved by the Institutional Review Board of the Pusan National University Hospital.
Laparoscopy-assisted or open gastrectomy was performed with lymphadenectomy, resection, and reconstruction. In laparoscopy-assisted gastrectomy, a laparoscope and trocars were inserted through small incisions in the abdominal wall under general anesthesia. The decision of range for surgical operation was based on the degree of cancer progression, histopathological diagnosis of the biopsy specimen, tumor location, and risk of LN metastasis, morbidity, and mortality. In cases with proximal tumor location, near-total or total gastrectomy was done with Billroth-I or Billroth-II and Roux-en-Y reconstruction methods. Subtotal gastrectomy was done for tumors located in the middle third or lower third of the stomach. D1 or D2 lymphadenectomies were performed on a case-by-case basis.
ESD procedures were performed by two experienced endoscopists (GH Kim and GA Song) using a single-channel endoscope (GIF-H260 or GIF-Q260; Olympus Co., Ltd., Tokyo, Japan). ESD was performed under conscious sedation with cardiorespiratory monitoring. For sedation, 5-10 mg of midazolam and 25 mg of meperidine were administered intravenously; propofol was administered as needed during the procedure. First, argon plasma coagulation was used to mark the borders of the lesion, which had been identified by conventional endoscopy or chromoendoscopy with the application of an indigo carmine solution. After marking, a saline solution (0.9% saline with a small amount of epinephrine and indigo carmine) was injected submucosally around the lesion to lift it off the muscular layer. Next, a circumferential mucosal incision was made around the marking dots with an IT knife (Olympus) and/or Flex knife (Olympus). The lesion was completely removed by submucosal dissection with these knives. If necessary, the submucosal injection was repeated and endoscopic hemostasis was achieved. A high-frequency electrosurgical current generator (Erbotom VIO 300D; ERBE, Tübingen, Germany) was used during marking, mucosal incision, submucosal dissection, and hemostasis.
The macroscopic shapes of lesions were categorized as either protruding (I), non-protruding and non-excavated (II), or excavated (III). Type II lesions were subclassified as slightly elevated (IIa), flat (IIb), or slightly depressed (IIc). Lesions were classified into 3 groups: elevated (I and IIa), flat (IIb), and depressed (IIc and III) types. Resected specimens were fixed in a 10% formalin solution and serially sectioned at 2-mm intervals to assess tumor involvement in the lateral and vertical margins. Tumor size, depth of invasion, presence of ulceration, lymphovascular invasion (LVI), and LN metastasis were evaluated microscopically.
The tumors were classified histopathologically according to the Japanese classification of gastric carcinoma[8]. Papillary adenocarcinoma was defined as a tumor in which more than 50% of the tumor area contained papillary structures composed of epithelial projections scaffolded by a central fibrovascular core. Histological types were classified as pure papillary (PP) and mixed papillary (MP) types. The MP type was subclassified as MP type with differentiated component (well and moderately differentiated tubular adenocarcinoma) and MP type with undifferentiated component (poorly differentiated adenocarcinoma and signet-ring cell carcinoma). The depth of mucosal invasion was classified as m1 (confined to the epithelial layer), m2 (invasion of the lamina propria), and m3 (invasion of the muscularis mucosa). The depth of submucosal invasion was classified as sm1 (submucosal invasion of ≤ 500 μm from the muscularis mucosa) and sm2 (submucosal invasion of > 500 μm from the muscularis mucosa).
In ESD cases, en bloc resection was defined as resection in a single piece as opposed to piecemeal resection (in multiple segments). Resection was regarded to be curative when en bloc resection was achieved with tumor-free lateral and vertical margins, with histopathological examinations revealing differentiated-type adenocarcinoma, submucosal invasion of ≤ 500 μm from the muscularis mucosa, and the absence of LVI. Resection was regarded to be non-curative if the above criteria were not met despite complete endoscopic removal of the lesion. For surgical operation, curative resection was defined as the compete removal of macroscopic tumor tissue and tumor-free resection margin on histological examination.
All patients who were treated with ESD underwent post-procedural chest and abdominal radiography and second-look endoscopy on the following day to detect any perforation or bleeding. Proton pump inhibitors and sucralfate were administered to relieve pain, prevent procedure-related bleeding, and promote ulcer healing. Patients without serious symptoms or adverse events were permitted to start food intake the day after the procedure and were discharged within 3-4 d.
In cases of curative resection, follow-up endoscopy was conducted 6 mo after ESD and annually thereafter. Abdominal CT, chest radiography, and laboratory measurements of tumor markers were performed 6 mo after ESD and annually thereafter. In cases of non-curative resection such as those with LVI, a positive vertical margin, or deep submucosal invasion, surgical operation was first recommended to all patients for curative resection. However, for patients who refused surgical operation, follow-up endoscopy with biopsies and abdominal CT were conducted 1-2 mo and 4-6 mo after ESD.
Variables were expressed as median (range) values and simple proportions. Fisher’s exact test or χ2 test was used to identify predictive factors of LN metastasis in papillary adenocarcinoma-type EGCs. Statistical calculations were performed using SPSS version 18.0 for Windows software (SPSS Inc., Chicago, IL, United States). A P value of < 0.05 was considered statistically significant.
Clinicopathological characteristics of the 49 patients who underwent surgical operation for papillary adenocarcinoma-type EGC are summarized in Table 1. Of the 49 patients, 35 were male and 14 were female, with a median age of 66 years (range: 48-80 years). Forty patients underwent primary surgical operation, with the remaining 9 undergoing an additional surgical operation for non-curative resection after ESD. Laparoscopy-assisted gastrectomy with D1 lymphadenectomy was performed in 9 patients, laparoscopy-assisted gastrectomy with D2 lymphadenectomy in 17 patients, and open gastrectomy with D2 lymphadenectomy in 23 patients. Most papillary adenocarcinomas (86%) were located in the lower third of the stomach. Macroscopically, elevated shape was more frequent than depressed shape (73% vs 27%). Regarding histological type, 28 patients (57%) had PP type and 21 patients (43%) had MP type. Median tumor size was 30 mm (range: 9-105 mm). Fourteen patients (29%) had mucosal cancer, and the other 35 patients (71%) had submucosal cancer. The frequencies of LVI and LN metastasis were 31% (15/49) and 18% (9/49), respectively.
| Characteristic | |
| No. of patients | 49 |
| Median age, yr (range) | 66 (48-80) |
| Gender | |
| Male | 35 (71) |
| Female | 14 (29) |
| Location | |
| Upper third | 5 (10) |
| Middle third | 2 (4) |
| Lower third | 42 (86) |
| Macroscopic shape | |
| Elevated | 36 (73) |
| Flat | 0 (0) |
| Depressed | 13 (27) |
| Ulceration | 12 (24) |
| Histologic type | |
| PP type | 28 (57) |
| MP with diff. | 14 (29) |
| MP with undiff. | 7 (14) |
| Median size, mm (range) | 30 (9-105) |
| ≤ 30 | 24 (49) |
| > 30 | 25 (51) |
| Depth of invasion | |
| Mucosa (m1:m2:m3) | 14 (0:0:14) (29) |
| Submucosa (sm1:sm2) | 35 (8:27) (71) |
| Lymphovascular invasion | 15 (31) |
| Lymph node metastasis | 9 (18) |
Clinicopathological characteristics of patients with LN metastasis are described in Table 2. Location, macroscopic shape, ulceration, histological type, and tumor size were not associated with LN metastasis (Table 3). The incidence of LN metastases was higher in submucosal cancers than in mucosal cancers; however, this difference did not reach statistical significance [22.9% (8/35) vs 7.1% (1/14), P = 0.195]. LVI was the only clinicopathological factor significantly associated with LN metastasis (P = 0.016).
| Patient No. | Gender/age | Tumor location | Macroscopic type | Ulceration | Histologic type | Tumor size (mm) | Depth of invasion | Lymphovascular invasion | Pre-treatment ESD indication | Primary treatment |
| 1 | F/63 | Lower third | IIa | - | PP | 35 | sm2 | + | Out | Op |
| 2 | M/58 | Lower third | IIa | - | MP + diff. | 48 | m3 | - | In (expanded) | Op |
| 3 | F/75 | Lower third | I + IIa | - | MP + undiff. | 105 | sm2 | + | Out | Op |
| 4 | F/71 | Lower third | IIc | + | PP | 25 | sm2 | - | Out | Op |
| 5 | M/67 | Lower third | I | - | MP + undiff. | 23 | sm2 | - | Out | Op |
| 6 | M/64 | Lower third | IIc | + | PP | 35 | sm2 | + | Out | Op |
| 7 | M/58 | Lower third | IIc + III | + | MP + undiff. | 55 | sm2 | + | Out | Op |
| 8 | M/68 | Lower third | IIa + IIb | - | PP | 27 | sm2 | + | Out | Op |
| 9 | F/63 | Lower third | IIa | - | MP + diff. | 30 | sm1 | + | In (expanded) | ESD |
| Factors | Lymph node metastasis | P value | |
| Present(n = 9) | Absent(n = 40) | ||
| Location | 0.712 | ||
| Upper third | 0 (0) | 5 (100) | |
| Middle third | 0 (0) | 2 (100) | |
| Lower third | 9 (21.4) | 33 (78.6) | |
| Macroscopic type | 0.683 | ||
| Elevated | 6 (16.7) | 30 (83.3) | |
| Depressed | 3 (23.1) | 10 (76.9) | |
| Ulceration | 0.498 | ||
| Absent | 6 (16.2) | 31 (83.8) | |
| Present | 3 (25.0) | 9 (75.0) | |
| Histologic type | 0.470 | ||
| PP type | 4 (14.3) | 24 (85.7) | |
| MP with diff. | 2 (16.7) | 10 (83.3) | |
| MP with undiff. | 3 (33.3) | 6 (66.7) | |
| Tumor size | 0.253 | ||
| ≤ 30 mm | 3 (12.5) | 21 (87.5) | |
| > 30 mm | 6 (24.0) | 19 (76.0) | |
| Depth of invasion | 0.195 | ||
| Mucosa | 1 (7.1) | 13 (92.9) | |
| Submucosa | 8 (22.9) | 27 (77.1) | |
| Lymphovascular invasion | 0.016 | ||
| Absent | 3 (8.6) | 32 (91.4) | |
| Present | 6 (42.9) | 8 (57.1) | |
Table 4 shows the prevalence of LN metastasis in 49 patients with papillary adenocarcinoma-type EGC according to the current indication criteria of ESD. Among the 49 patients, 6 had absolute indication for ESD (intramucosal cancer ≤ 20 mm in size without ulceration) and 11 had expanded indication for ESD (4 patients, intramucosal cancer > 20 mm in size without ulceration; 2 patients, intramucosal cancer ≤ 30 mm with ulceration; and 5 patients, sm1 cancer ≤ 30 mm). The other 32 patients were in out-of-ESD indication because of tumor size, ulceration, or deep submucosal invasion.
| Mucosal cancer (n = 14) | Submucosal cancer (n = 35) | ||||||
| Ulcer (-) (n = 10) | Ulcer (+) (n = 4) | sm1 (n = 8) | sm2 (n = 27) | ||||
| ≤20 mm | > 20 mm | ≤30 mm | > 30 mm | ≤30 mm | > 30 mm | Any size | |
| (n = 6) | (n = 4) | (n = 2) | (n = 2) | (n = 5) | (n = 3) | (n = 27) | |
| Lymph node metastasis | 0 (0) | 1 (25) | 0 (0) | 0 (0) | 1 (20) | 0 (0) | 7 (26) |
Among the 17 patients with EGC indicated for ESD, 2 patients (11.8%) had LN metastasis. One patient had intramucosal cancer (m3) of histological MP type (with differentiated component) 48 mm in size without ulceration, and the other patient had a submucosal cancer (sm1) of histological MP type (with differentiated component) 30 mm in size with LVI.
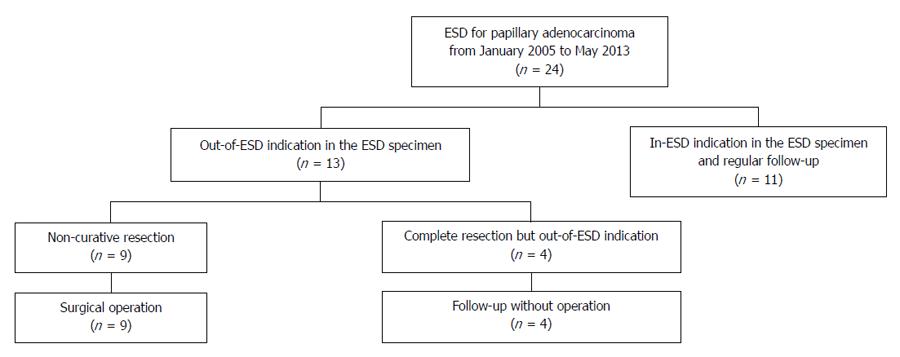
During the study period, 24 patients with papillary adenocarcinoma-type EGC underwent ESD as a primary treatment (Figure 2). The clinicopathological characteristics of these 24 patients are summarized in Table 5. In the pre-treatment work-up, 10 patients had absolute indication for ESD and 14 patients had expanded indication for ESD. In all cases, en bloc resection was obtained without treatment-related adverse events. In the final histopathology, 13 patients (54%) had out-of-ESD indication; 9 patients had LVI or deep submucosal invasion, 3 patients had intramucosal cancer > 30 mm in size with ulceration, and 1 patient had sm1 cancer > 30 mm in size (Table 6). Of these patients, 9 underwent additional surgical operation because of non-curative resection, and the other 4 patients were followed-up without operation. Recurrence was not observed during the median follow-up period of 19 mo (range: 6-51 mo) in the 15 patients who only underwent ESD.
| Characteristic | |
| No. of patients | 24 |
| Median age, yr (range) | 68 (56-80) |
| Gender | |
| Male | 19 (79) |
| Female | 5 (21) |
| Location | |
| Upper third | 4 (17) |
| Middle third | 0 (0) |
| Lower third | 20 (83) |
| Macroscopic shape | |
| Elevated | 23 (96) |
| Flat | 1 (4) |
| Depressed | 0 (0) |
| Ulceration | 5 (21) |
| Histologic type | |
| PP type | 13 (54) |
| MP with diff. | 9 (38) |
| MP with undiff. | 2 (8) |
| Median size, mm (range) | 22 (6-59) |
| ≤ 30 | 18 (75) |
| > 30 | 6 (25) |
| Depth of invasion | |
| Mucosa (m1:m2:m3) | 14 (0:4:10) (58) |
| Submucosa (sm1:sm2) | 10 (4:6) (42) |
| Lymphovascular invasion | 5 (21) |
| Lymph node metastasis | 1 (4) |
| Patient No. | Gender/age | Ulceration | Histologic type | Tumor size (mm) | Pre-treatment ESD indication | Depth of invasion | LVI | Additional surgery | Follow-up period (mo) | Recurrence |
| 1 | M/74 | - | PP | 18 | Absolute | m3 | + | Yes | 3 | No |
| 2 | M/80 | - | MP + undiff. | 18 | Absolute | sm2 | + | Yes | 7 | No |
| 3 | M/68 | - | PP | 18 | Absolute | sm2 | - | Yes | 18 | No |
| 4 | M/66 | - | PP | 13 | Absolute | sm2 | - | Yes | 20 | No |
| 5 | M/56 | + | PP | 32 | Expanded | m2 | - | No | 34 | No |
| 6 | M/78 | + | MP + diff. | 34 | Expanded | m3 | - | No | 3 | No |
| 7 | M/74 | + | PP | 59 | Expanded | m3 | - | No | 26 | No |
| 8 | M/56 | - | PP | 24 | Expanded | sm1 | + | Yes | 3 | No |
| 9 | F/63 | - | MP + diff. | 30 | Expanded | sm1 | + | Yes | 51 | No |
| 10 | F/71 | + | MP + diff. | 32 | Expanded | sm1 | - | No | 41 | No |
| 11 | M/65 | - | PP | 22 | Expanded | sm2 | - | Yes | 8 | No |
| 12 | M/66 | - | MP + diff. | 24 | Expanded | sm2 | + | Yes | 26 | No |
| 13 | F/73 | - | MP + undiff. | 31 | Expanded | sm2 | - | Yes | 24 | No |
In the present study, the overall incidence of LN metastasis in the 49 patients who underwent surgery for papillary adenocarcinoma-type EGC was 18.3%. Of the 17 patients who met the current indication criteria of ESD (6 patients, absolute indication; 11 patients, expanded indication), 2 patients with expanded indication of ESD had LN metastasis. Therefore, the rate of LN metastasis in patients with EGC indicated for ESD was somewhat high (11.8%). Furthermore, of the 24 patients who underwent ESD as a primary treatment for papillary carcinoma-type EGC, more than half (13 patients) achieved an out-of-ESD indication. Among the 13 patients in out-of-ESD indication, 9 underwent surgical operation because of LVI or deep submucosal invasion.
Papillary adenocarcinoma accounts for approximately 6%-11% of gastric carcinomas and 1% of EGCs[7,9,13,14]. There have been few reports on the clinicopathological characteristics of papillary adenocarcinoma because of its rarity[7,9]. Papillary adenocarcinomas tend to occur in older patients and have a proximal tumor location, elevated shape in the early stage, frequent liver metastasis, higher rate of LN involvement, and lower overall 5-year survival rate compared with non-papillary gastric carcinomas. In the present study, the frequency of papillary adenocarcinoma-type EGC was 4.2% (64/1510). Most papillary adenocarcinoma-type EGCs had an elevated shape (73%) and were located in the lower third of the stomach (86%).
Despite the more aggressive features of papillary adenocarcinoma, it is currently classified as a differentiated-type adenocarcinoma and, therefore, papillary adenocarcinoma-type and tubular adenocarcinoma-type EGCs have been treated according to the same ESD indication criteria. Many recent studies have reported a high curative resection rate (84%-95%) and excellent long-term outcomes (a 5-year survival rate of 92%-100%) for ESD in EGC based on the absolute and expanded indications[1-4,11]. However, the number of patients with papillary adenocarcinoma included in these studies was small. Therefore, it is possible that the safety risks of ESD for papillary adenocarcinoma-type EGC have been overlooked.
Known predictive factors of LN metastasis in EGC include tumor size, histological type, depth of invasion, and LVI[15]. The overall incidence of LN metastasis in EGC is known to exceed 10% (2%-5% for mucosal cancer vs > 20% for submucosal cancer; 0.4% for differentiated-type intramucosal cancer vs 4.2%-7.3% for undifferentiated-type intramucosal cancer)[5,15,16]. In the present study, LVI was a predictive factor of LN metastasis in papillary adenocarcinoma-type EGC. The incidence of LN metastasis was 7.1% in mucosal cancer and 22.9% in submucosal cancer. Therefore, the rate of LN metastasis in papillary adenocarcinoma type-EGCs was higher in the present study than in previous reports.
The criteria of surgical operation after non-curative endoscopic resection of EGC have yet to be clearly defined. To date, the criteria of surgical operation after ESD for EGC include positive lateral or vertical margins, deep submucosal invasion (> 500 μm), LVI, or undifferentiated-type histology[10,11,17,18]. The frequency of surgical operation after ESD is 2.1%-14.6%, according to previous reports[19,20]. The rate of surgical operation for papillary adenocarcinoma-type EGC because of LVI or deep submucosal invasion was higher (37.5%) in our study than in previous reports. It is impossible to directly compare our results with those of previous studies because of the relatively small number of patients and the exclusion of other carcinoma types in our study. However, papillary adenocarcinoma-type EGCs tended to have a higher frequency of LN metastasis and additional surgical operation after ESD in the present study compared with other differentiated-type EGCs in the previous reports, even for tumors that met the current indication criteria of ESD[5,15,16,19,20].
Our study demonstrated that ESD for papillary adenocarcinoma-type EGC should be approached more carefully because of the higher frequency of LN metastasis, LVI, and deep invasion. To our knowledge, this is the first study to demonstrate the outcomes of ESD for papillary adenocarcinoma-type EGC alone. However, our study has some limitations. Firstly, potential selection biases may have been present because of the retrospective nature of the study. Treatment options were selected on a case-by-case basis according to clinical judgment and patient factors. Secondly, our study had a relatively small number of patients and a short follow-up period. Further large-scale prospective studies with longer follow-up periods are needed to clarify the clinicopathological characteristics and outcomes of ESD in papillary adenocarcinoma-type EGC. Lastly, because a tumor’s histopathological type is typically defined as the predominant type in cases with mixed histopathological components, we defined papillary adenocarcinoma as a tumor in which more than 50% of the tumor area contained papillary structure. However, it is possible that other histopathological components of papillary adenocarcinomas could confer different clinicopathological characteristics and ESD outcomes. Further investigation is required to determine the contribution of papillary and non-papillary components of EGC to clinicopathological characteristics and ESD outcomes.
In conclusion, papillary adenocarcinoma-type EGCs are classified as differentiated-type carcinoma and, therefore, treated with ESD according to the same indication criteria as other differentiated-type carcinomas such as tubular adenocarcinoma. However, our study demonstrated that the rate of LN metastasis under the current ESD indication criteria was somewhat high (11.8%). In addition, more than half of the patients who underwent ESD as a primary treatment for papillary carcinoma-type EGC ultimately achieved out-of-ESD indication. Therefore, the use of ESD should be more carefully considered for papillary adenocarcinoma-type EGCs with suspected ESD indication after pre-treatment work-up compared with other differentiated-type adenocarcinomas because of their higher frequency of LN metastasis and additional surgical operation.
Papillary adenocarcinoma of the stomach is associated with a higher frequency of lymph node (LN) and liver metastasis, as well as poor surgical outcome, compared with tubular adenocarcinoma. Given the more aggressive features of papillary adenocarcinoma, an inevitable question is whether papillary and tubular adenocarcinomas should be treated according to the same endoscopic submucosal dissection (ESD) indication criteria.
Studies investigating the safety and outcomes of ESD for papillary adenocarcinoma-type EGC alone are currently few. In this study, the authors investigated the clinicopathological predictors of LN metastasis and evaluated the outcomes of ESD in papillary adenocarcinoma-type early gastric cancers (EGCs).
The overall prevalence of LN metastasis in papillary adenocarcinoma-type EGCs was 18.3%. Of patients with papillary adenocarcinoma-type EGCs who met the current indication criteria of ESD, the rate of LN metastasis somewhat high (11.8%). Furthermore, of patients who underwent ESD as a primary treatment for papillary carcinoma-type EGC, more than half achieved an out-of-ESD indication.
The use of ESD should be more carefully considered for papillary adenocarcinoma-type EGCs with suspected ESD indication after pre-treatment work-up compared with other differentiated-type adenocarcinomas because of their higher frequency of LN metastasis and additional surgical operation.
Papillary adenocarcinoma is a rare histologic variant of gastric adenocarcinoma that is characterized histologically by epithelial projections scaffolded by a central fibrovascular core. Currently, papillary adenocarcinoma is classified as a differentiated-type adenocarcinoma.
The authors present a large retrospective study with 49 patients with papillary adenocarcinoma of the stomach treated with either surgical operation or endoscopic submucosal dissection. The study is the largest study to date to compare the outcome of surgical interventions and ESD for this rare histopathologic entity.
P- Reviewer: Moeschler O, Teoh AYB S- Editor: Ma YJ L- Editor: Rutherford A E- Editor: Liu XM
| 1. | Abe N, Gotoda T, Hirasawa T, Hoteya S, Ishido K, Ida Y, Imaeda H, Ishii E, Kokawa A, Kusano C. Multicenter study of the long-term outcomes of endoscopic submucosal dissection for early gastric cancer in patients 80 years of age or older. Gastric Cancer. 2012;15:70-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 2. | Choi MK, Kim GH, Park do Y, Song GA, Kim DU, Ryu DY, Lee BE, Cheong JH, Cho M. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a single-center experience. Surg Endosc. 2013;27:4250-4258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 3. | Abe S, Oda I, Suzuki H, Nonaka S, Yoshinaga S, Odagaki T, Taniguchi H, Kushima R, Saito Y. Short- and long-term outcomes of endoscopic submucosal dissection for undifferentiated early gastric cancer. Endoscopy. 2013;45:703-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 120] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 4. | Chung IK, Lee JH, Lee SH, Kim SJ, Cho JY, Cho WY, Hwangbo Y, Keum BR, Park JJ, Chun HJ. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc. 2009;69:1228-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 476] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 5. | Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T, Kato Y. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3:219-225. [PubMed] |
| 6. | Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31-49. [PubMed] |
| 7. | Hu B, El Hajj N, Sittler S, Lammert N, Barnes R, Meloni-Ehrig A. Gastric cancer: Classification, histology and application of molecular pathology. J Gastrointest Oncol. 2012;3:251-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 256] [Reference Citation Analysis (0)] |
| 8. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2390] [Cited by in RCA: 2872] [Article Influence: 205.1] [Reference Citation Analysis (0)] |
| 9. | Yasuda K, Adachi Y, Shiraishi N, Maeo S, Kitano S. Papillary adenocarcinoma of the stomach. Gastric Cancer. 2000;3:33-38. [PubMed] |
| 10. | Takizawa K, Ono H, Kakushima N, Tanaka M, Hasuike N, Matsubayashi H, Yamagichi Y, Bando E, Terashima M, Kusafuka K. Risk of lymph node metastases from intramucosal gastric cancer in relation to histological types: how to manage the mixed histological type for endoscopic submucosal dissection. Gastric Cancer. 2013;16:531-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 11. | Hanaoka N, Tanabe S, Mikami T, Okayasu I, Saigenji K. Mixed-histologic-type submucosal invasive gastric cancer as a risk factor for lymph node metastasis: feasibility of endoscopic submucosal dissection. Endoscopy. 2009;41:427-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 12. | Okada K, Fujisaki J, Yoshida T, Ishikawa H, Suganuma T, Kasuga A, Omae M, Kubota M, Ishiyama A, Hirasawa T. Long-term outcomes of endoscopic submucosal dissection for undifferentiated-type early gastric cancer. Endoscopy. 2012;44:122-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 13. | Xuan ZX, Ueyama T, Yao T, Tsuneyoshi M. Time trends of early gastric carcinoma. A clinicopathologic analysis of 2846 cases. Cancer. 1993;72:2889-2894. [PubMed] |
| 14. | Uefuji K, Ichikura T, Tamakuma S. Clinical and prognostic characteristics of papillary clear carcinoma of stomach. Surg Today. 1996;26:158-163. [PubMed] |
| 15. | Kim KJ, Park SJ, Moon W, Park MI. Analysis of factors related to lymph node metastasis in undifferentiated early gastric cancer. Turk J Gastroenterol. 2011;22:139-144. [PubMed] |
| 16. | Akagi T, Shiraishi N, Kitano S. Lymph node metastasis of gastric cancer. Cancers (Basel). 2011;3:2141-2159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Fujii M, Egashira Y, Akutagawa H, Nishida T, Nitta T, Edagawa G, Kurisu Y, Shibayama Y. Pathological factors related to lymph node metastasis of submucosally invasive gastric cancer: criteria for additional gastrectomy after endoscopic resection. Gastric Cancer. 2013;16:521-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Oda I, Gotoda T, Sasako M, Sano T, Katai H, Fukagawa T, Shimoda T, Emura F, Saito D. Treatment strategy after non-curative endoscopic resection of early gastric cancer. Br J Surg. 2008;95:1495-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 109] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 19. | Jung H, Bae JM, Choi MG, Noh JH, Sohn TS, Kim S. Surgical outcome after incomplete endoscopic submucosal dissection of gastric cancer. Br J Surg. 2011;98:73-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Noh H, Park JJ, Yun JW, Kwon M, Yoon DW, Chang WJ, Oh HY, Joo MK, Lee BJ, Kim JH. Clinicopathologic characteristics of patients who underwent curative additional gastrectomy after endoscopic submucosal dissection for early gastric cancer or adenoma. Korean J Gastroenterol. 2012;59:289-295. [PubMed] |