Published online Apr 7, 2015. doi: 10.3748/wjg.v21.i13.3928
Peer-review started: August 13, 2014
First decision: September 15, 2014
Revised: October 10, 2014
Accepted: November 7, 2014
Article in press: November 11, 2014
Published online: April 7, 2015
Processing time: 237 Days and 7.6 Hours
AIM: To determine the cutoff values and to compare the diagnostic role of alpha-fetoprotein (AFP) and prothrombin induced by vitamin K absence-II (PIVKA-II) in chronic hepatitis B (CHB).
METHODS: A total of 1255 patients with CHB, including 157 patients with hepatocellular carcinoma (HCC), 879 with non-cirrhotic CHB and 219 with cirrhosis without HCC, were retrospectively enrolled. The areas under the receiver operating characteristic (AUROC) curves of PIVKA-II, AFP and their combination were calculated and compared.
RESULTS: The optimal cutoff values for PIVKA-II and AFP were 40 mAU/mL and 10 ng/mL, respectively, for the differentiation of HCC from nonmalignant CHB. The sensitivity and specificity were 73.9% and 89.7%, respectively, for PIVKA-II and 67.5% and 90.3% for AFP, respectively. The AUROC curves of both PIVKA-II and AFP were not significantly different (0.854 vs 0.853, P = 0.965) for the differentiation of HCC from nonmalignant CHB, whereas the AUROC of PIVKA-II was significantly better than that of AFP in patients with cirrhosis (0.870 vs 0.812, P = 0.042). When PIVKA-II and AFP were combined, the diagnostic power improved significantly compared to either AFP or PIVKA-II alone for the differentiation of HCC from nonmalignant CHB (P < 0.05), especially when cirrhosis was present (P < 0.05).
CONCLUSION: Serum PIVKA-II might be a better tumor marker than AFP, and its combination with AFP may enhance the early detection of HCC in patients with CHB.
Core tip: Hepatocellular carcinoma (HCC) surveillance is crucial for patients with chronic hepatitis B (CHB). There have been few studies that have compared the levels of prothrombin induced by vitamin K absence-II (PIVKA-II) and AFP in hepatitis B virus-associated HCC. Serum PIVKA-II, at a level of 40 mAU/mL, is a useful tumor marker to distinguish patients with HCC from those with nonmalignant CHB, especially liver cirrhosis (LC). A combination of AFP and PIVKA-II could enhance early detection of HCC in patients with CHB. Therefore, serum PIVKA-II levels should be measured in combination with serum AFP levels during the follow-up of patients with CHB and particularly those with LC.
- Citation: Seo SI, Kim HS, Kim WJ, Shin WG, Kim DJ, Kim KH, Jang MK, Lee JH, Kim JS, Kim HY, Kim DJ, Lee MS, Park CK. Diagnostic value of PIVKA-II and alpha-fetoprotein in hepatitis B virus-associated hepatocellular carcinoma. World J Gastroenterol 2015; 21(13): 3928-3935
- URL: https://www.wjgnet.com/1007-9327/full/v21/i13/3928.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i13.3928
Hepatocellular carcinoma (HCC) is one of the most common cancers and is the leading cause of cancer-related deaths worldwide. HCC appears to be increasing in incidence[1-4] and still has a dismal prognosis in spite of recent advancements in therapeutic intervention because of its diagnosis at advanced stages. Previous studies have reported the benefits of HCC surveillance on survival thanks to the detection of HCC at earlier stages[5-7]. Thus, many guidelines recommend HCC surveillance for at-risk populations[8-10].
Of the known biomarkers, alpha-fetoprotein (AFP) has been the most widely used as a tumor marker for diagnosis and surveillance of HCC. The sensitivity and specificity of AFP, however, have been reported to vary from 39% to 64% and from 76% to 91%, respectively[11-13]. Furthermore, AFP levels may be elevated in a number of nonspecific conditions in patients with cirrhosis or when cases of chronic hepatitis are exacerbated[14].
Due to such limitations, ultrasonography (US) alone without concurrent detection of AFP levels has been recommended for the surveillance of HCC, according to the representative guidelines in the United States and Europe[8,10]. US is the primary surveillance tool that is used in patients with chronic liver diseases that can detect the development of HCC. The sensitivity and specificity have been reported to be 65% to 80% and 90% to 93%, respectively[8]. However, the interpretation is not only highly operator-dependent but is also insufficiently sensitive in the patients who are obese or who have underlying liver cirrhosis (LC). Additionally, the combined use of AFP and US not only increases the detection rates but also increases the false-positive rates[8]. Therefore, reliable biomarkers to complement the pitfalls of US are needed, especially in patients with cirrhotic patients.
Prothrombin induced by vitamin K absence-II (PIVKA-II) is an abnormal prothrombin protein that is elevated in HCC. The overall sensitivity and specificity of serum PIVKA-II in the detection of HCC has been reported to be 48%-62% and 81%-98%, respectively[15]. Unlike AFP, the serum levels of PIVKA-II are not elevated in patients with chronic liver disease such as exacerbations of chronic hepatitis and cirrhosis. That PIVKA-II is more specific that AFP represents a highly specific feature of this protein[16,17].
To date, many studies have been conducted to determine the role of PIVKA-II in patients with HCC, but most included only small numbers of patients or patients with heterogeneous etiologies, with a predominance of individuals with hepatitis C virus (HCV) infection[15,18-23]. Considering that the clinical features and mechanisms of hepatocarcinogenesis vary according to the etiologies[24-26], the roles of PIVKA-II in hepatitis B virus (HBV)-associated HCC might be different from those of HCV-related HCC.
To the best of our knowledge, there have been few reports that have evaluated the role of PIVKA-II in HBV-associated HCC. Therefore, additional studies are warranted to determine the role of PIVKA-II in the diagnosis of HBV-associated HCC. The aim of this study was to compare the diagnostic role of PIVKA and AFP and to determine the best cutoff values of both tumor markers in patients with chronic hepatitis B (CHB).
A total of 1255 patients with CHB were retrospectively included at Hallym University Medical Center, Seoul, Korea, from January 2005 to December 2012. All patients who enrolled in this study demonstrated positivity for hepatitis B surface antigen for at least 6 mo.
Demographic and clinical information was collected from the medical records of the subjects. LC was diagnosed by histology and/or ultrasonographic/CT imaging features and was supplemented by clinically relevant portal hypertension (e.g., esophageal and/or gastric varices, ascites, splenomegaly with a platelet count of < 100000/mm3) or hepatic encephalopathy. All patients with HCC were newly diagnosed, and the diagnosis of HCC was based on liver histology or appropriate imaging characteristics as defined by accepted guidelines[8]. The HCC stage was determined according to the TNM staging system by the Liver Cancer Study Group of Japan[27]. Early stage HCC was defined as a single tumor nodule < 3 cm in diameter with no evidence of vascular invasion or metastasis.
All patients were divided into three groups: (1) non-cirrhotic CHB (G1); (2) cirrhosis without HCC (G2); and (3) HCC (G3). In the non-HCC groups, laboratory tests were performed on the most recent clinic visit, and the following parameters were assessed: albumin, total bilirubin, alanine aminotransferase (ALT), international normalized ratio of prothrombin time, model for end-stage liver disease score, and the levels of AFP and PIVKA-II. The same laboratory data were obtained at the time of diagnosis of HCC in the HCC group.
The exclusion criteria were as follows: the patients who (1) were positive for other markers of hepatitis such as hepatitis C virus or human immunodeficiency virus; (2) were heavy alcoholics (more than 80 g of ethanol daily); and (3) were taking warfarin or antibiotics that might influence the metabolism of vitamin K.
This study was approved by the Investigation and Ethics Committee for Human Research at Hallym University Medical Center, Seoul, Korea.
The serum AFP concentrations were determined with a commercially available electrochemiluminescence immunoassay kit (Elecsys AFP immunoassay, Roche, Mannheim, Germany). The serum PIVKA-II level was measured by a revised enzyme immunoassay (Eitest PIVKA-II; Eisai, Tokyo, Japan).
A one-way analysis of variance (ANOVA) test was used for continuous variables and a χ2 test was used for categorical variables in order to compare variables between the three groups. Four groups were compared with Kruskal-Wallis tests and a χ2 test. Log transformation was used for the AFP and PIVKA-II values to account for the large range of values among the groups for both markers. We used a new variable, the combination of AFP and PIVKA-II levels (logAFP + 4.6*logPIVKA-II), which was conceived in a previous study[15]. To find the optimal cutoff value of AFP and PIVKA-II in the diagnosis of HCC, the receiver operating characteristic (ROC) curves were plotted using all possible cutoff values for each assay. The areas under the ROC (AUROC) curves of PIVKA-II, AFP and the combination of the two were calculated and compared. Youden’s index was calculated as an index of sensitivity and specificity. A P value < 0.05 was considered significant. Statistical analyses were performed using SPSS, version 16 and Medcalc, version 12.3.
A total of 1255 patients were divided into three subgroups: (1) non-cirrhotic CHB (G1, n = 879); (2) cirrhosis without HCC (G2, n = 219); and (3) HCC (G3, n =157). The median levels (range) of both PIVKA-II and AFP were significantly higher in the HCC group compared to the non-cirrhotic CHB group and the cirrhosis without HCC group [PIVKA-II; 202 (10-2000) mAU/mL vs 23 (6-162) mAU/mL vs 19 (4-312) mAU/mL, AFP; 55.9 (0.6-121000.0) ng/mL vs 2.5 (0.6-602.8) ng/mL vs 3.3 (0.6-233.6) ng/mL, P < 0.001]. Additionally, patients in the HCC group (G3) showed advanced liver dysfunction compared with patients in the non-HCC group (G1 and G2). The characteristics of these patients are summarized in Table 1.
| Non-cirrhotic CHB (n = 879) | Cirrhosis without HCC (n = 219) | HCC (n = 157) | P value | |
| Age (yr) | 45 (17-97) | 54 (26-92) | 57 (34-89) | 0.0001 |
| Gender (M:F) | 549:330 | 151:68 | 124:33 | 0.0001 |
| AFP (ng/mL) | 2.5 (0.6-602.8) | 3.3 (0.6-233.6) | 55.9 (0.6-121000.0) | 0.0001 |
| PIVKA-II (mAU/mL) | 23 (6-162) | 19 (4-312) | 202 (10-2000) | 0.0001 |
| INR | 1.00 (0.84-3.24) | 1.11 (0.92-2.38) | 1.12 (0.79-8.93) | 0.0001 |
| Albumin (g/dL) | 4.5 (2.3-5.3) | 4.2 (0.5-5.2) | 3.6 (2.0-5.2) | 0.0001 |
| Total bilirubin (mg/dL) | 0.7 (0.2-19.2) | 0.9 (0.2-18.9) | 1.0 (0.3-14.7) | 0.0001 |
| ALT (IU/L) | 26.5 (2-3112) | 31 (8-886) | 39 (6-1155) | 0.1220 |
| MELD score | 7.1 | 10.1 | 9.9 | 0.0001 |
| TNM stage n (%) | NA | NA | ||
| I | 22 (14.0) | |||
| II | 58 (36.9) | |||
| III | 32 (20.4) | |||
| IV | 45 (28.7) | |||
| Early HCC n (%) | NA | NA | 46 (29.3) |
Ninety-two (10.5%), 21 (9.6%), and 116 (73.9%) patients in G1, G2, and G3, respectively, had PIVKA-II values above 40 mAU/mL, which was previously reported as the upper limit of normal. Elevated AFP (current clinical cutoff level, > 20 ng/mL,) was observed in 40 (4.6%), 22 (10.0%), and 94 (61.0%) patients of G1, G2, and G3, respectively. Of the patients with HCC, 26 patients (16.6%) had an AFP level < 20 ng/mL and a PIVKA-II level < 40 mAU/mL, 37 (23.6%) had isolated PIVKA-II elevation, and 15 (9.6%) had isolated AFP elevation.
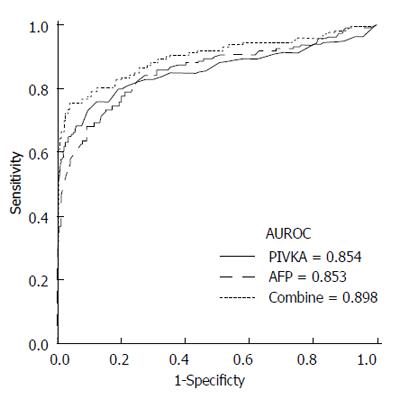
To determine the optimal cutoff values in the differentiation of HCC (G3) from nonmalignant CHB (G1 and G2), ROC curves were drawn (Figure 1). The best cutoff values for PIVKA-II and AFP were 40 mAU/mL and 10 ng/mL, respectively. The sensitivity and specificity at these cutoff values were 73.9% and 89.7%, respectively, for PIVKA-II and 67.5% and 90.3%, respectively, for AFP.
The AUROC curves of PIVKA-II and AFP were not significantly different for the differentiation of HCC from nonmalignant CHB (0.854 vs 0.853, P = 0.965). After the combined levels of PIVKA-II and AFP were considered, a significant improvement was observed in the diagnostic power compared with either PIVKA-II or AFP alone (AUROC = 0.898; vs PIVKA-II, P < 0.001; vs AFP, P = 0.03, respectively). The combination of these tumor markers yielded a sensitivity and specificity of 75.2% and 95.4%, respectively.
We compared the baseline characteristics of all of the patients according to the cutoff levels that we calculated from the ROC curve. A comparison of these baseline characteristics is represented in Table 2.
| PIVKA-II < 40 AFP < 10 | PIVKA-II≥40 AFP < 10 | PIVKA-II < 40 AFP < 10 | PIVKA-II≥40 AFP≥10 | P value | |
| Number | 918 | 125 | 108 | 104 | |
| Age (yr) | 47.9 (17-97) | 48.4 (18-83) | 51.4 (23-92) | 55 (25-88) | 0.0001 |
| Gender (M:F) | 578:340 | 97:28 | 67:41 | 82:22 | 0.0001 |
| INR | 1.0 (0.8-3.2) | 1.0 (0.8-2.3) | 1.1 (0.9-2.1) | 1.13 (0.7-8.9) | 0.0001 |
| Albumin (g/dL) | 4.5 (0.5-5.3) | 2.9 (0.6-9.6) | 3.8 (2-5.2) | 3.5 (1.7-4.9) | 0.0001 |
| TB (mg/dL) | 0.7 (0.2-18.9) | 0.7 (0.2-19.2) | 1.1 (0.2-17.3) | 1.1 (0.3-14.7) | 0.0001 |
| ALT (IU/L) | 25.4 (2-803) | 30.1 (5-1664) | 50 (9-1531) | 43.4 (9-3112) | 0.0001 |
| MELD score | 7.4 | 8.2 | 9.9 | 10.5 | 0.0001 |
| LC, n (%) | 188 (20.5) | 42 (33.6) | 50 (46.3) | 89 (85.6) | 0.0001 |
| HCC, n (%) | 21 (2.3) | 30 (24) | 20 (18.5) | 86 (82.7) | 0.0001 |
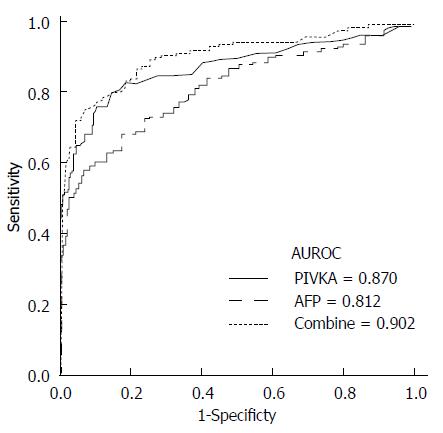
The optimal cutoff values of PIVKA-II and AFP for the differentiation of HCC (G3) from LC (G2) were 40 mAU/mL and 25 ng/mL, respectively (Figure 2). These values gave a sensitivity and specificity of 73.9% and 90.4%, respectively, for PIVKA-II and 58.6% and 92.7%, respectively, for AFP. Interestingly, the AUROC curve indicated a better sensitivity and specificity for PIVKA-II than for AFP in the differentiation of HCC from LC (0.870 vs 0.812, P = 0.042). When PIVKA-II and AFP were combined, the diagnostic power was significantly enhanced compared with that of either marker alone (AUROC = 0.902; vs PIVKA-II, P = 0.001; vs AFP, P < 0.001). The combination of these tumor markers yielded a sensitivity and specificity of 75.2% and 92.7%, respectively.
After an analysis of the diagnostic power of PIVKA-II and AFP for the differentiation of early HCC from LC, the AUROC curve of PIVKA-II tended to be better than that of AFP (AUROC = 0.752 vs 0.712, P = 0.512). Additionally, the combination of these two markers demonstrated a significant improvement in the diagnostic power compared with each marker alone (Combination vs PIVKA-II, P = 0.033; Combination vs AFP, P = 0.019, respectively).
Table 3 shows the sensitivity and specificity of PIVKA-II alone, AFP alone, and the combination of both markers in the differentiation of HCC from nonmalignant CHB at the various cutoff values based on our study and on previously published studies: 10, 20, 200, 400 ng/mL for AFP[11-13,15,23,28,29] and 40, 100, 125, 150 mAU/mL for PIVKA-II[15,20,21,30]. PIVKA-II values > 40 mAU/mL had better sensitivity, specificity, positive predictive value, and negative predictive value than PIVKA-II values of 100, 125 and 150 mAU/mL that were accepted in other studies. In addition, PIVKA-II values > 40 mAU/mL demonstrated a better diagnostic performance than AFP, regardless of the cutoff value that was chosen.
| Sensitivity | Specificity | PPV | NPV | |
| PIVKA-II (mAU/mL) | ||||
| > 40 | ||||
| HCC vs CHB | 73.9% | 89.7% | 50.7% | 96.0% |
| HCC vs LC | 73.9% | 90.4% | 84.7% | 82.8% |
| > 100 | ||||
| HCC vs CHB | 57.3% | 98.8% | 87.4% | 94.2% |
| HCC vs LC | 57.3% | 96.8% | 82.8% | 76.0% |
| > 125 | ||||
| HCC vs CHB | 56.1% | 99.2% | 90.7% | 94.0% |
| HCC vs LC | 56.1% | 97.3% | 93.6% | 75.5% |
| > 150 | ||||
| HCC vs CHB | 52.9% | 99.4% | 92.2% | 93.6% |
| HCC vs LC | 52.9% | 97.3% | 93.3% | 74.2% |
| AFP (ng/mL) | ||||
| > 10 | ||||
| HCC vs CHB | 67.5% | 90.3% | 50.0% | 95.1% |
| HCC vs LC | 67.5% | 82.6% | 73.6% | 78.0% |
| > 20 | ||||
| HCC vs CHB | 59.9% | 94.4% | 60.3% | 94.3% |
| HCC vs LC | 59.9% | 90.0% | 81.0% | 75.8% |
| > 200 | ||||
| HCC vs CHB | 36.3% | 99.3% | 87.7% | 91.6% |
| HCC vs LC | 36.3% | 99.1% | 96.6% | 68.5% |
| > 400 | ||||
| HCC vs CHB | 29.9% | 99.7% | 94.0% | 90.9% |
| HCC vs LC | 29.9% | 100.0% | 100.0% | 66.6% |
| Combination1 | ||||
| HCC vs CHB | 75.2% | 95.4% | 69.8% | 96.4% |
| HCC vs LC | 75.2% | 92.7% | 88.1% | 83.9% |
When the two markers were combined, the sensitivity increased from 73.9% for PIVKA-II alone and 67.5% for AFP alone to 75.2%; similarly, the specificity also increased from 89.7% for PIVKA-II alone and 90.3% for AFP alone to 95.4% in the differentiation of patients with HCC from those without HCC.
PIVKA-II is an abnormal prothrombin molecule, known as des-gamma-carboxy prothrombin, which is generated as a result of an acquired defect in the posttranslational carboxylation of the prothrombin precursor in malignant cells. Since the first report by Liebman et al[31] in 1984, many studies have demonstrated the usefulness of PIVKA-II for the diagnosis of HCC[17-22,31-33]. Earlier studies showed that PIVKA-II had a low sensitivity compared with AFP[30,34]. However, after the introduction of a revised enzyme immunoassay kit, more recent studies revealed that PIVKA-II is comparable or more sensitive than AFP for the differentiation of HCC from nonmalignant chronic liver disease; moreover, most studies have shown that PIVKA-II is more specific than AFP[15,17-22,32,33].
Most studies that have been concerned with the role of PIVKA-II were conducted in Japan and in Western countries where the etiology of liver disease varied greatly, primarily associated with HCV infection. Therefore, we focused on the role of PIVKA-II in HBV-related HCC after a consideration of the differences in hepatocarcinogenesis and in the clinical features between HBV-related HCC and HCV-related HCC. Actually, HBV-related HCC typically presents more frequently as an aggressive tumor compared with HCV-related HCC, and the levels of AFP are higher in HBV-related HCC than in HCV-related HCC[24-26].
In this study, we showed that PIVKA-II is more accurate than AFP in the ability to distinguish patients with HCC from those with nonmalignant CHB. The sensitivity and specificity at the cutoff values which were identified by the ROC curve were 73.9% and 89.7%, respectively, for PIVKA-II and 67.5% and 90.3%, respectively, for AFP. Although the AUROC curves of PIVKA-II and AFP showed a similar diagnostic efficacy for the differentiation of HCC from nonmalignant CHB, when the analysis was limited to patients with cirrhosis, the AUROC curve indicated a significantly better sensitivity and specificity for PIVKA-II than for AFP for differentiation of HCC from LC; this was also the case for the differentiation of early HCC from LC. These data are in contrast to those of previous studies that showed that the sensitivity of PIVKA-II was inferior to AFP in the detection of small HCC[33,34], and this result suggests that PIVKA-II is a more reliable tumor marker than AFP for the detection of early HCC in patients with CHB.
This difference might be due to the difference in the etiology of liver diseases of the patients. Previous studies have included patients with heterogeneous etiologies of liver diseases (mainly HCV infection or alcohol) and were case-controlled studies that were conducted in relatively small numbers of selected patients of the population. In contrast, our study included patients with only HBV as an etiology of liver disease and nearly all patients with CHB who visited our institute. Therefore, our study represents a real clinical situation, and indeed, advanced liver diseases such as LC or HCC account for approximately 30% in this study. This is in accordance with the disease progression that occurs as part of the natural history of CHB. To our knowledge, our study is the first large-scale study that has demonstrated the diagnostic role of PIVKA-II in HBV-associated HCC.
The ROC curve identified the optimal cutoff value of PIVKA-II as 40 mAU/mL for the differentiation of patients with HCC from those with nonmalignant CHB in our study. This value is comparable with the result of a Japanese study, but is lower than that of a previous American study. It is possible that the cutoff value of PIVKA-II may vary among different ethnic groups. Indeed, American patients typically have PIVKA-II values up to 63 mAU/mL, whereas studies in Japan have used 40 mAU/mL as the upper limit of normal[15,18-22].
We found that the optimal cutoff value for the level of AFP for the diagnosis of HCC was 10 ng/mL. This result was substantially lower than the current clinical cutoff level (20 ng/mL), but was in accordance with the results of recent studies with cutoff values of 10.9 ng/mL and 11 ng/mL[15,23]. In addition, the optimal cutoff value of AFP for the differentiation of HCC from LC was 25 ng/mL. These results are in agreement with those of previous prospective studies that have included patients with LC[11].
Because AFP and PIVKA-II levels do not correlate in patients with HCC and are complementary tumor markers, it would be reasonable to determine that using both markers might improve the accuracy of a diagnosis of HCC. Indeed, a few studies have demonstrated an increased sensitivity and specificity when these tumor markers are combined[35-37]. We also demonstrated that the combination of these two markers showed a significant improvement with respect to the diagnostic power compared with each marker alone for the differentiation of HCC from nonmalignant CHB, especially in patients with cirrhosis. Clinically, this is very important because US is the primary surveillance tool for LC, but this method is not sensitive enough to detect HCC in many patients with cirrhosis. Although AFP is no longer considered in surveillance tests for HCC in American and European practice guidelines, our study suggests that the combination of AFP and PIVKA-II could enhance the diagnostic accuracy. A prospective study of patients with LC is warranted to further validate the utility of this combination for the detection of early HCC.
Our results are interesting, but there are some potential limitations to our study. First, this is a single-center study with a retrospective design. Second, the number of patients with HCC (n = 157) is relatively small compared to those with nonmalignant CHB (n = 1098); nonetheless, it reflects the natural course of CHB progression. Finally, our study cannot be generalized to patients with liver diseases that are not caused by HBV or to patients who are not Asian.
In conclusion, serum PIVKA-II, at the level of 40 mAU/mL, is a useful tumor marker to distinguish patients with HCC from those with nonmalignant CHB, especially LC. A combination of AFP and PIVKA-II could enhance the early detection of HCC in patients with CHB. Therefore, the measurement of the serum levels of PIVKA-II should be applied in combination with the measurement of the AFP levels in the follow-up of patients with CHB, particularly those with LC. Further large-scale prospective studies are needed to verify the utility of PIVKA-II for the detection of early HCC.
Hepatocellular carcinoma (HCC) is one of the most common cancers, and therefore, the early detection of HCC is crucial for patients with chronic liver disease. Ultrasonography is the primary tool that is used for surveillance, but there are limitations associated with this method.
Alpha-fetoprotein (AFP) has been most widely used as a tumor marker for the diagnosis and surveillance of HCC; however, the sensitivities and specificities that have been reported have been varied. Prothrombin induced by vitamin K absence-II (PIVKA-II) can be more specific than AFP, and therefore, additional studies are needed in order to determine the role of PIVKA-II in the diagnosis of hepatitis B virus-associated HCC.
Previous studies regarding the role of PIVKA-II in patients with HCC were conducted in small numbers of patients or in patients with heterogeneous etiologies of liver disease (primarily those with hepatitis C virus). Hence, the authors compared the diagnostic roles of PIVKA-II and AFP and determined the best cutoff value of both tumor markers in patients with chronic hepatitis B (CHB).
The study results suggest that serum PIVKA-II levels might be a useful tumor marker to distinguish patients with HCC from those with nonmalignant CHB, especially liver cirrhosis. The combination of AFP and PIVKA-II may also enhance the early detection of HCC in patients with CHB.
PIVKA-II is an abnormal prothrombin molecule, known as des-gamma-carboxy prothrombin, which is generated as a result of an acquired defect in the posttranslational carboxylation of the prothrombin precursor in malignant cells.
The research is important and the research findings are significant. The novelty and innovative nature of the research is acceptable because the other similar reports but this one depicts an interesting number of patients.
P- Reviewer: Quarleri J S- Editor: Qi Y L- Editor: A E- Editor: Ma S
| 1. | Bosch FX, Ribes J, Díaz M, Cléries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5-S16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1799] [Cited by in RCA: 1816] [Article Influence: 86.5] [Reference Citation Analysis (0)] |
| 2. | Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2651] [Cited by in RCA: 2596] [Article Influence: 108.2] [Reference Citation Analysis (0)] |
| 3. | El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2221] [Cited by in RCA: 2140] [Article Influence: 82.3] [Reference Citation Analysis (0)] |
| 4. | Benhamiche AM, Faivre C, Minello A, Clinard F, Mitry E, Hillon P, Faivre J. Time trends and age-period-cohort effects on the incidence of primary liver cancer in a well-defined French population: 1976-1995. J Hepatol. 1998;29:802-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Stravitz RT, Heuman DM, Chand N, Sterling RK, Shiffman ML, Luketic VA, Sanyal AJ, Habib A, Mihas AA, Giles HC. Surveillance for hepatocellular carcinoma in patients with cirrhosis improves outcome. Am J Med. 2008;121:119-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 115] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 6. | Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:417-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 751] [Cited by in RCA: 937] [Article Influence: 46.9] [Reference Citation Analysis (1)] |
| 7. | McMahon BJ, Bulkow L, Harpster A, Snowball M, Lanier A, Sacco F, Dunaway E, Williams J. Screening for hepatocellular carcinoma in Alaska natives infected with chronic hepatitis B: a 16-year population-based study. Hepatology. 2000;32:842-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 242] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 8. | Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6554] [Article Influence: 468.1] [Reference Citation Analysis (1)] |
| 9. | Omata M, Lesmana LA, Tateishi R, Chen PJ, Lin SM, Yoshida H, Kudo M, Lee JM, Choi BI, Poon RT. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int. 2010;4:439-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 797] [Cited by in RCA: 840] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 10. | European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [PubMed] |
| 11. | Oka H, Tamori A, Kuroki T, Kobayashi K, Yamamoto S. Prospective study of alpha-fetoprotein in cirrhotic patients monitored for development of hepatocellular carcinoma. Hepatology. 1994;19:61-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 246] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 12. | Colombo M, de Franchis R, Del Ninno E, Sangiovanni A, De Fazio C, Tommasini M, Donato MF, Piva A, Di Carlo V, Dioguardi N. Hepatocellular carcinoma in Italian patients with cirrhosis. N Engl J Med. 1991;325:675-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 588] [Cited by in RCA: 536] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 13. | Pateron D, Ganne N, Trinchet JC, Aurousseau MH, Mal F, Meicler C, Coderc E, Reboullet P, Beaugrand M. Prospective study of screening for hepatocellular carcinoma in Caucasian patients with cirrhosis. J Hepatol. 1994;20:65-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 188] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 14. | Di Bisceglie AM, Hoofnagle JH. Elevations in serum alpha-fetoprotein levels in patients with chronic hepatitis B. Cancer. 1989;64:2117-2120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 15. | Marrero JA, Su GL, Wei W, Emick D, Conjeevaram HS, Fontana RJ, Lok AS. Des-gamma carboxyprothrombin can differentiate hepatocellular carcinoma from nonmalignant chronic liver disease in american patients. Hepatology. 2003;37:1114-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 293] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 16. | Malaguarnera G, Giordano M, Paladina I, Berretta M, Cappellani A, Malaguarnera M. Serum markers of hepatocellular carcinoma. Dig Dis Sci. 2010;55:2744-2755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 140] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 17. | Inagaki Y, Tang W, Makuuchi M, Hasegawa K, Sugawara Y, Kokudo N. Clinical and molecular insights into the hepatocellular carcinoma tumour marker des-γ-carboxyprothrombin. Liver Int. 2011;31:22-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 18. | Ikoma J, Kaito M, Ishihara T, Nakagawa N, Kamei A, Fujita N, Iwasa M, Tamaki S, Watanabe S, Adachi Y. Early diagnosis of hepatocellular carcinoma using a sensitive assay for serum des-gamma-carboxy prothrombin: a prospective study. Hepatogastroenterology. 2002;49:235-238. [PubMed] |
| 19. | Okuda H, Nakanishi T, Takatsu K, Saito A, Hayashi N, Takasaki K, Takenami K, Yamamoto M, Nakano M. Serum levels of des-gamma-carboxy prothrombin measured using the revised enzyme immunoassay kit with increased sensitivity in relation to clinicopathologic features of solitary hepatocellular carcinoma. Cancer. 2000;88:544-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 20. | Okuda H, Nakanishi T, Takatsu K, Saito A, Hayashi N, Watanabe K, Magario N, Yokoo T, Naraki T. Measurement of serum levels of des-gamma-carboxy prothrombin in patients with hepatocellular carcinoma by a revised enzyme immunoassay kit with increased sensitivity. Cancer. 1999;85:812-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 21. | Mita Y, Aoyagi Y, Yanagi M, Suda T, Suzuki Y, Asakura H. The usefulness of determining des-gamma-carboxy prothrombin by sensitive enzyme immunoassay in the early diagnosis of patients with hepatocellular carcinoma. Cancer. 1998;82:1643-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Nomura F, Ishijima M, Kuwa K, Tanaka N, Nakai T, Ohnishi K. Serum des-gamma-carboxy prothrombin levels determined by a new generation of sensitive immunoassays in patients with small-sized hepatocellular carcinoma. Am J Gastroenterol. 1999;94:650-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 90] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Marrero JA, Feng Z, Wang Y, Nguyen MH, Befeler AS, Roberts LR, Reddy KR, Harnois D, Llovet JM, Normolle D. Alpha-fetoprotein, des-gamma carboxyprothrombin, and lectin-bound alpha-fetoprotein in early hepatocellular carcinoma. Gastroenterology. 2009;137:110-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 583] [Cited by in RCA: 568] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 24. | Cantarini MC, Trevisani F, Morselli-Labate AM, Rapaccini G, Farinati F, Del Poggio P, Di Nolfo MA, Benvegnù L, Zoli M, Borzio F. Effect of the etiology of viral cirrhosis on the survival of patients with hepatocellular carcinoma. Am J Gastroenterol. 2006;101:91-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 72] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Tanabe G, Nuruki K, Baba Y, Imamura Y, Miyazono N, Ueno K, Arima T, Nakajyou M, Aikou T. A comparison of hepatocellular carcinoma associated with HBV or HCV infection. Hepatogastroenterology. 1999;46:2442-2446. [PubMed] |
| 26. | Anzola M. Hepatocellular carcinoma: role of hepatitis B and hepatitis C viruses proteins in hepatocarcinogenesis. J Viral Hepat. 2004;11:383-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 137] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 27. | Liver Cancer Study Group of Japan. General Rules for the Clinical and Pathological Study of Primary Liver Cancer, 2nd ed. Tokyo: Kanehara 2003; . |
| 28. | Gupta S, Bent S, Kohlwes J. Test characteristics of alpha-fetoprotein for detecting hepatocellular carcinoma in patients with hepatitis C. A systematic review and critical analysis. Ann Intern Med. 2003;139:46-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 294] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 29. | Borzio M, Bruno S, Roncalli M, Mels GC, Ramella G, Borzio F, Leandro G, Servida E, Podda M. Liver cell dysplasia is a major risk factor for hepatocellular carcinoma in cirrhosis: a prospective study. Gastroenterology. 1995;108:812-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 140] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 30. | Ozaki H, McLaughlin LW. Fluorescence resonance energy transfer between specific-labeled sites on DNA. Nucleic Acids Symp Ser. 1992;67-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Liebman HA, Furie BC, Tong MJ, Blanchard RA, Lo KJ, Lee SD, Coleman MS, Furie B. Des-gamma-carboxy (abnormal) prothrombin as a serum marker of primary hepatocellular carcinoma. N Engl J Med. 1984;310:1427-1431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 419] [Article Influence: 10.2] [Reference Citation Analysis (16)] |
| 32. | Durazo FA, Blatt LM, Corey WG, Lin JH, Han S, Saab S, Busuttil RW, Tong MJ. Des-gamma-carboxyprothrombin, alpha-fetoprotein and AFP-L3 in patients with chronic hepatitis, cirrhosis and hepatocellular carcinoma. J Gastroenterol Hepatol. 2008;23:1541-1548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 134] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 33. | Yoon YJ, Han KH, Kim do Y. Role of serum prothrombin induced by vitamin K absence or antagonist-II in the early detection of hepatocellular carcinoma in patients with chronic hepatitis B virus infection. Scand J Gastroenterol. 2009;44:861-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Nakamura S, Nouso K, Sakaguchi K, Ito YM, Ohashi Y, Kobayashi Y, Toshikuni N, Tanaka H, Miyake Y, Matsumoto E. Sensitivity and specificity of des-gamma-carboxy prothrombin for diagnosis of patients with hepatocellular carcinomas varies according to tumor size. Am J Gastroenterol. 2006;101:2038-2043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 117] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 35. | Fujiyama S, Tanaka M, Maeda S, Ashihara H, Hirata R, Tomita K. Tumor markers in early diagnosis, follow-up and management of patients with hepatocellular carcinoma. Oncology. 2002;62 Suppl 1:57-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 106] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 36. | Ishii M, Gama H, Chida N, Ueno Y, Shinzawa H, Takagi T, Toyota T, Takahashi T, Kasukawa R. Simultaneous measurements of serum alpha-fetoprotein and protein induced by vitamin K absence for detecting hepatocellular carcinoma. South Tohoku District Study Group. Am J Gastroenterol. 2000;95:1036-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 54] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 37. | Weitz IC, Liebman HA. Des-gamma-carboxy (abnormal) prothrombin and hepatocellular carcinoma: a critical review. Hepatology. 1993;18:990-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 106] [Article Influence: 3.3] [Reference Citation Analysis (0)] |