Published online Apr 7, 2015. doi: 10.3748/wjg.v21.i13.3826
Peer-review started: August 23, 2014
First decision: October 29, 2014
Revised: December 11, 2014
Accepted: February 12, 2015
Article in press: February 13, 2015
Published online: April 7, 2015
Processing time: 228 Days and 20.4 Hours
Hepatocellular carcinoma (HCC) is one of the leading causes of cancer death, especially in Eastern areas. With advancements in diagnosis and treatment modalities for HCC, the survival and prognosis of HCC patients are improving. However, treatment patterns are not uniform between areas despite efforts to promote a common protocol. Although many hepatologists in Asian countries may adopt the principles of the Barcelona Clinic Liver Cancer staging system, they are also independently making an effort to expand the indications of each treatment and to combine therapies for better outcomes. Several expanded criteria for liver transplantation in HCC have been developed in Asian countries. Living donor liver transplantation is much more commonly performed in these countries than deceased donor liver transplantation, and it may be preceded by other treatments such as the down-staging of tumors. Local ablation therapies are often combined with transarterial chemoembolization (TACE) and the outcome is comparable to that of surgical resection. The indications of TACE are expanding, and there are new types of transarterial therapies. Although data on drug-eluting beads, TACE, and radioembolization in Asian countries are still relatively sparse compared with Western countries, these methods are gradually gaining popularity because of better tolerability and the possibility of improved response rates. Hepatic arterial infusion chemotherapy and radiotherapy are not included in Western guidelines, but are currently being used actively in several Asian countries. For more advanced HCCs, appropriate combinations of TACE, radiotherapy, and sorafenib can be considered, and emerging data indicate improved outcomes of combination therapies compared with single therapies. To include these paradigm shifts into newer treatment guidelines, more studies may be needed, but they are certainly in progress.
Core tip: This article describes the current status of the management of hepatocellular carcinoma, focusing on the changing trends of treatment modalities in Eastern countries. Newly adopted therapies as well as emerging combination strategies are discussed based on recent data.
- Citation: Yim HJ, Suh SJ, Um SH. Current management of hepatocellular carcinoma: An Eastern perspective. World J Gastroenterol 2015; 21(13): 3826-3842
- URL: https://www.wjgnet.com/1007-9327/full/v21/i13/3826.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i13.3826
Worldwide, hepatocellular carcinoma (HCC) is the sixth most prevalent cancer[1]. More than 600000 people are newly diagnosed every year and approximately the same number die due to HCC annually. The main etiology of HCC is liver cirrhosis caused by chronic hepatitis B or C, alcohol, fatty liver diseases, or less commonly, autoimmune or genetic metabolic liver diseases[2]. The incidence, characteristics, and prognosis of HCC vary from region to region according to the prevalence of underlying chronic liver diseases as well as the screening and treatment strategies for HCC. Currently, efforts are being made to promote the use of common protocols, but the patterns of treatment are still not uniform as the therapeutic approach to HCC mainly depends on the availability of treatment modalities as well as the preferences of physicians[2-7]. As three-quarters of HCC cases occur in East Asia, the experiences and data in this area should have been substantially accumulated, and the treatment trends would have characteristic features. This article aims to review the current status of the management of HCC from an Eastern perspective. The first section introduces the principles and current trends of different treatment modalities, and the second section summarizes the findings on multidisciplinary treatments based on recently available data.
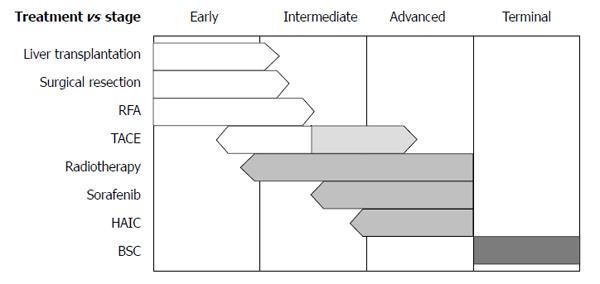
For decisions regarding initial treatments, the Barcelona Clinic Liver Cancer (BCLC) staging system from Western guidelines is frequently applied[3,4]. This system has very strict guidelines for treatments; only very early stage and early stage HCCs are indicated for the curative therapies, and only one treatment option is assigned to each of intermediate stage and advanced stage disease. Furthermore, no combination therapy is recommended according to the BCLC algorithm. Hence, despite the worldwide use of the BCLC guidelines, debates regarding their practicality are ongoing. The Asian Pacific Association for the Study of the Liver has guidelines for HCC treatment similar to those in the BCLC system[5]; both consider hepatic function as well as tumor size, number, and its extent, and the treatment options are not much different from BCLC. However, indications of pre-existing therapies are expanding and newly emerging therapies are currently being implemented (Figure 1). In addition, alternative therapies or combination therapies for each stage are available as determined by clinical situations in real practice. In this section, current status of surgical, interventional, medical, and radiation therapies are reviewed with newly available data, particularly, from Asian countries.
Liver transplantation: Transplanting a healthy liver provides the most favorable survival outcomes in HCC patients[8]. If the patients have underlying decompensated liver cirrhosis, no other option exists. However, the availability of organs limits access to this best curative therapy. The annual incidence of deceased organ donors does not exceed 5 per million in most Asian countries[9]. Compared with those in Western countries, patients with HCC in Asia have a low probability of receiving a deceased donor liver transplantation (DDLT) in a timely manner and thus have a higher risk of drop-out because of tumor progression[10]. For this reason, living donor liver transplantation (LDLT) has been promoted. In Korea, the proportion of adult LDLT recipients with HCC has increased to 30%-40% of all HCC liver transplant recipients[11]. Despite technical complexity, LDLT is replacing DDLT in other Asian countries as well[8]. Donor mortality and morbidity rates of LDLT were 0.2% and 24%, respectively, according to a report of a worldwide survey[12]. Most LDLT centers develop their own criteria for maximizing donor safety[13]. Although the right lobe is the most suitable graft for the recipient, its procurement is limited by size of donor liver. When the right lobe cannot be used alone, a dual graft from 2 donors containing the left lobe can be utilized[14]. Despite this method, the donor pool has not significantly expanded because of the technical complexity of the surgery and ethical concerns. To further overcome organ shortage, ABO-incompatible LDLT was attempted and became successful after the implementation of rituximab, which decreased antibody-mediated rejection rates from 23.5% to 6.3%, as shown in a Japanese multicenter study[15].
The Milan criteria (solitary tumor < 5 cm, 2 or 3 tumors < 3 cm each, and absence of vascular invasion and extrahepatic metastasis) have been applied for the selection of candidates for liver transplantation[16-18]. However, these criteria have been criticized because many patients missed opportunities for transplants because of the strictness of the criteria. Therefore, Yao et al[19] proposed their own set of criteria, permitting the listing of patients with somewhat larger-sized tumors. In Asia, several independent criteria have also been proposed, expanding indications without increasing the risk of HCC recurrence significantly[20-25] (Table 1). Five-year survival rates were as high as 80% after transplantation using these criteria. However, these criteria should be applied very carefully to DDLT candidates until a consensus is achieved.
| Criteria (city, country, reference) | Tumor number | Tumor diameter (cm) | Additional criteria | Overall survivalwithin criteria | |
| Hong Kong, China[20] | 1 | ≤ 6.5 | No diffuse type, | 3 yr | 78% |
| ≤ 3 | ≤ 4.5 | no vascular invasion | 5 yr | 66% | |
| Hangzhou, China[21] | NC | Total ≤ 8 | Histopathologic grade I or II with AFP ≤ 400 ng/dL if tumor > 8 cm | 3 yr | 70.7% |
| 5 yr | 70.7% | ||||
| Seoul (AMC), Korea[22] | ≤ 6 | ≤ 5 | No gross vascular invasion | 3 yr | 87.5% |
| 5 yr | 81.6% | ||||
| Seoul (CMC), Korea[23] | ≤ 7 | ≤ 7 | NC | 5 yr | 86.3% |
| Tokyo, Japan[24] | ≤ 5 | ≤ 5 | NC | 3 yr | 82% |
| 5 yr | 75% | ||||
| Kyoto, Japan[25] | ≤ 10 | ≤ 5 | PIVKA-II ≤ 400 mAU/mL | 5 yr | 87% |
Current issues related to “bridging therapy” and “downstaging” are discussed in the section on multidisciplinary treatments.
Hepatic resection: As hepatic resection is a potentially curative therapy, it has been considered a first-line option for HCC patients with well-preserved hepatic function, especially when there is only one tumor or when tumors are confined to a single lobe. To assess hepatic function, a Japanese group measured the indocyanine green retention rate at 15 min (ICG15)[26]. Feasibility and the extent of the resection are decided according to the degree of retention of the dye[27]. Although the BCLC algorithm mandates Child-Pugh A liver function without portal hypertension for hepatic resection, selective resection has been attempted in HCC patients exhibiting upper Child-Pugh B liver function or mild portal hypertension in Asian countries, with reference to the ICG15 value[6,26].
Prognosis after hepatic resection is determined by number and size of tumor, vascular invasion, and level of alpha-fetoprotein[28-30]. Five-year survival rates are > 50% after the resection of solitary tumors, whereas rates of 20%-30% have been reported for 3 or more nodules[28-30]. With respect to tumor size, 5-year survival rates for patients with HCCs < 2 cm, 2-5 cm, and > 5 cm are 66%, 52%, and 37%, respectively[28-30]. However, in selected cases with proper hepatic function, large single HCCs can be surgically removed with favorable long-term survival outcomes[29]. More advanced stages of HCCs have been resected in 511 Chinese patients, yielding a 5-year survival rate of 30.5%[31]. The presence of vascular invasion or extrahepatic metastasis resulted in poor outcomes[31].
Recently, laparoscopic liver resection has been implemented for the treatment of HCC. This is a minimally invasive surgery, so postoperative morbidity and duration of hospitalization are reduced with no changes in surgical margin status, tumor recurrence, and overall survival[32]. This technique is successfully being applied for the resection of large tumors between 5 and 10 cm and lesions at difficult-to-approach locations[33-35] as well as intra-abdominal metastatic HCCs in Asian countries[36].
Local ablative therapies: Local ablation can be categorized as chemical or thermal. Chemical ablation includes percutaneous ethanol injection (PEI) and acetic acid injection, whereas thermal ablation includes radiofrequency ablation (RFA), the use of microwaves, cryotherapy, and high-intensity focused ultrasound[3,5]. As these are considered potentially curative therapies, patients with early stage HCCs are the candidates, especially when surgical treatments are not available. Among these modalities, RFA is currently the most commonly used. Excellent long-term results of RFA, up to 10 years, were reported in Korean HCC patients meeting the Milan criteria[37]. The results at 5 and 10 years were as follows: cumulative local tumor progression rates, 27.0% and 36.9%; cumulative intrahepatic distant recurrence rates, 73.1% and 88.5%; and overall survival rates, 59.7% and 32.3%, respectively[37]. Comparison of the efficacy of RFA with other local therapies showed that RFA was substantially superior to PEI, especially in tumors with a diameter > 2 cm[38,39]. Nevertheless, PEI is associated with a necrosis rate of 90%-100% in tumors < 2 cm and is still useful in selected patients when RFA is not technically feasible[40-42]. Recently, it was reported that in cases where the tumor is located under the diaphragm or near the surface of the liver, creating artificial ascites or pleural effusion is helpful in performing RFA and avoiding burns on adjacent organs[43,44]. This technique is being applied in several Asian countries with good results[43,44].
Several randomized controlled trials compared the efficacy of RFA with that of resection in Asian patients with HCC meeting the Milan criteria[45,46]. Pooled data demonstrated no significant differences in overall survival or recurrence-free survival between the treatments at 1 and 3 years. The 5-year overall survival [relative risk (RR), RR = 0.72, 95% confidence interval (CI): 0.60-0.88] and recurrence-free survival (RR = 0.56, 95%CI: 0.40-0.78) rates were higher in the resection group[46]; however, the 5-year data were provided by only one study, which advocated surgery. Complication rates were lower and hospitalization period shorter in patients who received RFA rather than resection[46]. Although the efficacy of RFA appears to be comparable to that of hepatic resection with lower complication rates, additional data may be needed and the need for long-term surveillance should be re-enforced.
Transarterial chemoembolization: Patients with either large tumors or multinodular tumors and a good performance status are candidates for transarterial chemoembolization (TACE). However, the presence of decompensated liver disease, severe hepatic dysfunction, portal vein thrombosis, or extrahepatic tumor spread precludes TACE. Although TACE is associated with a complete response rate of only 40%, it improved survival compared with supportive treatment in 2 independently performed randomized controlled trials in Eastern and Western countries[47,48]. A meta-analysis of 7 trials that included 545 HCC patients showed similar results [odds ratio (OR), OR = 0.42, 95%CI: 0.20-0.88][49]. Importantly, when the tumor size is ≤ 2 cm, prognosis is even better; a Korean study of TACE in small HCCs reported cumulative survival rates of 93.4%, 75.4%, 63.1%, and 51.1% at 1, 3, 5, and 8 years, respectively, for TACE, which were not significantly different from those of 97.6%, 86.7%, 74.5%, and 60.0%, respectively, for RFA[50]. Therefore, TACE may have a potential role as a curative therapy for small HCCs when surgical or local ablative therapies are not feasible.
Although TACE has been contraindicated in cases of HCC with portal vein invasion, multiple studies reported that it can be safely performed and may have better survival benefits than supportive care in patients with compensated liver function[51-54]. Notably, when a tumor is nodular and restricted to 1 lobe or 1-2 segments and hepatic function is classified as Child-Pugh class A, median survival after TACE is as long as 22-30 mo even in the presence of main portal vein tumor thrombosis[51,52]. When compared with sorafenib, which is a current standard treatment for advanced HCC, median overall survival rates for TACE were not significantly different from those of sorafenib (9.2 and 7.4 mo, respectively; P = 0.377)[55]. Therefore, TACE could be an alternative therapeutic option for advanced HCC.
TACE was originally intended to maintain intratumoral concentrations of chemotherapeutic agents by transiently obstructing supply vessels and thus minimizing systemic exposure. The strategy for TACE was recently refined after the introduction of microspheres that can increase the duration of drug retention in the tumor without blocking blood flow, which reduces hepatic derangement and systemic toxicity[56]. In a multicenter phase II randomized study of 201 HCC patients, TACE with drug-eluting beads (DEB) was compared with conventional TACE, and hepatic toxicity and drug-related adverse events were significantly less observed in the DEB-TACE arm[57]. Although this study showed a nonsignificant trend toward better antitumoral effects with DEB-TACE, a case-control study conducted in Korea reported a significantly better objective response rate with DEB-TACE (85%) than with conventional TACE (30%, P < 0.01) as assessed by modified Response Evaluation Criteria in Solid Tumors. A systemic review of the published data demonstrated the superiority of DEB over conventional TACE in terms of overall disease control, especially in patients with more advanced stage disease[58]. To summarize, the indications of TACE are expanding, and new types of transarterial therapy are currently available in Eastern areas.
Cytotoxic chemotherapies: Cytotoxic chemotherapy has been attempted continuously since treatment of HCC began but has failed to improve overall survival in most clinical trials to date[59,60]. The main problem of cytotoxic chemotherapy in HCC is the co-existence of liver cirrhosis. Cirrhosis can delay the metabolism of chemotherapeutic agents and may enhance their toxicity[61]. In addition, HCC is relatively chemoresistant to most cytotoxic anticancer drugs. An early randomized trial of doxorubicin conducted in Hong Kong showed a tumor response of less than 10% and borderline improvement in overall survival (10.6 wk) compared with no treatment (7.5 wk, P = 0.036)[61]. Notably, 25% of patients died due to doxorubicin-related complications, including septicemia and cardiotoxicity. The antitumor activity of other cytotoxic agents such as gemcitabine[62,63], oxaliplatin[64], and capecitabine[65] in clinical and retrospective studies was modest with objective responses of < 20%. In randomized controlled trials, combination therapies such as PIAF (cisplatin, interferon, adriamycin, fluorouracil) and FOLFOX (5-fluorouracil, folic acid, and oxaliplatin) did not significantly improve survival compared with doxorubicin[59,60]. Moreover, a high rate of myelotoxicity was reported in the PIAF group[59]. Therefore, no cytotoxic chemotherapy regimen has provided strong evidence of improving the survival of HCC patients, and regular practice of chemotherapy is not advised. Nonetheless, a current retrospective study in Korea indicated that ECF (epirubicin, cisplatin, and 5-fluorouracil) combination therapy prolonged overall survival in sorafenib-refractory patients with metastatic HCC if a tumor response was observed; overall survival periods were 20.4 mo in responders and 4.9 mo in nonresponders (P < 0.001)[66]. Thus, ECF may be an alternative or rescue therapy for patients who failed sorafenib therapy, but further prospective evaluations will be needed.
Hepatic arterial infusion chemotherapy (HAIC) has been used for treatment of advanced HCC with portal vein tumor thrombosis in Asian countries[67-72]. Traditionally, the presence of tumor thrombus is assumed to aggravate ischemic injuries after TACE, so alternative modalities were sought. HAIC does not use embolic material, and the chemotherapeutic agent is infused into the hepatic artery via an implanted catheter, which reduces systemic side effects by first-pass effects and maximizes drug delivery to the tumor. Although this is considered an experimental treatment modality and is not recommended for treatment of HCC in Western countries, a large amount of clinical data on HAIC have been accumulated in Eastern countries[67-72]. A small retrospective study showed survival benefits of HAIC using low doses of cisplatin and 5-fluorouracil compared with systemic cytotoxic chemotherapy or supportive care (median survival, 6, 4, and 2 mo, respectively; P = 0.003) in cases of advanced HCC with portal vein tumor thrombosis[73]. A subsequent prospective study showed better efficacy of HAIC when a higher dose of cisplatin was used[74]. Importantly, a recent retrospective study by the same group in Korea compared HAIC and sorafenib in advanced HCC patients with portal vein tumor thrombosis and showed better overall survival (7.1 and 5.5 mo, respectively; P = 0.011) and longer median time to progression (3.3 and 2.1 mo, respectively; P = 0.034) in the HAIC group[75]. These findings are consistent with those of a Japanese study[76]. Although well-designed prospective studies are warranted to confirm these results, HAIC at least appears to be an alternative therapy for patients with portal vein tumor thrombosis when sorafenib is not available or is intolerable. Further research is also needed regarding the use of HAIC as salvage therapy in patients with advanced HCC who do not respond to standard therapy.
Molecular target therapies: Sorafenib is the only approved systemic agent for the treatment of advanced HCC. It is a multikinase inhibitor whose targets include Raf-1 and B-Raf serine/threonine kinases, vascular endothelial growth factor receptor (VEGFR) and platelet-derived growth factor receptor (PDGFR) tyrosine kinases, and c-kit receptors[77]. The Sorafenib Hepatocellular Carcinoma Assessment Randomized Protocol trial, which enrolled 602 patients with advanced stage HCC, showed improved median overall survival in the sorafenib group compared with the placebo group (10.7 and 7.9 mo, respectively; P < 0.001)[78]. A subsequent study conducted in the Asia-Pacific region showed a similar trend (overall survival of 6.5 and 4.2 mo in the sorafenib and placebo groups, respectively; P = 0.014)[79]. On the basis of the results of these trials, sorafenib became the standard treatment for advanced HCC with well-preserved liver function. The most significant adverse effects were diarrhea and hand-foot skin reactions[78,79]. Interestingly, these toxicities were associated with better survival in patients receiving sorafenib[80,81]. Therefore, despite the occurrence of adverse reactions, the use of sorafenib should not be discouraged when tolerable.
Sorafenib had not been compared with other treatment modalities before its approval. Currently, its efficacy in a real-life setting was compared with the efficacy of other treatments (TACE, radiation, and cytotoxic chemotherapy) in Korean patients with advanced HCC[82,83]. Overall survival times were 8.4 and 8.2 mo for sorafenib and other treatments, respectively, and the difference was not significant[82,83]. To improve the efficacy of sorafenib, combination therapy or a multidisciplinary approach may be needed[84].
Several newer molecular target therapeutic agents were evaluated in clinical trials. Sunitinib, an orally administered multikinase inhibitor of receptor tyrosine kinases, showed modest activity against HCC. Although an overall survival time of 9.8 mo was observed in a phase II study[85], sunitinib did not outperform sorafenib in a phase III randomized study (overall survival, 8.1 and 10.0 mo, respectively; P = 0.0019)[86]. Brivanib, a selective dual inhibitor of fibroblast growth factor (FGF) and vascular endothelial growth factor (VEGF) signaling, was associated with a median overall survival of 10 mo in a phase II trial[87] and was considered a promising new drug for advanced HCC. However, the primary endpoint of brivanib not being non-inferior to sorafenib was not met in a subsequent phase III trial (overall survival, 9.5 and 9.9 mo, respectively, P value is nonsignificant)[88]. The efficacy of brivanib in advanced HCC patients who were intolerant to sorafenib or failed to respond to sorafenib previously was also tested. The results of this study showed no significant improvement in overall survival compared with placebo (9.4 and 8.2 mo for brivanib and placebo, respectively)[89]. Linifanib (ABT-869), a receptor tyrosine kinase inhibitor targeting VEGFRs, also failed to significantly improve survival compared with sorafenib in a phase III trial (overall survival, 9.1 and 9.8 mo, respectively)[90]. The reasons for most of these novel agents failing to improve survival may be diverse and include lack of understanding of critical drivers of cancer progression, unpredicted toxicity, and marginal antitumor effects[91]. To overcome these obstacles, the clinical trial design should be modified to focus on biomarker-based subpopulation targeting strategies, and thereby, personalized therapies should be pursued in the future. In addition, efficacy and toxicity need to be evaluated in detail in phase I and II studies before moving to phase III studies[91]. Currently, several novel molecular targeting agents are being evaluated in phase III trials as first-line or second-line therapies including lenvatinib [VEGFR1-3, FGF receptor (FGFR) 1-3, PDGFR-β, RET, KIT], ramucirumab (VEGFR2), regorafenib (VEGFR, TIE-2, PDGFR-β, FGFR, KIT, RET, RAF), cabozantinib (MET, VEGFR-2), and tivantinib (MET)[92]. Table 2 summarizes the current status of the randomized controlled trials of molecular target therapies.
| Status | |
| First line | |
| Comparison with placebo | |
| Sorafenib (SHARP, Asian-Pacific) | Proven benefit |
| Sorafenib in Child B (BOOST) | Phase III Ongoing |
| Comparison study between sorafenib and single agent (head to head) | |
| Sunitinib -> endpoint not met | Terminated |
| Brivanib (BRISK-FL) -> endpoint not met | Failed |
| Linifanib -> endpoint not met | Terminated |
| Lenvatinib | Phase III ongoing |
| Combination of sorafenib and another agent | |
| Sorafenib + Erlotinib (SEARCH) -> endpoint not met | Failed |
| Sorafenib + Doxorubicin (CALGB-80802) | Phase III ongoing |
| Sorafenib + Everolimus | R-Phase II; Failed |
| Second line | |
| Sorafenib failure | |
| Brivanib (BRISK-PS) -> endpoint not met | Failed |
| Brivanib (BRISK-APS) | Terminated |
| Everolimus (EVOLVE-1) -> endpoint not met | Failed |
| Ramucirumab (REACH) | Phase III ongoing |
| Regorafenib (RESORCE) | Phase III ongoing |
| Cabozantinib (CELESTAL) | Phase III ongoing |
| Tivantinib (Metiv-HCC) | Phase III ongoing |
| Combination or addition to standard therapies | |
| Adjuvant setting after surgery or RFA: Sorafenib (STORM) | |
| Failed | |
| Combination with TACE: Sorafenib (SPACE) -> endpoint not met | |
| Failed | |
| Brivanib (BRISK-TA) -> endpoint not met | Failed |
| Sorafenib (TACTICS) | R-Phase II ongoing |
In summary, there are no currently available first-line molecular targeted agents other than sorafenib and no standard second-line treatments for patients intolerant or nonresponsive to sorafenib. If underlying liver function is well preserved, novel molecular target therapies, HAIC, or systemic cytotoxic chemotherapy may have a role as second-line treatment, but further studies are warranted. If a patient with advanced HCC has poor hepatic function, aggressive anticancer treatments are not indicated.
External radiation therapies: Radiotherapy techniques for the treatment of HCC have substantially evolved over the past decades. Delivery of radiation energy became more precise, which enabled the exposure of tumors to higher doses of radiation, while saving non-tumorous liver parenchyma[93]. In the past, the role of radiation therapy was limited to alleviation of bone pain due to bone metastasis and to emergency use in spine and brain metastasis[94-96]. Radiation therapy has currently been adopted as a definitive therapy with curative intent if the tumor is at an early stage. Particularly, stereotactic body radiation therapy can achieve high rates of locoregional tumor control as it can deliver high doses of radiation in a single treatment session or in a small number of fractions[97,98]. In locally advanced HCCs, radiation therapy can be used to relieve obstruction and improve portal blood flow if the tumor invades the biliary tree or portal vein[99,100]. A large multicenter study in Korea of 994 HCC patients with portal vein tumor thrombosis showed a median survival of 9.2 mo[101]. This was a relatively longer survival time than that of advanced HCC patients who did not receive any treatment in previous trials[78,79]. Studies from Japan and China also reported the efficacy of radiotherapy for HCC with portal vein thrombosis, and overall survival was significantly better in patients receiving radiotherapy than in patients receiving sorafenib (10.9 and 4.8 mo, respectively; P = 0.025) or undergoing surgery (12.3 and 10.3 mo, respectively; P = 0.029)[102,103]. Although these studies were retrospective, they suggest the usefulness of radiotherapy in advanced HCC. However, radiotherapy has not been incorporated into the international guidelines for HCC despite its efficacy. This may be attributed to the paucity of well-designed randomized controlled studies, which are urgently needed. In addition, guidelines for optimal dose fractionation and protocols for avoiding radiation toxicity should be further established[93].
Proton beam therapy (PBT) can dramatically reduce damage to surrounding liver tissue by modulation of the Bragg peak of protons in energy and time, and thereby, maximizes the effects of radiation on the tumor. In Eastern areas, studies of PBT in HCC patients have been reported mainly by Japanese groups[104-106]. A retrospective study of PBT in 162 surgically unresectable patients reported a local control rate of 89% and an overall survival rate of 23.5% at 5 years[106]. Although the tumor stages of the patients were diverse and TACE or PEI may have also been administered, the overall efficacy seems quite favorable. PBT showed a good response rate even for large tumors (> 10 cm) and HCCs with main portal tumor thrombosis[107,108].
Radioembolization: Radioembolization is a modality involving the use of a transarterial approach to the hepatic tumor and subsequent infusion of radioactive substances. The rationale for this approach is that the efficacy of external beam radiation therapy is limited by the low tolerability of cirrhotic livers leading to radiation hepatitis or decompensation. To avoid exposure of non-tumorous parenchyma to radiation, microspheres emitting high-energy and low-penetration radiation are selectively delivered to the tumor[109]. The most commonly used radioembolic agents are iodine-131 and yttrium-90 glass beads, both of which showed favorable antitumoral effects with an acceptable safety profile[109,110]. The benefits of radioembolization over the other types of transarterial therapies still need to be validated. A retrospective analysis showed no significant differences in efficacy between radioembolization and TACE for intermediate stage HCC; median survival times were 15.0 and 14.4 mo, respectively[111]. However, patients receiving radioembolization needed less hospitalization and fewer treatments. Fewer treatment sessions should improve quality of life and reduce the possibility of liver derangement; therefore, in these respects, radioembolization is considered better than conventional TACE. The efficacy of radioembolization in patients with advanced HCC patients has also been evaluated. Sixty- three patients with portal vein thrombosis were analyzed from an European HCC cohort according to underlying liver function[112]. Median overall survival and time to progression were 13.8 and 5.6 mo, respectively, for Child-Pugh A patients and 6.5 and 4.9 mo, respectively, for Child-Pugh B patients[112]. Although these data appear very promising, there are still no randomized controlled trials comparing radioembolization with standard treatments for each stage. Data from Asian countries are limited, but a multicenter prospective study in Korea showed a median time to progression of 18 mo and a 3-year survival rate of 75%[113]. This is an improved result compared with data from Western countries[114,115], but future well-designed studies are needed.
Emerging therapies: Recently, the oncolytic and immunotherapeutic vaccinia virus has been reported to induce antibody-mediated, complement-dependent cancer cell lysis in humans[116]. Immunotherapy may benefit patients with advanced stage HCC who do not have further treatment options. The results of a phase III trial need to be confirmed.
Currently, several target delivery systems has been exploited for the treatment of HCC. New formulations including polymeric nanoparticles, nanocapsules, liposomes, nanoemulsions, microsphere, and polymeric micelles have been reported[117,118]. Novel drug delivery systems are expected to improve treatment efficacy and to decrease toxicity by drug targeting to the specific site of action[118]. For example, the asialoglycoprotein (ASPG) receptor is expressed on hepatocyte, and a synthetic ligand, lactosylated liposomes can be used for effective delivery vehicles of doxorubicin in HCC therapy[119]. In a previous report, lactosylated liposomes encapsulating doxorubicin showed stronger anti-tumor response than the non-targeted liposomal doxorubicin and free doxorubicin. A galactose ligand with chitosan modifications, galactosylated chitosan, is also a promising carrier of chemotherapeutic agent, such as 5-fluorouracil, to the ASPG receptor, and its in vitro and in vivo efficacy was well described[120]. It is thought that efficacy of anticancer therapy utilizing target delivery system will be more synergized by combination of molecular target therapy. Further studies are warrantied.
As treatment modalities for HCC are very diverse, not only hepatologists but also surgeons, intervention radiologists, medical oncologists, and radiation oncologists should jointly discuss the best treatment options for HCC patients. Treatment may not necessarily be a sole modality; combinations of multiple treatments can be considered. Although current guidelines do not recommend multiple treatments, emerging data indicate better outcomes with multidisciplinary treatments for HCC. Furthermore, several newer clinical trials aim to properly evaluate such strategies. In this context, multimodality treatment options based on currently available evidence, especially from Eastern countries, are described in this section, according to HCC stage.
Guidelines recommend liver transplantation, hepatic resection, or RFA/PEI for very early or early stage HCC[3-5]. However, treatment may be diversified according to the status of the patient or the tumor. In addition to the single treatments described above, the following treatments can be considered as an adjuvant or a combination.
Bridging therapy for liver transplantation: Although the best outcome can be achieved with liver transplantation, HCCs may progress while patients are on the waiting list. To avoid dropout, the rate of which approaches 20%, bridging therapies may be needed[121]. Most commonly applied therapies are RFA, TACE, and surgical resection, although data from Asian countries are rather sparse. If the tumor is within the Milan criteria and liver function is not decompensated, RFA should be the first bridging therapy attempted because its post-procedural intratumoral necrosis rate is higher than that of other locoregional therapies, and it is associated with the lowest drop-out rate[122]. PEI appears to be less efficacious than RFA, but can be chosen if the lesions are close to adjacent organs, where RFA is dangerous to perform. If the tumor size is > 3 cm, TACE or TACE plus RFA may be favored as tumors become more vascularized and the effect of RFA may be diminished[121]. Surgical resection can precede liver transplantation, and salvage transplantation can be performed in the event of recurrence, without a decrease in overall post-transplant survival[123]. However, most data on bridging therapy data are uncontrolled, so it is difficult to strongly recommend this therapeutic strategy, especially if patients are eligible for LDLT.
Adjuvant therapy after resection: Hepatic resection is the preferred treatment for patients with early stage tumors and well-compensated liver function, but recurrence is the main obstacle to improving long-term prognosis. To reduce the recurrence rate, various neoadjuvant and adjuvant therapies were evaluated, but they failed to demonstrate any benefits to recurrence-free survival[124-126]. Recently, sorafenib was tested for prevention of recurrence after curative therapy, including resection and ablation (the STORM study), but again no benefits to recurrence-free survival were observed[127]. A Japanese group previously showed positive effects of acyclic retinoids and vitamin K analogues on recurrence-free survival, but overall survival was not improved and large-scale studies were not performed appropriately[128,129]. Interferon has been suggested as an adjuvant therapy after resection[130]. According to a large current database in Taiwan, antiviral therapies reduce the recurrence of HCC after surgery in patients with chronic hepatitis B or C[131,132]. In agreement, a randomized controlled trial conducted in China showed better recurrence-free survival (RR = 0.651, 95%CI: 0.451-0.938) and overall survival (RR = 0.420, 95%CI: 0.271-0.651) in patients receiving antiviral therapy, especially in terms of prevention of late recurrence[133]. It would be reasonable to recommend nucleoside or nucleotide analogues or interferon therapy to patients with hepatitis B and pegylated interferon-based therapy to patients with hepatitis C after curative hepatic resection[130-133].
RFA/PEI combined with TACE: Local ablative therapies, which are curative modalities for HCC as mentioned previously[3,5], have been very useful in the treatment of patients reluctant to undergo or ineligible for surgery because of issues other than liver diseases. As the size of the tumor limits the efficacy of RFA or PEI, the combination of RFA or PEI and vaso-occlusive therapies such as TACE has been attempted to overcome the limitations of interventional therapies and to maximize synergistic effects[134]. A retrospective study conducted in Korea evaluated the therapeutic efficacy of RFA plus TACE in patients with medium-sized (3.1-5.0 cm) HCCs and found that it significantly lowered the local tumor progression rate compared with RFA alone (55% and 86% at 5 years, respectively; P < 0.001)[135]. Subsequently, several randomized controlled trials compared RFA and RFA plus TACE in Japan and China[136,137], and a meta-analysis of these studies showed that the combined treatment was significantly associated with higher overall survival (OR = 1.85, 95%CI: 1.26-2.71) and recurrence-free survival (OR = 2.13, 95%CI: 1.41-3.20) rates[138]. The benefits of the combination therapy could be attributed to the avoidance of the heat sink effect and the subsequent increase in the size of the thermal coagulation zone. In addition, synergism between hyperthermia and high concentrations of chemotherapeutic agents may enhance the destruction of microscopic satellite lesions. Recently, a non-randomized controlled study compared RFA plus TACE and surgical resection for the treatment of single HCCs ranging in size from 2 to 5 cm[139]. The study showed that the combination therapy was as effective as resection in terms of recurrence-free survival (69.4% and 65%, respectively, at 4 years, P value is nonsignificant) and overall survival (78.4% and 80.3%, respectively, at 4 years, P value is nonsignificant). Collectively, the available data suggest that RFA plus TACE provides better outcomes than RFA alone and may be as efficacious as surgical resection for medium-sized HCCs.
PEI may be also combined with TACE, thereby peripheral micrometastasis will be better controlled and diffusion of the ethanol can be more facilitated compared with PEI alone[140]. This combination has been reported to be associated with superior efficacy in terms of local control, but no survival benefits compared with PEI monotherapy have been confirmed[141].
Most HCCs beyond the Milan criteria correspond to intermediate stage HCCs if vascular invasion and distant metastasis are absent. TACE is the recommended therapy for this stage[3,5], but the beneficial effects of TACE on long-term survival are limited. Therefore, further treatment would be necessary even in the presence of an initial tumor response.
Liver transplantation after downstaging: As liver transplantation is associated with the best treatment outcome of HCC, listing patients for transplantation should be considered whenever available; patients in the intermediate stage will be eligible if effective treatment was achieved and their HCC status was shifted to meet the Milan criteria[142]. TACE is the most commonly used modality for downstaging, and local ablative therapies may be combined[142]. The expected 5-year overall survival rate in patients who received liver transplants after downstaging is comparable to that of HCC patients who met the Milan criteria without downstaging[143]. However, the 5-year disease-free survival rate is lower in the downstaged patients[143]. Stricter follow-ups would be necessary for these patients.
TACE combined with RFA or radiotherapy: Combination therapy with RFA or PEI and TACE is being used to treat early stage HCC as mentioned earlier. This therapy can also be applied to intermediate stage HCC. However, tumor may be too extensive or multiple to combine ablative therapies in intermediate stages. For best results, modalities commonly used for more advanced stage HCC can be adopted in combination with TACE (e.g., sorafenib and radiotherapy). The efficacy of TACE plus radiotherapy has been studied in 12 non-randomized and 5 randomized controlled trials in Korea, Japan, and China. A meta-analysis of these trials showed significantly improved survival at 1 year (OR = 2.23, 95%CI: 1.76-2.83) and 5 years (OR = 4.47, 95%CI: 2.08-9.61) and a better tumor response (OR = 2.58, 95%CI: 1.64-4.06) in patients receiving TACE plus radiotherapy compared with patients receiving TACE alone[144]. In this analysis, most although not all patients in the individual studies had intermediate stage HCC. Therefore, the combination of TACE and radiotherapy should be beneficial for this stage, but consensus is needed for routine recommendation in practice guidelines.
TACE combined with sorafenib: TACE may upregulate circulating VEGF, which is associated with vascular invasion, tumor growth, metastasis, and poor survival. Therefore, control of VEGF signaling and of other tumor growth factors is necessary to prevent the progression and recurrence of HCC in patients receiving TACE[145]. A randomized controlled trial was conducted in Japan and Korea to assess the effects of TACE plus sorafenib[146]. In that study, time to progression was significantly longer in Korean patients receiving TACE plus sorafenib than in those receiving TACE alone, but not in Japanese patients. To clarify the clinical results, a meta-analysis of 6 studies was performed, and the pooled results showed that overall survival [hazard ratio (HR) = 0.65, 95%CI: 0.47-0.89] and time to progression (HR = 0.68, 95%CI: 0.52-0.87) were significantly longer in patients who received TACE plus sorafenib than in patients who received TACE only[147]. Another recent meta-analysis of 9 studies mostly from China reached the same conclusions[148]. These results indicate that appropriate combination therapies will improve clinical outcomes in patients with unresectable HCCs.
This stage encompasses locally advanced HCCs with vascular invasion and HCCs with extrahepatic metastasis. Whether the tumor has advanced locally or distantly, the BCLC guidelines uniformly recommend treatment with sorafenib. Although survival benefits were observed compared with no treatment, the efficacy of sorafenib is limited[78,79]. Therefore, it would be appropriate to search for more effective methods. For instance, sorafenib may be combined with other type of therapies (e.g., TACE, radioembolization, and external radiation) and TACE or HAIC may be combined with radiotherapy in patients with advanced HCC.
Sorafenib combined with TACE: A relatively large retrospective study compared the efficacy of TACE plus sorafenib and sorafenib alone in 355 advanced stage HCC patients (164 and 191 patients, respectively)[149]. Overall survival was significantly longer in the combination group than in the sorafenib monotherapy group (8.9 and 5.9 mo, respectively; P = 0.009) as was median time to progression (2.5 and 2.1 mo, respectively; P = 0.008). The difference in time to progression was still significant after propensity score matching, whereas the difference in overall survival was not. Another study compared the efficacy of TACE plus sorafenib and TACE alone in 246 advanced stage HCC patients (82 and 164 patients, respectively) after propensity score matching. Overall survival was significantly longer in the combination group than in the TACE monotherapy group (7.0 and 4.9 mo, respectively; P = 0.003) as was time to progression (2.6 and 1.9 mo, respectively; P = 0.001)[150]. These data suggest that the combination of TACE and sorafenib is most likely more efficacious than either therapy alone in advanced HCC. Other types of transarterial therapies, including DEB-TACE or radioembolization, are emerging modalities for the treatment of advanced HCC as mentioned above[112,151]. To potentially improve their efficacy, combining new modalities with sorafenib are being evaluated in clinical trials[152,153]; a phase II study which combined DEB-TACE with sorafenib showed objective response rate of 58% and disease control rate of 100% in advanced HCC patients[152]. The combination is a promising HCC treatment strategy considering the current data, but its benefits compared with monotherapy needs to be confirmed in a future phase III trial.
Sorafenib combined with radiotherapy: Sorafenib was reported to enhance the radiosensitivity of human HCC cell lines by inhibiting radiation-induced activation of VEGFRs, a downstream kinase (extracellular signal-regulated kinase), and nuclear factor-κB and by increasing radiation-induced apoptosis[154]. Therefore, combining sorafenib and radiotherapy, in the form of either radioembolization or external beam radiation, is expected to be synergistic. A multicenter phase II study evaluated safety and efficacy of combining sorafenib therapy and radioembolization in several Asian-Pacific countries[155]. Sorafenib was administered after radioembolization, and the median overall survival time was 8.6 mo in patients with advanced stage HCC[155]. Most of toxicities were associated with sorafenib therapy. Considering phase III Asian-Pacific trial data of sorafenib which showed median survival time of 6.5 mo in advanced HCC, the data of radioembolization plus sorafenib combination therapy appears favorable[79]. Data of sorafenib plus external beam radiation are emerging, recently. A phase II study of sorafenib therapy plus external beam radiation reported an initial complete or partial response rate of 55% and a 2-year overall survival rate of 32% in 40 Taiwanese patients with advanced HCC[156]. These efficacy data seem encouraging, but further investigations are warranted.
TACE combined with radiotherapy: As mentioned in the intermediate stage section, TACE plus radiotherapy is an effective synergistic strategy. Most of the previous randomized and non-randomized clinical trials of TACE plus radiotherapy included both intermediate stage and advanced stage HCC[100,144], whereas studies of advanced stage HCC only are few[157,158]. A retrospective study assessed outcome of patients with locally advanced HCC; 27 patients who were treated with TACE plus radiotherapy and another 27 patients who received sorafenib alone were compared after propensity score matching. Interestingly, overall survival was better in the former group than in the latter one (6.7 and 3.1 mo, respectively; P < 0.001)[158]. Although this was a small study, the results iterate that universal application of sorafenib for advanced stage patients may not be the best option. Further well-designed studies are warranted.
HAIC combined with radiotherapy: To facilitate the efficacy of HAIC for treatment of advanced HCC with portal vein thrombosis, radiotherapy may be combined. In a previous pilot clinical trial, infusion of 5-fluorouracil was performed at 1st and 5th wk of radiotherapy, and then continued every 4 wk. Objective response rate was 45% and median survival time was 13.1 mo[99]. More recently, HAIC combined with radiotherapy was compared with HAIC in advanced HCC patients, and the combination therapy was shown to be better than HAIC monotherapy in terms of time to progression (5.0 and 2.7 mo, respectively; P = 0.0024) and overall survival (8.6 and 5.0 mo, respectively; P = 0.0002), particularly, among the HAIC non-responders[159]. Although, these studies are retrospectively performed, HAIC combined with radiotherapy appears to have more benefits than monotherapy, suggesting synergistic effects of the therapies.
Sequential therapy with metastasectomy: In cases of extrahepatic metastasis, radiotherapy is considered if the lesions cause severe symptoms[160]. Metastatic lesions are often surgically removed if: (1) liver function is well preserved; (2) intrahepatic lesions are adequately controlled by surgery or locoregional therapy; and (3) extrahepatic metastatic lesions are confined to a single organ[161-164]. Studies from Asian countries showed 5-year survival rates ranging from 26% to 37% in HCC patients with lung metastasis who underwent metastasectomy[162-164], which are surprising survival data at the patients’ tumor stage despite the selection of surgical candidates. Although sorafenib became currently the first-line treatment for HCC with distant metastasis, uniform application of sorafenib monotherapy does not seem to be the best way because of its low objective response rate. Therefore, when possible, treating intrahepatic and metastatic lesions via metastasectomy, locoregional therapy or radiotherapy before the administration of sorafenib would be a reasonable plan[165]. However, because no data exist regarding this strategy, it needs to be evaluated further in the future.
At this stage, best supportive care (BSC) is recommended. BSC includes management of cirrhotic complications such as ascites, hepatic encephalopathy, variceal hemorrhage, and hepatorenal syndrome. Another important aspect would be management of cancer pain. Indeed, pain management has been frequently neglected at many tertiary hospitals in Asian countries. However, a systematic approach to cancer pain control is important.
Non-opioid drugs (paracetamol) and mild opioids (codeine, tramadol, and dihydrocodeine) may be useful for mild to moderate pain if administered on a regular basis[166]. Nonsteroidal ant-inflammatory drugs, which can cause renal derangement, should be avoided. Transdermal patches (fentanyl and buprenorphine) are considered if patient’s requirements of opioid are stable[166]. Breakthrough pain can be managed with rapid-acting rescue therapies administered via intravenous or subcutaneous routes. Strong opioids (morphine, oxycodone, hydromorphone, oxymorphone, and fentanyl) are used to control severe pain[166], but expose the patient to the risk of developing hepatic encephalopathy. Hence, close monitoring is essential. Emotional and nutritional support is also important for terminal stage care, so collaboration between the hospice team and the clinical nutrition team would be helpful[167]. Until the final round, a multidisciplinary approach should be maintained.
With the advancement of therapeutic modalities and aggressive treatment by either mono- or combination therapy as reviewed so far, the prognosis of HCC has improved remarkably; survival benefits are better observed in more advanced stage diseases. In this regard, reevaluation of preexisting staging systems and refinement of the best-fit models have been performed in Korea, Japan, Taiwan, and China[168-171]. Most recently, a newer staging system was pronounced from a single center in Hong Kong, reflecting recent improved survival outcomes in subsets of intermediate and advanced stage patients with more radical therapies[172]. In addition, the Hong Kong Liver Cancer staging was better than BCLC staging in stratifying HCC patients with different prognostic groups. Although further validation may be needed in non-Asian patients, the system will be helpful for identifying patients who are suitable for more aggressive treatments than what BCLC staging system recommends.
There is an increasing demand that international HCC treatment guidelines should be updated properly. Still, combinations of treatment modalities have not been incorporated into recent guidelines, and there are several unmet needs. Treatment intervals, strategies in the event of recurrence, and the timing of retreatment have not been properly studied, and no established recommendations are available. Therefore, to further improve the outcomes of HCC patients, strategies for surveillance, diagnosis, initial treatment, recurrence monitoring, and treatment after recurrence should be more organized. Close collaboration between specialists in multiple fields is of utmost importance in achieving these aims.
P- Reviewer: Decena Sollano JD, Yu CY S- Editor: Yu J L- Editor: A E- Editor: Wang CH
| 1. | Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3249] [Cited by in RCA: 3597] [Article Influence: 276.7] [Reference Citation Analysis (4)] |
| 2. | Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430. [PubMed] |
| 3. | European Association For The Study Of The Liver, European Organisation For Research Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4059] [Cited by in RCA: 4521] [Article Influence: 347.8] [Reference Citation Analysis (2)] |
| 4. | Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6573] [Article Influence: 469.5] [Reference Citation Analysis (1)] |
| 5. | Omata M, Lesmana LA, Tateishi R, Chen PJ, Lin SM, Yoshida H, Kudo M, Lee JM, Choi BI, Poon RT. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int. 2010;4:439-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 797] [Cited by in RCA: 841] [Article Influence: 56.1] [Reference Citation Analysis (0)] |
| 6. | Korean Liver Cancer Study G, National Cancer Center K. [Practice guidelines for management of hepatocellular carcinoma 2009]. Korean J Hepatol. 2009;15:391-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 221] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 7. | Kudo M, Izumi N, Kokudo N, Matsui O, Sakamoto M, Nakashima O, Kojiro M, Makuuchi M. Management of hepatocellular carcinoma in Japan: Consensus-Based Clinical Practice Guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis. 2011;29:339-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 664] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 8. | Chan SC. Liver transplantation for hepatocellular carcinoma. Liver Cancer. 2013;2:338-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | de Villa V, Lo CM. Liver transplantation for hepatocellular carcinoma in Asia. Oncologist. 2007;12:1321-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 101] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 10. | Hwang S, Lee SG, Belghiti J. Liver transplantation for HCC: its role: Eastern and Western perspectives. J Hepatobiliary Pancreat Sci. 2010;17:443-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Hwang S, Lee SG, Ahn CS, Kim KH, Moon DB, Ha TY, Song GW, Jung DH, Kim KW, Choi NK. An increase in deceased donor incidence alleviated the need for urgent adult living donor liver transplantation in a Korean high-volume center. Transplant Proc. 2010;42:1497-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Cheah YL, Simpson MA, Pomposelli JJ, Pomfret EA. Incidence of death and potentially life-threatening near-miss events in living donor hepatic lobectomy: a world-wide survey. Liver Transpl. 2013;19:499-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 212] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 13. | Hwang S, Lee SG, Lee YJ, Sung KB, Park KM, Kim KH, Ahn CS, Moon DB, Hwang GS, Kim KM. Lessons learned from 1,000 living donor liver transplantations in a single center: how to make living donations safe. Liver Transpl. 2006;12:920-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 282] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 14. | Lee SG, Hwang S, Park KM, Kim KH, Ahn CS, Lee YJ, Cheon JY, Joo SH, Moon DB, Joo CW. Seventeen adult-to-adult living donor liver transplantations using dual grafts. Transplant Proc. 2001;33:3461-3463. [PubMed] |
| 15. | Egawa H, Teramukai S, Haga H, Tanabe M, Mori A, Ikegami T, Kawagishi N, Ohdan H, Kasahara M, Umeshita K. Impact of rituximab desensitization on blood-type-incompatible adult living donor liver transplantation: a Japanese multicenter study. Am J Transplant. 2014;14:102-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 141] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 16. | Todo S, Furukawa H. Living donor liver transplantation for adult patients with hepatocellular carcinoma: experience in Japan. Ann Surg. 2004;240:451-459; discussion 459-461. [PubMed] |
| 17. | Hwang S, Lee SG, Joh JW, Suh KS, Kim DG. Liver transplantation for adult patients with hepatocellular carcinoma in Korea: comparison between cadaveric donor and living donor liver transplantations. Liver Transpl. 2005;11:1265-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 166] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 18. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5110] [Cited by in RCA: 5313] [Article Influence: 183.2] [Reference Citation Analysis (0)] |
| 19. | Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1594] [Cited by in RCA: 1695] [Article Influence: 70.6] [Reference Citation Analysis (0)] |
| 20. | Ng KK, Lo CM, Chan SC, Chok KS, Cheung TT, Fan ST. Liver transplantation for hepatocellular carcinoma: the Hong Kong experience. J Hepatobiliary Pancreat Sci. 2010;17:548-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Zheng SS, Xu X, Wu J, Chen J, Wang WL, Zhang M, Liang TB, Wu LM. Liver transplantation for hepatocellular carcinoma: Hangzhou experiences. Transplantation. 2008;85:1726-1732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 379] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 22. | Lee SG, Hwang S, Moon DB, Ahn CS, Kim KH, Sung KB, Ko GY, Park KM, Ha TY, Song GW. Expanded indication criteria of living donor liver transplantation for hepatocellular carcinoma at one large-volume center. Liver Transpl. 2008;14:935-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 260] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 23. | Choi HJ, Kim DG, Na GH, Hong TH, You YK. Extended criteria for living donor liver transplantation in patients with advanced hepatocellular carcinoma. Transplant Proc. 2012;44:399-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Sugawara Y, Tamura S, Makuuchi M. Living donor liver transplantation for hepatocellular carcinoma: Tokyo University series. Dig Dis. 2007;25:310-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 201] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 25. | Ito T, Takada Y, Ueda M, Haga H, Maetani Y, Oike F, Ogawa K, Sakamoto S, Ogura Y, Egawa H. Expansion of selection criteria for patients with hepatocellular carcinoma in living donor liver transplantation. Liver Transpl. 2007;13:1637-1644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 201] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 26. | Makuuchi M, Kosuge T, Takayama T, Yamazaki S, Kakazu T, Miyagawa S, Kawasaki S. Surgery for small liver cancers. Semin Surg Oncol. 1993;9:298-304. [PubMed] |
| 27. | Torzilli G, Minagawa M, Takayama T, Inoue K, Hui AM, Kubota K, Ohtomo K, Makuuchi M. Accurate preoperative evaluation of liver mass lesions without fine-needle biopsy. Hepatology. 1999;30:889-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 218] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 28. | Ikai I, Arii S, Kojiro M, Ichida T, Makuuchi M, Matsuyama Y, Nakanuma Y, Okita K, Omata M, Takayasu K. Reevaluation of prognostic factors for survival after liver resection in patients with hepatocellular carcinoma in a Japanese nationwide survey. Cancer. 2004;101:796-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 353] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 29. | Poon RT, Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, Yeung C, Wong J. Extended hepatic resection for hepatocellular carcinoma in patients with cirrhosis: is it justified? Ann Surg. 2002;236:602-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 30. | Ishizawa T, Hasegawa K, Aoki T, Takahashi M, Inoue Y, Sano K, Imamura H, Sugawara Y, Kokudo N, Makuuchi M. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology. 2008;134:1908-1916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 583] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 31. | Yang T, Lin C, Zhai J, Shi S, Zhu M, Zhu N, Lu JH, Yang GS, Wu MC. Surgical resection for advanced hepatocellular carcinoma according to Barcelona Clinic Liver Cancer (BCLC) staging. J Cancer Res Clin Oncol. 2012;138:1121-1129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 32. | Li N, Wu YR, Wu B, Lu MQ. Surgical and oncologic outcomes following laparoscopic versus open liver resection for hepatocellular carcinoma: A meta-analysis. Hepatol Res. 2012;42:51-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 33. | Ai JH, Li JW, Chen J, Bie P, Wang SG, Zheng SG. Feasibility and safety of laparoscopic liver resection for hepatocellular carcinoma with a tumor size of 5-10 cm. PLoS One. 2013;8:e72328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 34. | Yoon YS, Han HS, Cho JY, Ahn KS. Totally laparoscopic central bisectionectomy for hepatocellular carcinoma. J Laparoendosc Adv Surg Tech A. 2009;19:653-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Han HS, Yoon YS, Cho JY, Ahn KS. Laparoscopic right hemihepatectomy for hepatocellular carcinoma. Ann Surg Oncol. 2010;17:2090-2091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 36. | Yim HJ, Yeon JE, Byun KS, Lee CH, Choi SY, Kim SK. Laparoscopic resection of HCC implanted in the peritoneal cavity: a case detected by PET after hepatic resection. Hepatogastroenterology. 2008;55:1549-1552. [PubMed] |
| 37. | Kim YS, Lim HK, Rhim H, Lee MW, Choi D, Lee WJ, Paik SW, Koh KC, Lee JH, Choi MS. Ten-year outcomes of percutaneous radiofrequency ablation as first-line therapy of early hepatocellular carcinoma: analysis of prognostic factors. J Hepatol. 2013;58:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 300] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 38. | Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Randomised controlled trial comparing percutaneous radiofrequency thermal ablation, percutaneous ethanol injection, and percutaneous acetic acid injection to treat hepatocellular carcinoma of 3 cm or less. Gut. 2005;54:1151-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 431] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 39. | Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Radiofrequency ablation improves prognosis compared with ethanol injection for hepatocellular carcinoma & lt; or =4 cm. Gastroenterology. 2004;127:1714-1723. [PubMed] |
| 40. | Okada S. Local ablation therapy for hepatocellular carcinoma. Semin Liver Dis. 1999;19:323-328. [PubMed] |
| 41. | Ishii H, Okada S, Nose H, Okusaka T, Yoshimori M, Takayama T, Kosuge T, Yamasaki S, Sakamoto M, Hirohashi S. Local recurrence of hepatocellular carcinoma after percutaneous ethanol injection. Cancer. 1996;77:1792-1796. [PubMed] |
| 42. | Livraghi T, Bolondi L, Lazzaroni S, Marin G, Morabito A, Rapaccini GL, Salmi A, Torzilli G. Percutaneous ethanol injection in the treatment of hepatocellular carcinoma in cirrhosis. A study on 207 patients. Cancer. 1992;69:925-929. [PubMed] |
| 43. | Uehara T, Hirooka M, Ishida K, Hiraoka A, Kumagi T, Kisaka Y, Hiasa Y, Onji M. Percutaneous ultrasound-guided radiofrequency ablation of hepatocellular carcinoma with artificially induced pleural effusion and ascites. J Gastroenterol. 2007;42:306-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 82] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 44. | Song I, Rhim H, Lim HK, Kim YS, Choi D. Percutaneous radiofrequency ablation of hepatocellular carcinoma abutting the diaphragm and gastrointestinal tracts with the use of artificial ascites: safety and technical efficacy in 143 patients. Eur Radiol. 2009;19:2630-2640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 142] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 45. | Feng K, Yan J, Li X, Xia F, Ma K, Wang S, Bie P, Dong J. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol. 2012;57:794-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 598] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 46. | Wang Y, Luo Q, Li Y, Deng S, Wei S, Li X. Radiofrequency ablation versus hepatic resection for small hepatocellular carcinomas: a meta-analysis of randomized and nonrandomized controlled trials. PLoS One. 2014;9:e84484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 47. | Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 1987] [Article Influence: 86.4] [Reference Citation Analysis (0)] |
| 48. | Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2502] [Cited by in RCA: 2611] [Article Influence: 113.5] [Reference Citation Analysis (0)] |
| 49. | Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2207] [Cited by in RCA: 2271] [Article Influence: 103.2] [Reference Citation Analysis (0)] |
| 50. | Kim JW, Kim JH, Sung KB, Ko HK, Shin JH, Kim PN, Choi HK, Ko GY, Yoon HK, Chun SY. Transarterial chemoembolization vs. radiofrequency ablation for the treatment of single hepatocellular carcinoma 2 cm or smaller. Am J Gastroenterol. 2014;109:1234-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 51. | Chung JW, Park JH, Han JK, Choi BI, Han MC. Hepatocellular carcinoma and portal vein invasion: results of treatment with transcatheter oily chemoembolization. AJR Am J Roentgenol. 1995;165:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 109] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 52. | Lee HS, Kim JS, Choi IJ, Chung JW, Park JH, Kim CY. The safety and efficacy of transcatheter arterial chemoembolization in the treatment of patients with hepatocellular carcinoma and main portal vein obstruction. A prospective controlled study. Cancer. 1997;79:2087-2094. [PubMed] |
| 53. | Kim KM, Kim JH, Park IS, Ko GY, Yoon HK, Sung KB, Lim YS, Lee HC, Chung YH, Lee YS. Reappraisal of repeated transarterial chemoembolization in the treatment of hepatocellular carcinoma with portal vein invasion. J Gastroenterol Hepatol. 2009;24:806-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 118] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 54. | Kim JH, Yoon HK, Kim SY, Kim KM, Ko GY, Gwon DI, Sung KB. Transcatheter arterial chemoembolization vs. chemoinfusion for unresectable hepatocellular carcinoma in patients with major portal vein thrombosis. Aliment Pharmacol Ther. 2009;29:1291-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 55. | Pinter M, Hucke F, Graziadei I, Vogel W, Maieron A, Königsberg R, Stauber R, Grünberger B, Müller C, Kölblinger C. Advanced-stage hepatocellular carcinoma: transarterial chemoembolization versus sorafenib. Radiology. 2012;263:590-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 165] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 56. | Varela M, Real MI, Burrel M, Forner A, Sala M, Brunet M, Ayuso C, Castells L, Montañá X, Llovet JM. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007;46:474-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 719] [Article Influence: 39.9] [Reference Citation Analysis (1)] |
| 57. | Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, Pitton M, Sergent G, Pfammatter T, Terraz S. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33:41-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1063] [Cited by in RCA: 1208] [Article Influence: 75.5] [Reference Citation Analysis (0)] |
| 58. | Martin R, Geller D, Espat J, Kooby D, Sellars M, Goldstein R, Imagawa D, Scoggins C. Safety and efficacy of trans arterial chemoembolization with drug-eluting beads in hepatocellular cancer: a systematic review. Hepatogastroenterology. 2012;59:255-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 59. | Yeo W, Mok TS, Zee B, Leung TW, Lai PB, Lau WY, Koh J, Mo FK, Yu SC, Chan AT. A randomized phase III study of doxorubicin versus cisplatin/interferon alpha-2b/doxorubicin/fluorouracil (PIAF) combination chemotherapy for unresectable hepatocellular carcinoma. J Natl Cancer Inst. 2005;97:1532-1538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 467] [Cited by in RCA: 455] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 60. | Qin S, Bai Y, Ye S, Fan J, Lim H, Cho J, Thongprasert S, Chao Y, Rau K, Sun Y. Phase III study of oxaliplatin plus 5-fluorouracil/leucovorin (FOLFOX4) versus doxorubicin as palliative systemic chemotherapy in advanced HCC in Asian patients. J Clin Oncol. 2010;28:4008. |
| 61. | Lai CL, Wu PC, Chan GC, Lok AS, Lin HJ. Doxorubicin versus no antitumor therapy in inoperable hepatocellular carcinoma. A prospective randomized trial. Cancer. 1988;62:479-483. [PubMed] |
| 62. | Yang TS, Lin YC, Chen JS, Wang HM, Wang CH. Phase II study of gemcitabine in patients with advanced hepatocellular carcinoma. Cancer. 2000;89:750-756. [PubMed] |
| 63. | Guan Z, Wang Y, Maoleekoonpairoj S, Chen Z, Kim WS, Ratanatharathorn V, Reece WH, Kim TW, Lehnert M. Prospective randomised phase II study of gemcitabine at standard or fixed dose rate schedule in unresectable hepatocellular carcinoma. Br J Cancer. 2003;89:1865-1869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 64. | Yen Y, Lim DW, Chung V, Morgan RJ, Leong LA, Shibata SI, Wagman LD, Marx H, Chu PG, Longmate JA. Phase II study of oxaliplatin in patients with unresectable, metastatic, or recurrent hepatocellular cancer: a California Cancer Consortium Trial. Am J Clin Oncol. 2008;31:317-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 65. | Patt YZ, Hassan MM, Aguayo A, Nooka AK, Lozano RD, Curley SA, Vauthey JN, Ellis LM, Schnirer II, Wolff RA. Oral capecitabine for the treatment of hepatocellular carcinoma, cholangiocarcinoma, and gallbladder carcinoma. Cancer. 2004;101:578-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 139] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 66. | Lee JE, Bae SH, Choi JY, Yoon SK, You YK, Lee MA. Epirubicin, cisplatin, 5-FU combination chemotherapy in sorafenib-refractory metastatic hepatocellular carcinoma. World J Gastroenterol. 2014;20:235-241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 67. | Inaba Y, Arai Y, Yamaura H, Sato Y, Najima M, Aramaki T, Sone M, Kumada T, Tanigawa N, Anai H. Phase I/II study of hepatic arterial infusion chemotherapy with gemcitabine in patients with unresectable intrahepatic cholangiocarcinoma (JIVROSG-0301). Am J Clin Oncol. 2011;34:58-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 68. | Jeong SW, Jang JY, Lee JE, Lee SH, Kim SG, Cha SW, Kim YS, Cho YD, Kim HS, Kim BS. The efficacy of hepatic arterial infusion chemotherapy as an alternative to sorafenib in advanced hepatocellular carcinoma. Asia Pac J Clin Oncol. 2012;8:164-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 69. | Kirikoshi H, Yoneda M, Mawatari H, Fujita K, Imajo K, Kato S, Suzuki K, Kobayashi N, Kubota K, Maeda S. Is hepatic arterial infusion chemotherapy effective treatment for advanced hepatocellular carcinoma resistant to transarterial chemoembolization? World J Gastroenterol. 2012;18:1933-1939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 70. | Ueda H, Fukuchi H, Tanaka C. Toxicity and efficacy of hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma (Review). Oncol Lett. 2012;3:259-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 71. | Ueshima K, Kudo M, Takita M, Nagai T, Tatsumi C, Ueda T, Kitai S, Ishikawa E, Yada N, Inoue T. Hepatic arterial infusion chemotherapy using low-dose 5-fluorouracil and cisplatin for advanced hepatocellular carcinoma. Oncology. 2010;78 Suppl 1:148-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 72. | Yamashita T. Current status of hepatocellular carcinoma treatment in Japan: hepatic arterial infusion chemotherapy. Clin Drug Investig. 2012;32 Suppl 2:15-23. [PubMed] |
| 73. | Cheong JY, Lee KM, Cho SW, Won JH, Kim JK, Wang HJ, Hahm KB, Kim JH. Survival benefits of intra-arterial infusion chemotherapy in patients with advanced hepatocellular carcinoma with portal vein tumor thrombosis. Hepatol Res. 2005;32:127-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 74. | Woo HY, Bae SH, Park JY, Han KH, Chun HJ, Choi BG, Im HU, Choi JY, Yoon SK, Cheong JY. A randomized comparative study of high-dose and low-dose hepatic arterial infusion chemotherapy for intractable, advanced hepatocellular carcinoma. Cancer Chemother Pharmacol. 2010;65:373-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 75. | Song DS, Song MJ, Bae SH, Chung WJ, Jang JY, Kim YS, Lee SH, Park JY, Yim HJ, Cho SB. A comparative study between sorafenib and hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis. J Gastroenterol. 2014;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 115] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 76. | Hiramine Y, Uto H, Imamura Y, Tabu K, Baba Y, Hiwaki T, Sho Y, Tahara K, Higashi H, Tamai T. Sorafenib and hepatic arterial infusion chemotherapy for unresectable advanced hepatocellular carcinoma: A comparative study. Exp Ther Med. 2011;2:433-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 77. | Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, Lynch M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther. 2008;7:3129-3140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1149] [Cited by in RCA: 1109] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 78. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10271] [Article Influence: 604.2] [Reference Citation Analysis (2)] |
| 79. | Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3854] [Cited by in RCA: 4653] [Article Influence: 273.7] [Reference Citation Analysis (0)] |
| 80. | Vincenzi B, Santini D, Russo A, Addeo R, Giuliani F, Montella L, Rizzo S, Venditti O, Frezza AM, Caraglia M. Early skin toxicity as a predictive factor for tumor control in hepatocellular carcinoma patients treated with sorafenib. Oncologist. 2010;15:85-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 143] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 81. | Cho JY, Paik YH, Lim HY, Kim YG, Lim HK, Min YW, Gwak GY, Choi MS, Lee JH, Koh KC. Clinical parameters predictive of outcomes in sorafenib-treated patients with advanced hepatocellular carcinoma. Liver Int. 2013;33:950-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 82. | Kim HY, Park JW, Nam BH, Kim HK, Choi JI, Kim TH, Kim HB, Kim CM. Survival of patients with advanced hepatocellular carcinoma: sorafenib versus other treatments. J Gastroenterol Hepatol. 2011;26:1612-1618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 83. | Yoon EL, Yeon JE, Lee HJ, Suh SJ, Lee SJ, Kang SH, Kang K, Yoo YJ, Kim JH, Yim HJ. Systemic cytotoxic chemotherapy of patients with advanced hepatocellular carcinoma in the era of sorafenib nonavailability. J Clin Gastroenterol. 2014;48:e22-e29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 84. | Kim HY, Park JW. Clinical trials of combined molecular targeted therapy and locoregional therapy in hepatocellular carcinoma: past, present, and future. Liver Cancer. 2014;3:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 85. | Zhu AX, Sahani DV, Duda DG, di Tomaso E, Ancukiewicz M, Catalano OA, Sindhwani V, Blaszkowsky LS, Yoon SS, Lahdenranta J. Efficacy, safety, and potential biomarkers of sunitinib monotherapy in advanced hepatocellular carcinoma: a phase II study. J Clin Oncol. 2009;27:3027-3035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 367] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 86. | Cheng AL, Kang YK, Lin DY, Park JW, Kudo M, Qin S, Chung HC, Song X, Xu J, Poggi G. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J Clin Oncol. 2013;31:4067-4075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 594] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 87. | Park JW, Finn RS, Kim JS, Karwal M, Li RK, Ismail F, Thomas M, Harris R, Baudelet C, Walters I. Phase II, open-label study of brivanib as first-line therapy in patients with advanced hepatocellular carcinoma. Clin Cancer Res. 2011;17:1973-1983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 121] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 88. | Johnson PJ, Qin S, Park JW, Poon RT, Raoul JL, Philip PA, Hsu CH, Hu TH, Heo J, Xu J. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK-FL study. J Clin Oncol. 2013;31:3517-3524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 597] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 89. | Llovet JM, Decaens T, Raoul JL, Boucher E, Kudo M, Chang C, Kang YK, Assenat E, Lim HY, Boige V. Brivanib in patients with advanced hepatocellular carcinoma who were intolerant to sorafenib or for whom sorafenib failed: results from the randomized phase III BRISK-PS study. J Clin Oncol. 2013;31:3509-3516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 434] [Cited by in RCA: 486] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 90. | Chan SL, Yeo W. Development of systemic therapy for hepatocellular carcinoma at 2013: updates and insights. World J Gastroenterol. 2014;20:3135-3145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 91. | Llovet JM, Hernandez-Gea V. Hepatocellular carcinoma: reasons for phase III failure and novel perspectives on trial design. Clin Cancer Res. 2014;20:2072-2079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 323] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 92. | Suh SJ, Yim HJ. [Current status of molecular targeted therapies in hepatocellular carcinoma]. Korean J Gastroenterol. 2013;61:136-146. [PubMed] |
| 93. | Seong J. Challenge and hope in radiotherapy of hepatocellular carcinoma. Yonsei Med J. 2009;50:601-612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 94. | Choi HJ, Cho BC, Sohn JH, Shin SJ, Kim SH, Kim JH, Yoo NC. Brain metastases from hepatocellular carcinoma: prognostic factors and outcome: brain metastasis from HCC. J Neurooncol. 2009;91:307-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 92] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 95. | Nakamura N, Igaki H, Yamashita H, Shiraishi K, Tago M, Sasano N, Shiina S, Omata M, Makuuchi M, Ohtomo K. A retrospective study of radiotherapy for spinal bone metastases from hepatocellular carcinoma (HCC). Jpn J Clin Oncol. 2007;37:38-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 96. | Seong J, Koom WS, Park HC. Radiotherapy for painful bone metastases from hepatocellular carcinoma. Liver Int. 2005;25:261-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 85] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 97. | Kang JK, Kim MS, Cho CK, Yang KM, Yoo HJ, Kim JH, Bae SH, Jung da H, Kim KB, Lee DH. Stereotactic body radiation therapy for inoperable hepatocellular carcinoma as a local salvage treatment after incomplete transarterial chemoembolization. Cancer. 2012;118:5424-5431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 245] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 98. | Kwon JH, Bae SH, Kim JY, Choi BO, Jang HS, Jang JW, Choi JY, Yoon SK, Chung KW. Long-term effect of stereotactic body radiation therapy for primary hepatocellular carcinoma ineligible for local ablation therapy or surgical resection. Stereotactic radiotherapy for liver cancer. BMC Cancer. 2010;10:475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 195] [Cited by in RCA: 197] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 99. | Han KH, Seong J, Kim JK, Ahn SH, Lee do Y, Chon CY. Pilot clinical trial of localized concurrent chemoradiation therapy for locally advanced hepatocellular carcinoma with portal vein thrombosis. Cancer. 2008;113:995-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 123] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 100. | Seo YS, Kim JN, Keum B, Park S, Kwon YD, Kim YS, Jeen YT, Chun HJ, Kim CY, Kim CD. Radiotherapy for 65 patients with advanced unresectable hepatocellular carcinoma. World J Gastroenterol. 2008;14:2394-2400. [PubMed] |
| 101. | Yu JI, Yoon SM, Park HC, Kim JH, Kim TH, Park JW, Seong J, Lee IJ, Jang HS, Kay CS. Multicenter validation study of a prognostic index for portal vein tumor thrombosis in hepatocellular carcinoma. Cancer Res Treat. 2014;46:348-357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 102. | Nakazawa T, Hidaka H, Shibuya A, Okuwaki Y, Tanaka Y, Takada J, Minamino T, Watanabe M, Kokubu S, Koizumi W. Overall survival in response to sorafenib versus radiotherapy in unresectable hepatocellular carcinoma with major portal vein tumor thrombosis: propensity score analysis. BMC Gastroenterol. 2014;14:84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 98] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 103. | Tang QH, Li AJ, Yang GM, Lai EC, Zhou WP, Jiang ZH, Lau WY, Wu MC. Surgical resection versus conformal radiotherapy combined with TACE for resectable hepatocellular carcinoma with portal vein tumor thrombus: a comparative study. World J Surg. 2013;37:1362-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 104. | Bush DA, Kayali Z, Grove R, Slater JD. The safety and efficacy of high-dose proton beam radiotherapy for hepatocellular carcinoma: a phase 2 prospective trial. Cancer. 2011;117:3053-3059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 138] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 105. | Kawashima M, Furuse J, Nishio T, Konishi M, Ishii H, Kinoshita T, Nagase M, Nihei K, Ogino T. Phase II study of radiotherapy employing proton beam for hepatocellular carcinoma. J Clin Oncol. 2005;23:1839-1846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 156] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 106. | Chiba T, Tokuuye K, Matsuzaki Y, Sugahara S, Chuganji Y, Kagei K, Shoda J, Hata M, Abei M, Igaki H. Proton beam therapy for hepatocellular carcinoma: a retrospective review of 162 patients. Clin Cancer Res. 2005;11:3799-3805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 171] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 107. | Lee SU, Park JW, Kim TH, Kim YJ, Woo SM, Koh YH, Lee WJ, Park SJ, Kim DY, Kim CM. Effectiveness and safety of proton beam therapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis. Strahlenther Onkol. 2014;190:806-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 108. | Sugahara S, Oshiro Y, Nakayama H, Fukuda K, Mizumoto M, Abei M, Shoda J, Matsuzaki Y, Thono E, Tokita M. Proton beam therapy for large hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2010;76:460-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 109. | Lau WY, Lai EC, Leung TW. Current role of selective internal irradiation with yttrium-90 microspheres in the management of hepatocellular carcinoma: a systematic review. Int J Radiat Oncol Biol Phys. 2011;81:460-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 110. | Ahmadzadehfar H, Sabet A, Wilhelm K, Biersack HJ, Risse J. Iodine-131-lipiodol therapy in hepatic tumours. Methods. 2011;55:246-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 111. | Moreno-Luna LE, Yang JD, Sanchez W, Paz-Fumagalli R, Harnois DM, Mettler TA, Gansen DN, de Groen PC, Lazaridis KN, Narayanan Menon KV. Efficacy and safety of transarterial radioembolization versus chemoembolization in patients with hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2013;36:714-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 112. | Memon K, Kulik L, Lewandowski RJ, Mulcahy MF, Benson AB, Ganger D, Riaz A, Gupta R, Vouche M, Gates VL. Radioembolization for hepatocellular carcinoma with portal vein thrombosis: impact of liver function on systemic treatment options at disease progression. J Hepatol. 2013;58:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 113. | Kim DY, Park BJ, Kim YH, Han KH, Cho SB, Cho KR, Uhm SH, Choe JG, Choi JY, Chun HJ. Radioembolization With Yttrium-90 Resin Microspheres in Hepatocellular Carcinoma: A Multicenter Prospective Study. Am J Clin Oncol. 2013;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 114. | Salem R, Lewandowski RJ, Mulcahy MF, Riaz A, Ryu RK, Ibrahim S, Atassi B, Baker T, Gates V, Miller FH. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010;138:52-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 738] [Cited by in RCA: 781] [Article Influence: 52.1] [Reference Citation Analysis (0)] |
| 115. | Sangro B, Carpanese L, Cianni R, Golfieri R, Gasparini D, Ezziddin S, Paprottka PM, Fiore F, Van Buskirk M, Bilbao JI. Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: a European evaluation. Hepatology. 2011;54:868-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 505] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 116. | Heo J, Reid T, Ruo L, Breitbach CJ, Rose S, Bloomston M, Cho M, Lim HY, Chung HC, Kim CW. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat Med. 2013;19:329-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 490] [Cited by in RCA: 614] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 117. | Wang Y, Du H, Zhai G. Recent advances in active hepatic targeting drug delivery system. Curr Drug Targets. 2014;15:573-599. [PubMed] |
| 118. | Sharma P, Pandita A, Murthy RS. Concepts and Strategies for the Site Specific Delivery of Nanocarrier Based Delivery Systems for Treating Hepatocellular Carcinoma. Curr Drug Deliv. 2013;Epub ahead of print. [PubMed] |
| 119. | Zhou X, Zhang M, Yung B, Li H, Zhou C, Lee LJ, Lee RJ. Lactosylated liposomes for targeted delivery of doxorubicin to hepatocellular carcinoma. Int J Nanomedicine. 2012;7:5465-5474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 120. | Cheng MR, Li Q, Wan T, He B, Han J, Chen HX, Yang FX, Wang W, Xu HZ, Ye T. Galactosylated chitosan/5-fluorouracil nanoparticles inhibit mouse hepatic cancer growth and its side effects. World J Gastroenterol. 2012;18:6076-6087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 40] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 121. | Ashoori N, Bamberg F, Paprottka P, Rentsch M, Kolligs FT, Siegert S, Peporte A, Al-Tubaikh JA, D’Anastasi M, Hoffmann RT. Multimodality treatment for early-stage hepatocellular carcinoma: a bridging therapy for liver transplantation. Digestion. 2012;86:338-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 122. | Huo TI, Huang YH, Su CW, Lin HC, Chiang JH, Chiou YY, Huo SC, Lee PC, Lee SD. Validation of the HCC-MELD for dropout probability in patients with small hepatocellular carcinoma undergoing locoregional therapy. Clin Transplant. 2008;22:469-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 123. | Hwang S, Lee SG, Moon DB, Ahn CS, Kim KH, Lee YJ, Ha TY, Song GW. Salvage living donor liver transplantation after prior liver resection for hepatocellular carcinoma. Liver Transpl. 2007;13:741-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 107] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 124. | Zhou Y, Zhang X, Wu L, Ye F, Su X, Shi L, Li B. Meta-analysis: preoperative transcatheter arterial chemoembolization does not improve prognosis of patients with resectable hepatocellular carcinoma. BMC Gastroenterol. 2013;13:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 125. | Ono T, Yamanoi A, Nazmy El Assal O, Kohno H, Nagasue N. Adjuvant chemotherapy after resection of hepatocellular carcinoma causes deterioration of long-term prognosis in cirrhotic patients: metaanalysis of three randomized controlled trials. Cancer. 2001;91:2378-2385. [PubMed] |
| 126. | Kim do Y, Ahn SH, Kim SU, Choi SB, Lee KH, Park MS, Park JY, Lee do Y, Han KH, Kim KS. Adjuvant hepatic arterial infusional chemotherapy with 5-fluorouracil and cisplatin after curative resection of hepatocellular carcinoma. Oncology. 2011;81:184-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 127. | Bruix J, Takayama T, Mazzaferro V, Chau G-Y, Yang J, Kudo M, Cai J, Poon RT-P, Han K-H, Tak W-Y. STORM: A phase III randomized, double-blind, placebo-controlled trial of adjuvant sorafenib after resection or ablation to prevent recurrence of hepatocellular carcinoma (HCC). J Clin Oncol. 2014;32 Suppl:abstract 4006. |
| 128. | Muto Y, Moriwaki H, Ninomiya M, Adachi S, Saito A, Takasaki KT, Tanaka T, Tsurumi K, Okuno M, Tomita E. Prevention of second primary tumors by an acyclic retinoid, polyprenoic acid, in patients with hepatocellular carcinoma. Hepatoma Prevention Study Group. N Engl J Med. 1996;334:1561-1567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 509] [Cited by in RCA: 477] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 129. | Kakizaki S, Sohara N, Sato K, Suzuki H, Yanagisawa M, Nakajima H, Takagi H, Naganuma A, Otsuka T, Takahashi H. Preventive effects of vitamin K on recurrent disease in patients with hepatocellular carcinoma arising from hepatitis C viral infection. J Gastroenterol Hepatol. 2007;22:518-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 130. | Jiang S, Liu Y, Wang L, Duan C, Liu M. A meta-analysis and systematic review: adjuvant interferon therapy for patients with viral hepatitis-related hepatocellular carcinoma. World J Surg Oncol. 2013;11:240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 131. | Hsu YC, Ho HJ, Wu MS, Lin JT, Wu CY. Postoperative peg-interferon plus ribavirin is associated with reduced recurrence of hepatitis C virus-related hepatocellular carcinoma. Hepatology. 2013;58:150-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 132. | Wu CY, Chen YJ, Ho HJ, Hsu YC, Kuo KN, Wu MS, Lin JT. Association between nucleoside analogues and risk of hepatitis B virus-related hepatocellular carcinoma recurrence following liver resection. JAMA. 2012;308:1906-1914. [PubMed] |
| 133. | Huang G, Lau WY, Wang ZG, Pan ZY, Yuan SX, Shen F, Zhou WP, Wu MC. Antiviral therapy improves postoperative survival in patients with hepatocellular carcinoma: a randomized controlled trial. Ann Surg. 2015;261:56-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 192] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 134. | Kim JH, Yim HJ, Lee KG, Kim SY, Jung ES, Jung YK, Seo YS, Yeon JE, Lee HS, Um SH. Recurrence rates and factors for recurrence after radiofrequency ablation combined with transarterial chemoembolization for hepatocellular carcinoma: a retrospective cohort study. Hepatol Int. 2011;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 135. | Kim JH, Won HJ, Shin YM, Kim SH, Yoon HK, Sung KB, Kim PN. Medium-sized (3.1-5.0 cm) hepatocellular carcinoma: transarterial chemoembolization plus radiofrequency ablation versus radiofrequency ablation alone. Ann Surg Oncol. 2011;18:1624-1629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 136. | Peng ZW, Zhang YJ, Chen MS, Xu L, Liang HH, Lin XJ, Guo RP, Zhang YQ, Lau WY. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J Clin Oncol. 2013;31:426-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 394] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 137. | Morimoto M, Numata K, Kondou M, Nozaki A, Morita S, Tanaka K. Midterm outcomes in patients with intermediate-sized hepatocellular carcinoma: a randomized controlled trial for determining the efficacy of radiofrequency ablation combined with transcatheter arterial chemoembolization. Cancer. 2010;116:5452-5460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 249] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 138. | Liu Z, Gao F, Yang G, Singh S, Lu M, Zhang T, Zhong Z, Zhang F, Tang R. Combination of radiofrequency ablation with transarterial chemoembolization for hepatocellular carcinoma: an up-to-date meta-analysis. Tumour Biol. 2014;35:7407-7413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 139. | Kim JW, Shin SS, Kim JK, Choi SK, Heo SH, Lim HS, Hur YH, Cho CK, Jeong YY, Kang HK. Radiofrequency ablation combined with transcatheter arterial chemoembolization for the treatment of single hepatocellular carcinoma of 2 to 5 cm in diameter: comparison with surgical resection. Korean J Radiol. 2013;14:626-635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 140. | Kawamura R, Seki T, Umehara H, Ikeda K, Inokuchi R, Asayama T, Yamaguchi T, Takahashi Y, Sakao M, Lencioni R. Combined treatment of large hepatocellular carcinoma with transcatheter arterial chemoembolization and percutaneous ethanol injection with a multipronged needle: experimental and clinical investigation. Cardiovasc Intervent Radiol. 2012;35:325-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 141. | Mizuki A, Tatemichi M, Tsukada N, Nagamatsu R, Kawaguchi M, Itoshima T, Maruyama S, Satou A, Imari Y, Kawatoko T. Addition of transcatheter arterial chemoembolization decreased local recurrence but had no survival benefit to percutaneous ethanol injection therapy for patients with small hepatocellular carcinoma: A multicenter randomized control study. Oncol Lett. 2010;1:855-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 142. | Ravaioli M, Grazi GL, Piscaglia F, Trevisani F, Cescon M, Ercolani G, Vivarelli M, Golfieri R, D’Errico Grigioni A, Panzini I. Liver transplantation for hepatocellular carcinoma: results of down-staging in patients initially outside the Milan selection criteria. Am J Transplant. 2008;8:2547-2557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 299] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 143. | Ahn CS, Moon DB, Lee SG, Hwang S, Kim KH, Ha TY, Song GW, Jung DH, Park GC, Park YH. Survival differences between Milan criteria after down-staging and De novo Milan in living donor liver transplantation for hepatocellular carcinoma. Hepatogastroenterology. 2014;61:187-191. [PubMed] |
| 144. | Meng MB, Cui YL, Lu Y, She B, Chen Y, Guan YS, Zhang RM. Transcatheter arterial chemoembolization in combination with radiotherapy for unresectable hepatocellular carcinoma: a systematic review and meta-analysis. Radiother Oncol. 2009;92:184-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 145. | Schoenleber SJ, Kurtz DM, Talwalkar JA, Roberts LR, Gores GJ. Prognostic role of vascular endothelial growth factor in hepatocellular carcinoma: systematic review and meta-analysis. Br J Cancer. 2009;100:1385-1392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 165] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 146. | Kudo M, Imanaka K, Chida N, Nakachi K, Tak WY, Takayama T, Yoon JH, Hori T, Kumada H, Hayashi N. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer. 2011;47:2117-2127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 459] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 147. | Zhang L, Hu P, Chen X, Bie P. Transarterial chemoembolization (TACE) plus sorafenib versus TACE for intermediate or advanced stage hepatocellular carcinoma: a meta-analysis. PLoS One. 2014;9:e100305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 148. | Fu QH, Zhang Q, Bai XL, Hu QD, Su W, Chen YW, Su RG, Liang TB. Sorafenib enhances effects of transarterial chemoembolization for hepatocellular carcinoma: a systematic review and meta-analysis. J Cancer Res Clin Oncol. 2014;140:1429-1440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 149. | Choi GH, Shim JH, Kim MJ, Ryu MH, Ryoo BY, Kang YK, Shin YM, Kim KM, Lim YS, Lee HC. Sorafenib alone versus sorafenib combined with transarterial chemoembolization for advanced-stage hepatocellular carcinoma: results of propensity score analyses. Radiology. 2013;269:603-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 116] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 150. | Hu H, Duan Z, Long X, Hertzanu Y, Shi H, Liu S, Yang Z. Sorafenib combined with transarterial chemoembolization versus transarterial chemoembolization alone for advanced-stage hepatocellular carcinoma: a propensity score matching study. PLoS One. 2014;9:e96620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 151. | Kalva SP, Pectasides M, Liu R, Rachamreddy N, Surakanti S, Yeddula K, Ganguli S, Wicky S, Blaszkowsky LS, Zhu AX. Safety and effectiveness of chemoembolization with drug-eluting beads for advanced-stage hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2014;37:381-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 152. | Pawlik TM, Reyes DK, Cosgrove D, Kamel IR, Bhagat N, Geschwind JF. Phase II trial of sorafenib combined with concurrent transarterial chemoembolization with drug-eluting beads for hepatocellular carcinoma. J Clin Oncol. 2011;29:3960-3967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 246] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 153. | Ricke J, Bulla K, Kolligs F, Peck-Radosavljevic M, Reimer P, Sangro B, Schott E, Schütte K, Verslype C, Walecki J, Malfertheiner P; the Soramic study group. Safety and toxicity of radioembolization plus Sorafenib in advanced hepatocellular carcinoma: analysis of the European multicentre trial SORAMIC. Liver Int. 2015;35:620-626. [PubMed] |
| 154. | Yu W, Gu K, Yu Z, Yuan D, He M, Ma N, Lai S, Zhao J, Ren Z, Zhang X. Sorafenib potentiates irradiation effect in hepatocellular carcinoma in vitro and in vivo. Cancer Lett. 2013;329:109-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 155. | Chow PK, Poon DY, Khin MW, Singh H, Han HS, Goh AS, Choo SP, Lai HK, Lo RH, Tay KH. Multicenter phase II study of sequential radioembolization-sorafenib therapy for inoperable hepatocellular carcinoma. PLoS One. 2014;9:e90909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 156. | Chen SW, Lin LC, Kuo YC, Liang JA, Kuo CC, Chiou JF. Phase 2 study of combined sorafenib and radiation therapy in patients with advanced hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2014;88:1041-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 157. | Yoon SM, Lim YS, Won HJ, Kim JH, Kim KM, Lee HC, Chung YH, Lee YS, Lee SG, Park JH. Radiotherapy plus transarterial chemoembolization for hepatocellular carcinoma invading the portal vein: long-term patient outcomes. Int J Radiat Oncol Biol Phys. 2012;82:2004-2011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 180] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 158. | Cho JY, Paik YH, Park HC, Yu JI, Sohn W, Gwak GY, Choi MS, Lee JH, Koh KC, Paik SW. The feasibility of combined transcatheter arterial chemoembolization and radiotherapy for advanced hepatocellular carcinoma. Liver Int. 2014;34:795-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 159. | Fujino H, Kimura T, Aikata H, Miyaki D, Kawaoka T, Kan H, Fukuhara T, Kobayashi T, Naeshiro N, Honda Y. Role of 3-D conformal radiotherapy for major portal vein tumor thrombosis combined with hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma. Hepatol Res. 2014;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 160. | Asahara T, Dohi K, Hino H, Nakahara H, Katayama K, Itamoto T, Shimamoto F, Honke Y. A case of hepatocellular carcinoma with bone metastasis responding to radiotherapy after successful hepatectomy of primary lesion. Hiroshima J Med Sci. 1999;48:35-39. [PubMed] |
| 161. | Kim CH, Chung CK, Jahng TA, Kim HJ. Surgical outcome of spinal hepatocellular carcinoma metastases. Neurosurgery. 2011;68:888-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 162. | Kuo SW, Chang YL, Huang PM, Hsu HH, Chen JS, Lee JM, Lee PH, Lee YC. Prognostic factors for pulmonary metastasectomy in hepatocellular carcinoma. Ann Surg Oncol. 2007;14:992-997. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 163. | Kwon JB, Park K, Kim YD, Seo JH, Moon SW, Cho DG, Kim YW, Kim DG, Yoon SK, Lim HW. Clinical outcome after pulmonary metastasectomy from primary hepatocellular carcinoma: analysis of prognostic factors. World J Gastroenterol. 2008;14:5717-5722. [PubMed] |
| 164. | Yoon YS, Kim HK, Kim J, Choi YS, Shim YM, Paik SW, Kim K. Long-term survival and prognostic factors after pulmonary metastasectomy in hepatocellular carcinoma. Ann Surg Oncol. 2010;17:2795-2801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 165. | Lee HS. Management of patients with hepatocellular carcinoma and extrahepatic metastasis. Dig Dis. 2011;29:333-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 166. | Ripamonti CI, Santini D, Maranzano E, Berti M, Roila F. Management of cancer pain: ESMO Clinical Practice Guidelines. Ann Oncol. 2012;23 Suppl 7:vii139-vii154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 269] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 167. | Hwang SJ, Chang HT, Hwang IH, Wu CY, Yang WH, Li CP. Hospice offers more palliative care but costs less than usual care for terminal geriatric hepatocellular carcinoma patients: a nationwide study. J Palliat Med. 2013;16:780-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 168. | Seo YS, Kim YJ, Um SH, Yoo H, Lee JW, Kim YS, Jeen YT, Chun HJ, Kim CD, Ryu HS. Evaluation of the prognostic powers of various tumor status grading scales in patients with hepatocellular carcinoma. J Gastroenterol Hepatol. 2008;23:1267-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 169. | Kitai S, Kudo M, Izumi N, Kaneko S, Ku Y, Kokudo N, Sakamoto M, Takayama T, Nakashima O, Kadoya M. Validation of three staging systems for hepatocellular carcinoma (JIS score, biomarker-combined JIS score and BCLC system) in 4,649 cases from a Japanese nationwide survey. Dig Dis. 2014;32:717-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 170. | Kee KM, Wang JH, Lin CY, Wang CC, Cheng YF, Lu SN. Validation of the 7th edition TNM staging system for hepatocellular carcinoma: an analysis of 8,828 patients in a single medical center. Dig Dis Sci. 2013;58:2721-2728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 171. | Zhang JF, Shu ZJ, Xie CY, Li Q, Jin XH, Gu W, Jiang FJ, Ling CQ. Prognosis of unresectable hepatocellular carcinoma: comparison of seven staging systems (TNM, Okuda, BCLC, CLIP, CUPI, JIS, CIS) in a Chinese cohort. PLoS One. 2014;9:e88182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 172. | Yau T, Tang VY, Yao TJ, Fan ST, Lo CM, Poon RT. Development of Hong Kong Liver Cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology. 2014;146:1691-1700.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 543] [Article Influence: 49.4] [Reference Citation Analysis (0)] |