Published online Apr 7, 2015. doi: 10.3748/wjg.v21.i13.3777
Peer-review started: November 26, 2014
First decision: December 26, 2014
Revised: January 8, 2015
Accepted: February 5, 2015
Article in press: February 5, 2015
Published online: April 7, 2015
Processing time: 133 Days and 1.5 Hours
Nonalcoholic fatty liver disease (NAFLD)/nonalcoholic steatohepatitis (NASH) is considered to be a hepatic manifestation of metabolic syndrome, and its incidence is rapidly increasing worldwide. It is currently the most common chronic liver disease. NASH can progress to liver cirrhosis and hepatocellular carcinoma, and may result in liver-related death. Currently, the principal treatment for NAFLD/NASH is lifestyle modification by diet and exercise. However, pharmacological therapy is indispensable because obese patients with NAFLD often have difficulty maintaining improved lifestyles. The pathogenesis of NAFLD/NASH has not been completely elucidated. However, insulin resistance, inflammatory cytokines, and oxidative stress are thought to be important in the development and/or progression of the disease. Currently, insulin sensitizers (thiazolidinediones) and antioxidants (vitamin E) seem to be the most promising therapeutic agents for NAFLD/NASH, and lipid-lowering drugs, pentoxifylline, angiotensin receptor blockers, and n-3 polyunsaturated fatty acids also have promise. However, there is a lack of consensus regarding the most effective and appropriate pharmacotherapy for NAFLD/NASH. Animal experiments suggest that herbal medicines and natural products may be promising therapeutic agents for NAFLD/NASH, but their efficacy and safety are yet to be investigated in human studies. In this paper, we review the existing and potential pharmacological therapies for NAFLD/NASH.
Core tip: Nonalcoholic fatty liver disease (NAFLD)/nonalcoholic steatohepatitis (NASH) is considered to be a hepatic manifestation of metabolic syndrome. Currently, the principal treatment for NAFLD/NASH is lifestyle modification by diet and exercise. However, establishment of pharmacological therapy is indispensable. Currently, insulin sensitizers (thiazolidinediones) and antioxidants (vitamin E) seem to be the most promising agents for treating NAFLD/NASH, and lipid-lowering drugs, pentoxifylline, angiotensin receptor blockers, and n-3 polyunsaturated fatty acids also have promise. However, there is a lack of consensus regarding the most effective and appropriate pharmacotherapy for NAFLD/NASH. Here, we review the existing and potential pharmacological therapies for NAFLD/NASH.
- Citation: Takahashi Y, Sugimoto K, Inui H, Fukusato T. Current pharmacological therapies for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol 2015; 21(13): 3777-3785
- URL: https://www.wjgnet.com/1007-9327/full/v21/i13/3777.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i13.3777
Nonalcoholic fatty liver disease (NAFLD) is characterized by accumulation of triglycerides in the liver of patients without a history of excessive alcohol consumption. NAFLD is classified into simple steatosis, in which only hepatic steatosis is observed, and nonalcoholic steatohepatitis (NASH), in which intralobular inflammation and ballooning degeneration of hepatocytes as well as hepatic steatosis are observed. NASH is a progressive disease and may lead to liver cirrhosis and hepatocellular carcinoma[1,2]. Twenty percent of NASH patients are reported to develop cirrhosis, and 30%-40% of patients with NASH cirrhosis experience liver-related death[3]. Recently, NASH has become the third most common indication for liver transplantation in the United States[4].
NAFLD/NASH is considered to be a hepatic manifestation of metabolic syndrome[5]. The incidence of NAFLD/NASH has been rapidly increasing globally in line with the increased prevalence of obesity, and is currently the most common chronic liver disease. Recently, the incidence of NAFLD and NASH was reported to be 46% and 12%, respectively, in a largely middle-aged population[6].
Currently, the principal treatment for NAFLD/NASH is lifestyle modification by diet and exercise. At present, there is a lack of consensus regarding the most useful and appropriate pharmacological therapy. However, establishment of pharmacological therapy is indispensable because obese patients with NAFLD often have difficulty maintaining improved lifestyles. In this paper, we review the existing and potential pharmacological therapies for NAFLD/NASH.
Understanding the pathogenesis of NAFLD/NASH is important for the development of suitable drugs. Although the pathogenesis of NAFLD/NASH has not been completely elucidated, the “two-hit”[7] and “multiple parallel hit”[8] hypotheses have been proposed. In the two-hit hypothesis, hepatic steatosis occurs first and progresses to NASH by subsequent second hits. Hepatic steatosis results from an imbalance between triglyceride accumulation and elimination in the liver. Insulin resistance (IR), which is frequently seen in obese individuals, is closely linked to this process, because it alters nutrient distribution among tissues and nutrient metabolism[9]. Peripheral IR leads to an influx of free fatty acids into the liver by decreased suppression of lipolysis and increased de novo lipogenesis[10]. The renin-angiotensin-aldosterone system plays a central role in IR and is associated with NAFLD/NASH[11]. Hepatic inflammation is caused by increased levels of inflammatory cytokines [e.g., tumor necrosis factor (TNF)-α, interleukin-6], decreased levels of anti-inflammatory cytokines (e.g., adiponectin), oxidative stress, and endotoxins originating from intestinal bacterial flora.
IR is a major mechanism in the development and progression of NAFLD, therefore, the potential therapeutic effect of insulin sensitizers on NAFLD/NASH has gathered much attention. Thiazolidinediones (TZDs) are peroxisome proliferator-activated receptor (PPAR)-γ agonists and increase insulin sensitivity. Rosiglitazone and pioglitazone are representative TZDs. Rosiglitazone was shown to improve steatosis and aminotransferase levels in patients with NASH in a randomized controlled trial[12]. However, usage of rosiglitazone is restricted because it can increase the risk of heart attack (ischemic heart disease). Usage of rosiglitazone is prohibited in Europe according to the recommendations of the European Medicines Agency, and it is highly restricted in the United States based on the recommendations of the Food and Drug Administration (FDA).
In randomized controlled trials, administration of 30 or 45 mg/d pioglitazone induced significant improvements in serum aminotransferase levels and liver histology (steatosis, inflammation, ballooning, and Mallory-Denk bodies) compared with placebo in NASH patients[13-15]. However, improvement in the extent of fibrosis was not significant. American guidelines for NAFLD have recommended the use of pioglitazone in patients with biopsy-proven NASH[16]. Pioglitazone has side effects including weight gain, edema, heart failure, and bone density reduction. In addition, it is reported that the risk of bladder cancer is increased if pioglitazone is used for > 2 years[17]. Therefore, the usage of pioglitazone for new patients is prohibited in France and Germany. In the United States, the FDA currently recommends avoidance of pioglitazone if active bladder cancer is present, and caution if there is history of the disease[18].
Metformin is classified as a biguanide, and is used to treat type 2 diabetes mellitus. Metformin increases insulin sensitivity by decreasing hepatic gluconeogenesis and limiting triglyceride production[19]. In pilot studies, metformin was shown to improve fatty liver disease and reverse hepatomegaly, steatosis, and aminotransferase abnormalities in a mouse model of NAFLD[20], as well as improve serum aminotransferase levels and liver histology including steatosis, necroinflammation, and fibrosis in NAFLD/NASH patients[21,22]. However, in a subsequent randomized controlled trial, treatment with metformin for 6 mo showed no significant benefits compared with placebo in terms of improvement in liver histology in patients with NAFLD, although it was associated with a reduction in serum levels of lipids and glucose[23]. In a recent randomized controlled trial, metformin was not superior to placebo in attaining sustained reduction of alanine aminotransferase (ALT) levels in patients with pediatric NAFLD[24]. American guidelines for NAFLD do not recommend metformin for the treatment of adult NAFLD[25].
Many studies have examined the therapeutic effects of antioxidants on NAFLD/NASH because oxidative stress is thought to be an important factor for the progression of NAFLD. Vitamin E (α-tocopherol) is a fat-soluble vitamin with antioxidant properties. In a pilot study, daily oral vitamin E administration was shown to normalize serum aminotransferase and alkaline phosphatase levels in children with NASH[26]. In a large randomized controlled trial, vitamin E administration (800 IU/d) for 96 wk significantly improved serum aminotransferase levels, hepatic steatosis, and lobular inflammation compared with placebo in adults with NASH and without diabetes. However, the extent of hepatic fibrosis was not significantly improved[15]. In another randomized controlled trial, administration of vitamin E and C (1000 IU/d and 1000 mg/d, respectively) for 6 mo resulted in significant improvement in hepatic fibrosis in patients with NASH[27]. In a recent randomized controlled trial, vitamin E (800 IU/d) administration for 96 wk significantly improved hepatocellular ballooning, but it was not superior to placebo in attaining sustained reduction in ALT level in patients with pediatric NAFLD[24]. Based on the results of earlier trials in non-diabetic patients with biopsy-proven NASH, the American guidelines for NAFLD recommend the use of vitamin E for non-diabetic patients with biopsy-proven NASH[16]. It is important to examine the effects of vitamin E in NASH patients with diabetes.
It is noteworthy that the long-term safety of vitamin E is questionable. It was reported that high-dosage (≥ 400 IU/d) vitamin E supplements may increase all-cause mortality[28]. In addition, it is reported that dietary supplementation with vitamin E significantly increases the risk of prostate cancer among healthy men[29]. It is necessary to investigate the long-term prognoses of NASH patients who take vitamin E supplements.
NAFLD is strongly associated with obesity and dyslipidemia. Statins prevent cholesterol synthesis by inhibiting 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, and are used to treat dyslipidemia. In addition, owing to their possible anti-inflammatory effects, statins are an option for treating NAFLD[30]. Atorvastatin and simvastatin are representative statins. In a pilot study, serum aminotransferase and lipid levels were reduced significantly in all patients with NAFLD by atorvastatin treatment; however, histological assessment was not performed[31]. In a subsequent open-label study, liver steatosis and NAFLD activity score (NAS) significantly improved, whereas four of 17 (24%) patients with biopsy-proven NASH with hyperlipidemia had increased fibrosis stage after atorvastatin administration[32]. In a randomized controlled trial, atorvastatin (20 mg/d) combined with antioxidants (vitamin C and E) was effective in reducing the risk of hepatic steatosis by 71% after 4 years of active therapy in individuals with NAFLD at baseline[33]. In a randomized controlled trial, simvastatin treatment did not induce significant improvement in serum aminotransferase levels, hepatic steatosis, necroinflammatory activity, or stage of fibrosis in NASH patients[34]. Thus, the efficacy of statins for NAFLD/NASH has not been fully validated. Statins decrease lipid levels both peripherally and viscerally, specifically in the liver[9]. Serum aminotransferase levels may increase transiently when fat is removed from the liver. This transient elevation of serum aminotransferase levels does not progress to liver injury, and decreased fat deposition in the liver eventually decreases serum aminotransferase levels. Therefore, even if serum aminotransferase levels increase shortly after administration of statins, it is not necessary to discontinue treatment. American guidelines recommend against the use of statins for treatment of NASH until randomized controlled trials have confirmed their histological efficacy[16]. However, administration of statins may be beneficial in improving metabolic status and reducing the risk of cardiovascular disease.
Ezetimibe is a newer agent that decreases serum lipid levels by inhibiting cholesterol absorption. It was reported that combination therapy with ezetimibe and acarbose (an α-glucosidase inhibitor) for 24 wk improved histopathological findings (steatosis, inflammation, and fibrosis) in a mouse model of NAFLD[35]. In an open-label pilot study, serum aspartate aminotransferase (AST), ALT, and low-density lipoprotein (LDL) cholesterol levels were significantly improved in NASH patients by treatment with ezetimibe (10 mg/d) for 6 mo. Follow-up liver biopsies revealed that steatosis grade and NAS also significantly improved; however, the fibrosis stage did not change significantly[36]. In a subsequent trial, long-term (10 mg/d for 24 mo) ezetimibe therapy significantly improved serum triglyceride, total cholesterol, LDL cholesterol, and ALT levels in NAFLD patients. In that study, histological features of steatosis, necroinflammation, and ballooning significantly improved from baseline, but fibrosis stage did not significantly improve[37]. In a recent randomized controlled trial, ezetimibe administration (10 mg/d for 6 mo) improved hepatic fibrosis but increased hepatic long-chain fatty acid and hemoglobin A1c levels in patients with NAFLD[38].
Pentoxifylline is a methylxanthine derivative and nonselective phosphodiesterase inhibitor that inhibits synthesis of TNF-α. TNF-α is thought to be important in the progression of NAFLD, thus, pentoxifylline has been investigated as a treatment option for NAFLD/NASH. In addition, pentoxifylline has recently been shown to decrease oxidized lipid product levels in NASH patients[39]. In pilot trials, administration of pentoxifylline for 12 mo significantly decreased serum AST and ALT levels compared to baseline, and this correlated well with histological resolution in NASH patients[40,41]. However, randomized controlled trials led to mixed results. Van Wagner et al[42] reported that administration of pentoxifylline (1200 mg/d) for 12 mo failed to reduce aminotransferase levels compared to placebo in NASH patients. However, Zein et al[43] reported that administration of pentoxifylline (1200 mg/d) for 12 mo improved histological features of NASH (steatosis, lobular inflammation, NAS, and fibrosis) compared to placebo. Larger randomized controlled trials are needed in the future to validate the effects of pentoxifylline on NAFLD/NASH. Administration of pentoxifylline requires caution because it causes adverse effects such as nausea and vomiting.
Ursodeoxycholic acid (UDCA) is a hydrophilic bile acid with antiapoptotic and cytoprotective properties. Its effects on NAFLD/NASH have therefore been examined. In a randomized controlled trial, 2 years of therapy with UDCA at a dose of 13-15 mg/kg per day did not have any significant benefit compared with placebo for patients with NASH[44]. In a subsequent randomized controlled trial, 23-28 mg/kg per day UDCA treatment for 18 mo failed to improve overall histology in patients with NASH compared with placebo[45]. A recent randomized controlled trial showed that treatment with high-dose (28-35 mg/kg per day) UDCA for 12 mo improved aminotransferase levels, serum fibrosis markers, and selected metabolic parameters in NASH patients, but histological assessment was not performed in this study[46]. In a randomized controlled trial, 2 years treatment with UDCA in combination with vitamin E improved serum AST and ALT levels and hepatic steatosis in patients with NASH[47]; however, this may be primarily due to the effects of vitamin E. American guidelines do not recommend UDCA for the treatment of NAFLD or NASH[25].
The renin-angiotensin-aldosterone system modulates insulin sensitivity and is associated with pathogenesis of NAFLD/NASH. Thus, the effects of angiotensin II type 1 blockers (e.g., telmisartan, valsartan and losartan) on NAFLD have been investigated. Telmisartan attenuated steatohepatitis progression by suppressing the infiltration of macrophages into the liver in a mouse model of NASH[48]. In a clinical trial, telmisartan and valsartan decreased serum ALT levels, homeostasis model assessment as an index of insulin resistance (HOMA-IR), and NAS in NASH patients with metabolic syndrome, with telmisartan showing a higher efficacy than valsartan for HOMA-IR and NAS[49]. Combined treatment with losartan and deferasirox (an oral iron chelator) attenuated progression of NASH in rats[50]. In a clinical trial, losartan improved serum aminotransferase levels and liver histology (necroinflammation and fibrosis)[51]. However, in an open-label trial, combination therapy with rosiglitazone and losartan conferred no greater benefit than rosiglitazone alone with respect to histopathology[52]. Well-designed randomized controlled trials are needed to confirm the effects of angiotensin receptor blockers on NAFLD/NASH. In addition, the use of angiotensin receptor blockers for normotensive patients requires caution because of their hypotensive effects.
N-3 polyunsaturated fatty acids (n-3 PUFAs) are PPARα ligands, and are suggested to play a role in improving NAFLD. Supplementation with n-3 PUFAs ameliorated hepatic steatosis and the degree of liver injury in a rat model of NASH[53]. In a pilot human study, n-3 PUFA supplementation significantly decreased serum AST, ALT, triglyceride, and fasting glucose levels in patients with NAFLD compared with those in controls. Moreover, ultrasonography demonstrated improvement of liver echotexture after n-3 PUFA supplementation[54]. Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are major n-3 PUFAs. In a pilot trial, after administration of highly purified EPA (2700 mg/d) for 12 mo, serum ALT levels and liver histology, including steatosis, lobular inflammation, ballooning, and fibrosis improved in most NASH patients[55]. However, in a recent phase 2 trial, ethyl-eicosapentaenoic acid (EPA-E), a synthetic n-3 PUFA, had no significant effect on the histological features of NASH[56]. Although many studies have suggested positive effects of n-3 PUFAs on NAFLD/NASH, conclusions and recommendations for n-3 PUFA supplementation are difficult to establish because the specific quantities and ratios of EPA and DHA were unclear in most published trials.
Probiotics are microorganisms that provide health benefits when consumed. Prebiotics are chemicals that induce the growth and/or activity of microorganisms that contribute to the well-being of their host. Synbiotics are nutritional supplements combining probiotics and prebiotics in a synergistic form. Gut microorganisms play a role in the development of insulin resistance, hepatic steatosis, necroinflammation, and fibrosis[57], therefore, the potential benefits of probiotics and synbiotics on NAFLD/NASH have been suggested. In 2003, treatment with VSL#3 probiotic was reported to improve liver histology, reduce hepatic total fatty acid content, and decrease serum ALT levels in an animal model of NAFLD[58]. Subsequently, various studies have shown the beneficial effects of probiotics or synbiotics on animal models of NAFLD/NASH[59-61]. In contrast, in an open label pilot trial, all subjects who received one sachet of VSL#3 probiotic daily for 4 mo experienced a significant increase in liver fat content[62]. However, the study had several limitations, including a small number of subjects and use of only one dose and preparation of the probiotic compound. No subsequent clinical studies have reported a similar harmful effect of probiotics. In a randomized controlled trial, the administration of probiotic Lactobacillus rhamnosus strain GG (12 billion colony forming unit/d for 8 wk) significantly decreased serum ALT levels compared with the placebo in pediatric obesity-related liver disease patients[63]. In a recent randomized controlled trial, administration of a synbiotic capsule twice daily for 28 wk in addition to lifestyle modification significantly decreased serum ALT and AST levels and fibrosis scores, as determined by transient elastography compared with placebo[64]. Although promising results have been obtained in most of the previous experimental and clinical studies, the effects of probiotics and synbiotics on NAFLD/NASH need to be confirmed in larger randomized controlled trials. In addition, the most effective preparations and dosages need to be established.
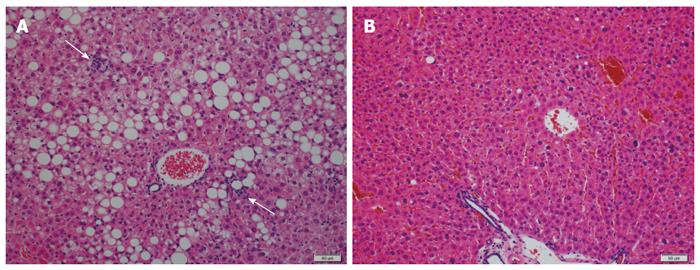
Various herbal medicines and natural products are known to possess anti-inflammatory and antioxidant properties, and their effects on NAFLD/NASH have therefore been anticipated. Japanese herbal medicines (JHMs) (Kampo medicines) are traditional Japanese medicines that are integrated into modern clinical practice. We investigated the effects of four kinds of JHMs [shosaikoto (TJ-9), inchinkoto (TJ-135), juzentaihoto (TJ-48), and keishibukuryogan (TJ-25)] on a mouse model of NASH (methionine- and choline-deficient diet-fed db/db mice), and showed that TJ-9 and TJ-48 inhibited necroinflammation and fibrosis in the liver[65] (Figure 1). We also found that TJ-9 and TJ-48, as well as TJ-135, inhibited necroinflammatory activity in another mouse model of NASH [high-fat diet (HFD)-fed db/db mice][66]. Recently, it was reported that bofutsushosan (TJ-62), an anti-obesity JHM, attenuated the progression of HFD-induced NASH in mice[67]. In a small retrospective study, TJ-25 administration led to a significant improvement in liver injury tests and blood cholesterol levels in all NAFLD patients examined[68].
Resveratrol is a polyphenol with antioxidant, anti-inflammatory, antiproliferative, and antiangiogenic effects, and plays a potentially important role in many disorders[69]. It was shown that resveratrol improves IR and NAFLD severity in rats, and this effect is suggested to be associated with activation of AMP-activated protein kinase[70,71].
In an epidemiological study, increased consumption of green tea was associated with decreased serum concentrations of total cholesterol and triglycerides and an increased concentration of HDL cholesterol, together with a decreased concentration of low- and very low-density lipoprotein cholesterol. Increased consumption of green tea is related to decreased concentrations of serum AST and ALT[72]. Green tea extracts attenuated hepatic steatosis by decreasing adipose lipogenesis and enhancing hepatic antioxidant defenses in a mouse model of NAFLD[73,74]. In addition, (-)-epigallocatechin-3-gallate, the major polyphenol found in green tea, improved plasma ALT concentrations and hepatic steatosis in HFD-fed mice[75].
The leaves of eucalyptus (Eucalyptus globulus) and banaba (Lagerstroemia speciosa L.) are used as traditional remedies for diabetes mellitus. We found that extracts of these leaves reduced lipogenesis, oxidative stress, and inflammatory cytokine expression, and thus inhibited NASH induced by excessive ingestion of fructose in rats (unpublished data). Reports on positive effects of herbal medicines and natural products on animal models of NAFLD/NASH have been increasing, and human studies are needed in the future.
As reviewed in this paper, many animal experiments and clinical studies have been performed to investigate the effects of various drugs on NAFLD/NASH. However, there is a lack of consensus regarding the most effective and appropriate pharmacotherapy for this disease. The mechanism of action and limitations/demerits of each pharmacotherapy are summarized in Table 1. Currently, insulin sensitizers (TZDs) and vitamin E seem to be the most promising. However, they cause side effects such as weight gain and increased all-cause mortality, respectively. Better understanding on the long-term safety and efficacy of these drugs is needed before they can be fully incorporated into clinical practice. Pentoxifylline, statins, angiotensin receptor blockers, and n-3 PUFAs have some promise, but their effects should be validated by large, well-designed clinical trials. Results of animal experiments suggest that herbal medicines and natural products may be promising as therapeutic agents for NAFLD/NASH. However, their efficacy and safety need to be investigated in clinical studies. Continuous clinical and preclinical studies on existing and potential drugs are needed to improve treatment for NAFLD/NASH, which is an increasingly prevalent disease.
| Drug | Mechanism of action | Limitations/demerits |
| Insulin sensitizers | Improve insulin sensitivity | Obvious side effects (heart attack with rosiglitazone; weight gain, edema, heart failure, and bone density reduction with pioglitazone) |
| Antioxidants (vitamin E) | Attenuate oxidative stress | Long-term safety is questionable (increased all-cause mortality and risk of prostate cancer) |
| Lipid-lowering drugs | Improve dyslipidemia | The efficacy has not been fully validated in clinical trials |
| Pentoxifylline | Inhibits synthesis of TNF-α | Randomized controlled trials led to inconsistent results. Side effects (nausea and vomiting) |
| UDCA | Antiapoptotic and cytoprotective properties | Most randomized controlled trials did not show positive effects |
| Angiotensin receptor blockers | Modulates insulin sensitivity | Randomized controlled trials are lacking. Side effects (hypotension) |
| n-3 PUFAs | Activate PPARα | Specific quantities and ratios of EPA and DHA are unclear in most trials |
| Probiotics and synbiotics | Control gut microbiota | The most effective preparation and dose have not yet been established |
| Herbal medicines/natural products | Anti-inflammatory and antioxidative properties | Effects have not been studied in humans |
P- Reviewer: Hekmatdoost A, Mikolasevic I S- Editor: Qi Y L- Editor: Kerr C E- Editor: Wang CH
| 1. | Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43:S99-S112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1756] [Cited by in RCA: 1822] [Article Influence: 95.9] [Reference Citation Analysis (0)] |
| 2. | Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332:1519-1523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1720] [Cited by in RCA: 1659] [Article Influence: 118.5] [Reference Citation Analysis (2)] |
| 3. | McCullough AJ. Pathophysiology of nonalcoholic steatohepatitis. J Clin Gastroenterol. 2006;40 Suppl 1:S17-S29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 139] [Reference Citation Analysis (0)] |
| 4. | Charlton MR, Burns JM, Pedersen RA, Watt KD, Heimbach JK, Dierkhising RA. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141:1249-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 835] [Cited by in RCA: 855] [Article Influence: 61.1] [Reference Citation Analysis (0)] |
| 5. | Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, Natale S, Vanni E, Villanova N, Melchionda N. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917-923. [PubMed] |
| 6. | Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1522] [Cited by in RCA: 1620] [Article Influence: 115.7] [Reference Citation Analysis (1)] |
| 7. | Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842-845. [PubMed] |
| 8. | Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52:1836-1846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1543] [Cited by in RCA: 1820] [Article Influence: 121.3] [Reference Citation Analysis (0)] |
| 9. | Tomeno W, Yoneda M, Imajo K, Ogawa Y, Kessoku T, Saito S, Eguchi Y, Nakajima A. Emerging drugs for non-alcoholic steatohepatitis. Expert Opin Emerg Drugs. 2013;18:279-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Ibrahim MA, Kelleni M, Geddawy A. Nonalcoholic fatty liver disease: current and potential therapies. Life Sci. 2013;92:114-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 11. | Georgescu EF. Angiotensin receptor blockers in the treatment of NASH/NAFLD: could they be a first-class option? Adv Ther. 2008;25:1141-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Ratziu V, Giral P, Jacqueminet S, Charlotte F, Hartemann-Heurtier A, Serfaty L, Podevin P, Lacorte JM, Bernhardt C, Bruckert E. Rosiglitazone for nonalcoholic steatohepatitis: one-year results of the randomized placebo-controlled Fatty Liver Improvement with Rosiglitazone Therapy (FLIRT) Trial. Gastroenterology. 2008;135:100-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 479] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 13. | Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, Balas B, Gastaldelli A, Tio F, Pulcini J. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297-2307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1307] [Cited by in RCA: 1329] [Article Influence: 69.9] [Reference Citation Analysis (0)] |
| 14. | Aithal GP, Thomas JA, Kaye PV, Lawson A, Ryder SD, Spendlove I, Austin AS, Freeman JG, Morgan L, Webber J. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology. 2008;135:1176-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 556] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 15. | Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675-1685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2642] [Cited by in RCA: 2469] [Article Influence: 164.6] [Reference Citation Analysis (2)] |
| 16. | Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005-2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2413] [Cited by in RCA: 2613] [Article Influence: 201.0] [Reference Citation Analysis (1)] |
| 17. | Lewis JD, Ferrara A, Peng T, Hedderson M, Bilker WB, Quesenberry CP, Vaughn DJ, Nessel L, Selby J, Strom BL. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care. 2011;34:916-922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 459] [Cited by in RCA: 470] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 18. | Lomonaco R, Sunny NE, Bril F, Cusi K. Nonalcoholic fatty liver disease: current issues and novel treatment approaches. Drugs. 2013;73:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 115] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 19. | Torres DM, Harrison SA. Diagnosis and therapy of nonalcoholic steatohepatitis. Gastroenterology. 2008;134:1682-1698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 264] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 20. | Lin HZ, Yang SQ, Chuckaree C, Kuhajda F, Ronnet G, Diehl AM. Metformin reverses fatty liver disease in obese, leptin-deficient mice. Nat Med. 2000;6:998-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 509] [Cited by in RCA: 523] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 21. | Marchesini G, Brizi M, Bianchi G, Tomassetti S, Zoli M, Melchionda N. Metformin in non-alcoholic steatohepatitis. Lancet. 2001;358:893-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 479] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 22. | Bugianesi E, Gentilcore E, Manini R, Natale S, Vanni E, Villanova N, David E, Rizzetto M, Marchesini G. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol. 2005;100:1082-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 487] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 23. | Haukeland JW, Konopski Z, Eggesbø HB, von Volkmann HL, Raschpichler G, Bjøro K, Haaland T, Løberg EM, Birkeland K. Metformin in patients with non-alcoholic fatty liver disease: a randomized, controlled trial. Scand J Gastroenterol. 2009;44:853-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 248] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 24. | Lavine JE, Schwimmer JB, Van Natta ML, Molleston JP, Murray KF, Rosenthal P, Abrams SH, Scheimann AO, Sanyal AJ, Chalasani N. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA. 2011;305:1659-1668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 864] [Cited by in RCA: 847] [Article Influence: 60.5] [Reference Citation Analysis (0)] |
| 25. | Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1226] [Cited by in RCA: 1357] [Article Influence: 104.4] [Reference Citation Analysis (4)] |
| 26. | Lavine JE. Vitamin E treatment of nonalcoholic steatohepatitis in children: a pilot study. J Pediatr. 2000;136:734-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 249] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 27. | Harrison SA, Torgerson S, Hayashi P, Ward J, Schenker S. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2003;98:2485-2490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 484] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 28. | Miller ER, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1820] [Cited by in RCA: 1627] [Article Influence: 81.4] [Reference Citation Analysis (0)] |
| 29. | Klein EA, Thompson IM, Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, Minasian LM, Ford LG, Parnes HL, Gaziano JM. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2011;306:1549-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1218] [Cited by in RCA: 1223] [Article Influence: 87.4] [Reference Citation Analysis (0)] |
| 30. | Dima A, Marinescu AG, Dima AC. Non-alcoholic fatty liver disease and the statins treatment. Rom J Intern Med. 2012;50:19-25. [PubMed] |
| 31. | Gómez-Domínguez E, Gisbert JP, Moreno-Monteagudo JA, García-Buey L, Moreno-Otero R. A pilot study of atorvastatin treatment in dyslipemid, non-alcoholic fatty liver patients. Aliment Pharmacol Ther. 2006;23:1643-1647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 141] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 32. | Hyogo H, Tazuma S, Arihiro K, Iwamoto K, Nabeshima Y, Inoue M, Ishitobi T, Nonaka M, Chayama K. Efficacy of atorvastatin for the treatment of nonalcoholic steatohepatitis with dyslipidemia. Metabolism. 2008;57:1711-1718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 169] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 33. | Foster T, Budoff MJ, Saab S, Ahmadi N, Gordon C, Guerci AD. Atorvastatin and antioxidants for the treatment of nonalcoholic fatty liver disease: the St Francis Heart Study randomized clinical trial. Am J Gastroenterol. 2011;106:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 203] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 34. | Nelson A, Torres DM, Morgan AE, Fincke C, Harrison SA. A pilot study using simvastatin in the treatment of nonalcoholic steatohepatitis: A randomized placebo-controlled trial. J Clin Gastroenterol. 2009;43:990-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 206] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 35. | Nozaki Y, Fujita K, Yoneda M, Wada K, Shinohara Y, Takahashi H, Kirikoshi H, Inamori M, Kubota K, Saito S. Long-term combination therapy of ezetimibe and acarbose for non-alcoholic fatty liver disease. J Hepatol. 2009;51:548-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 36. | Yoneda M, Fujita K, Nozaki Y, Endo H, Takahashi H, Hosono K, Suzuki K, Mawatari H, Kirikoshi H, Inamori M. Efficacy of ezetimibe for the treatment of non-alcoholic steatohepatitis: An open-label, pilot study. Hepatol Res. 2010;40:566-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 37. | Park H, Shima T, Yamaguchi K, Mitsuyoshi H, Minami M, Yasui K, Itoh Y, Yoshikawa T, Fukui M, Hasegawa G. Efficacy of long-term ezetimibe therapy in patients with nonalcoholic fatty liver disease. J Gastroenterol. 2011;46:101-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 144] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 38. | Takeshita Y, Takamura T, Honda M, Kita Y, Zen Y, Kato K, Misu H, Ota T, Nakamura M, Yamada K. The effects of ezetimibe on non-alcoholic fatty liver disease and glucose metabolism: a randomised controlled trial. Diabetologia. 2014;57:878-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 39. | Zein CO, Lopez R, Fu X, Kirwan JP, Yerian LM, McCullough AJ, Hazen SL, Feldstein AE. Pentoxifylline decreases oxidized lipid products in nonalcoholic steatohepatitis: new evidence on the potential therapeutic mechanism. Hepatology. 2012;56:1291-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 40. | Adams LA, Zein CO, Angulo P, Lindor KD. A pilot trial of pentoxifylline in nonalcoholic steatohepatitis. Am J Gastroenterol. 2004;99:2365-2368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 166] [Article Influence: 7.9] [Reference Citation Analysis (1)] |
| 41. | Satapathy SK, Sakhuja P, Malhotra V, Sharma BC, Sarin SK. Beneficial effects of pentoxifylline on hepatic steatosis, fibrosis and necroinflammation in patients with non-alcoholic steatohepatitis. J Gastroenterol Hepatol. 2007;22:634-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 42. | Van Wagner LB, Koppe SW, Brunt EM, Gottstein J, Gardikiotes K, Green RM, Rinella ME. Pentoxifylline for the treatment of non-alcoholic steatohepatitis: a randomized controlled trial. Ann Hepatol. 2011;10:277-286. [PubMed] |
| 43. | Zein CO, Yerian LM, Gogate P, Lopez R, Kirwan JP, Feldstein AE, McCullough AJ. Pentoxifylline improves nonalcoholic steatohepatitis: a randomized placebo-controlled trial. Hepatology. 2011;54:1610-1619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 291] [Cited by in RCA: 277] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 44. | Lindor KD, Kowdley KV, Heathcote EJ, Harrison ME, Jorgensen R, Angulo P, Lymp JF, Burgart L, Colin P. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology. 2004;39:770-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 516] [Cited by in RCA: 492] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 45. | Leuschner UF, Lindenthal B, Herrmann G, Arnold JC, Rössle M, Cordes HJ, Zeuzem S, Hein J, Berg T. High-dose ursodeoxycholic acid therapy for nonalcoholic steatohepatitis: a double-blind, randomized, placebo-controlled trial. Hepatology. 2010;52:472-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 230] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 46. | Ratziu V, de Ledinghen V, Oberti F, Mathurin P, Wartelle-Bladou C, Renou C, Sogni P, Maynard M, Larrey D, Serfaty L. A randomized controlled trial of high-dose ursodesoxycholic acid for nonalcoholic steatohepatitis. J Hepatol. 2011;54:1011-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 236] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 47. | Dufour JF, Oneta CM, Gonvers JJ, Bihl F, Cerny A, Cereda JM, Zala JF, Helbling B, Steuerwald M, Zimmermann A. Randomized placebo-controlled trial of ursodeoxycholic acid with vitamin e in nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2006;4:1537-1543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 256] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 48. | Kudo H, Yata Y, Takahara T, Kawai K, Nakayama Y, Kanayama M, Oya T, Morita S, Sasahara M, Mann DA. Telmisartan attenuates progression of steatohepatitis in mice: role of hepatic macrophage infiltration and effects on adipose tissue. Liver Int. 2009;29:988-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 49. | Georgescu EF, Ionescu R, Niculescu M, Mogoanta L, Vancica L. Angiotensin-receptor blockers as therapy for mild-to-moderate hypertension-associated non-alcoholic steatohepatitis. World J Gastroenterol. 2009;15:942-954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 135] [Cited by in RCA: 136] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 50. | Kaji K, Yoshiji H, Kitade M, Ikenaka Y, Noguchi R, Shirai Y, Aihara Y, Namisaki T, Yoshii J, Yanase K. Combination treatment of angiotensin II type I receptor blocker and new oral iron chelator attenuates progression of nonalcoholic steatohepatitis in rats. Am J Physiol Gastrointest Liver Physiol. 2011;300:G1094-G1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 51. | Yokohama S, Yoneda M, Haneda M, Okamoto S, Okada M, Aso K, Hasegawa T, Tokusashi Y, Miyokawa N, Nakamura K. Therapeutic efficacy of an angiotensin II receptor antagonist in patients with nonalcoholic steatohepatitis. Hepatology. 2004;40:1222-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 351] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 52. | Torres DM, Jones FJ, Shaw JC, Williams CD, Ward JA, Harrison SA. Rosiglitazone versus rosiglitazone and metformin versus rosiglitazone and losartan in the treatment of nonalcoholic steatohepatitis in humans: a 12-month randomized, prospective, open- label trial. Hepatology. 2011;54:1631-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 134] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 53. | Svegliati-Baroni G, Candelaresi C, Saccomanno S, Ferretti G, Bachetti T, Marzioni M, De Minicis S, Nobili L, Salzano R, Omenetti A. A model of insulin resistance and nonalcoholic steatohepatitis in rats: role of peroxisome proliferator-activated receptor-alpha and n-3 polyunsaturated fatty acid treatment on liver injury. Am J Pathol. 2006;169:846-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 201] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 54. | Capanni M, Calella F, Biagini MR, Genise S, Raimondi L, Bedogni G, Svegliati-Baroni G, Sofi F, Milani S, Abbate R. Prolonged n-3 polyunsaturated fatty acid supplementation ameliorates hepatic steatosis in patients with non-alcoholic fatty liver disease: a pilot study. Aliment Pharmacol Ther. 2006;23:1143-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 300] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 55. | Tanaka N, Sano K, Horiuchi A, Tanaka E, Kiyosawa K, Aoyama T. Highly purified eicosapentaenoic acid treatment improves nonalcoholic steatohepatitis. J Clin Gastroenterol. 2008;42:413-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 179] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 56. | Sanyal AJ, Abdelmalek MF, Suzuki A, Cummings OW, Chojkier M. No significant effects of ethyl-eicosapentanoic acid on histologic features of nonalcoholic steatohepatitis in a phase 2 trial. Gastroenterology. 2014;147:377-384.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 239] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 57. | Eslamparast T, Eghtesad S, Hekmatdoost A, Poustchi H. Probiotics and Nonalcoholic Fatty liver Disease. Middle East J Dig Dis. 2013;5:129-136. [PubMed] |
| 58. | Li Z, Yang S, Lin H, Huang J, Watkins PA, Moser AB, Desimone C, Song XY, Diehl AM. Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatology. 2003;37:343-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 658] [Cited by in RCA: 702] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 59. | Ma X, Hua J, Li Z. Probiotics improve high fat diet-induced hepatic steatosis and insulin resistance by increasing hepatic NKT cells. J Hepatol. 2008;49:821-830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 317] [Cited by in RCA: 306] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 60. | Esposito E, Iacono A, Bianco G, Autore G, Cuzzocrea S, Vajro P, Canani RB, Calignano A, Raso GM, Meli R. Probiotics reduce the inflammatory response induced by a high-fat diet in the liver of young rats. J Nutr. 2009;139:905-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 185] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 61. | Raso GM, Simeoli R, Iacono A, Santoro A, Amero P, Paciello O, Russo R, D’Agostino G, Di Costanzo M, Canani RB. Effects of a Lactobacillus paracasei B21060 based synbiotic on steatosis, insulin signaling and toll-like receptor expression in rats fed a high-fat diet. J Nutr Biochem. 2014;25:81-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 62. | Solga SF, Buckley G, Clark JM, Horska A, Diehl AM. The effect of a probiotic on hepatic steatosis. J Clin Gastroenterol. 2008;42:1117-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 63. | Vajro P, Mandato C, Licenziati MR, Franzese A, Vitale DF, Lenta S, Caropreso M, Vallone G, Meli R. Effects of Lactobacillus rhamnosus strain GG in pediatric obesity-related liver disease. J Pediatr Gastroenterol Nutr. 2011;52:740-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 248] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 64. | Eslamparast T, Poustchi H, Zamani F, Sharafkhah M, Malekzadeh R, Hekmatdoost A. Synbiotic supplementation in nonalcoholic fatty liver disease: a randomized, double-blind, placebo-controlled pilot study. Am J Clin Nutr. 2014;99:535-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 286] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 65. | Takahashi Y, Soejima Y, Kumagai A, Watanabe M, Uozaki H, Fukusato T. Inhibitory effects of Japanese herbal medicines sho-saiko-to and juzen-taiho-to on nonalcoholic steatohepatitis in mice. PLoS One. 2014;9:e87279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 66. | Takahashi Y, Soejima Y, Kumagai A, Watanabe M, Uozaki H, Fukusato T. Japanese herbal medicines shosaikoto, inchinkoto, and juzentaihoto inhibit high-fat diet-induced nonalcoholic steatohepatitis in db/db mice. Pathol Int. 2014;64:490-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 67. | Ono M, Ogasawara M, Hirose A, Mogami S, Ootake N, Aritake K, Higuchi T, Okamoto N, Sakamoto S, Yamamoto M. Bofutsushosan, a Japanese herbal (Kampo) medicine, attenuates progression of nonalcoholic steatohepatitis in mice. J Gastroenterol. 2014;49:1065-1073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 68. | Fujimoto M, Tsuneyama K, Kinoshita H, Goto H, Takano Y, Selmi C, Keen CL, Gershwin ME, Shimada Y. The traditional Japanese formula keishibukuryogan reduces liver injury and inflammation in patients with nonalcoholic fatty liver disease. Ann N Y Acad Sci. 2010;1190:151-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 69. | Catalgol B, Batirel S, Taga Y, Ozer NK. Resveratrol: French paradox revisited. Front Pharmacol. 2012;3:141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 301] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 70. | Shang J, Chen LL, Xiao FX, Sun H, Ding HC, Xiao H. Resveratrol improves non-alcoholic fatty liver disease by activating AMP-activated protein kinase. Acta Pharmacol Sin. 2008;29:698-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 232] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 71. | Bujanda L, Hijona E, Larzabal M, Beraza M, Aldazabal P, García-Urkia N, Sarasqueta C, Cosme A, Irastorza B, González A. Resveratrol inhibits nonalcoholic fatty liver disease in rats. BMC Gastroenterol. 2008;8:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 168] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 72. | Imai K, Nakachi K. Cross sectional study of effects of drinking green tea on cardiovascular and liver diseases. BMJ. 1995;310:693-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 246] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 73. | Bruno RS, Dugan CE, Smyth JA, DiNatale DA, Koo SI. Green tea extract protects leptin-deficient, spontaneously obese mice from hepatic steatosis and injury. J Nutr. 2008;138:323-331. [PubMed] |
| 74. | Park HJ, DiNatale DA, Chung MY, Park YK, Lee JY, Koo SI, O‘Connor M, Manautou JE, Bruno RS. Green tea extract attenuates hepatic steatosis by decreasing adipose lipogenesis and enhancing hepatic antioxidant defenses in ob/ob mice. J Nutr Biochem. 2011;22:393-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 113] [Article Influence: 7.5] [Reference Citation Analysis (0)] |