Published online Mar 14, 2015. doi: 10.3748/wjg.v21.i10.3055
Peer-review started: July 12, 2014
First decision: August 15, 2014
Revised: August 21, 2014
Accepted: October 20, 2014
Article in press: October 21, 2014
Published online: March 14, 2015
Processing time: 246 Days and 21.5 Hours
AIM: To investigate the involvement of decaprenyl diphosphate synthase subunit 2 (PDSS2) in development and progression of human hepatocellular carcinoma (HCC).
METHODS: PDSS2 protein expression was examined in well- and poorly differentiated HCC tumor samples. The levels of PDSS2 expression were compared with clinical features and prognosis of HCC patients. The effects of PDSS2 on cell proliferation, cell cycle, apoptosis, cell migration, and invasion in HCC HepG2 cells were also investigated.
RESULTS: PDSS2 was downregulated in poorly differentiated cancer samples compared with well-differentiated tumor samples, and the expression level was markedly lower in HCC tissues than in histologically normal tissue adjacent to the cancer. Reduced protein expression was negatively associated with the status of HCC progression. In addition, overexpression of PDSS2 dramatically suppressed cell proliferation and colony formation, and induced apoptosis in HepG2 cells by inducing G1-phase cell-cycle arrest. The migration and invasion capabilities of HepG2 cells were significantly decreased following PDSS2 overexpression.
CONCLUSION: Decreased PDSS2 expression is an unfavorable prognostic factor for HCC, and PDSS2 has potent anticancer activity in HCC tissues and HepG2 cells.
Core tip: We found that decaprenyl diphosphate synthase subunit 2 (PDSS2) was frequently downregulated in primary hepatocellular carcinoma (HCC), and the level of expression was markedly lower in poorly differentiated cancer samples compared with well-differentiated tumor tissues. Furthermore, the expression of PDSS2 was inversely correlated with clinical stage. Overexpression of PDSS2 in HepG2 cells decreased cell proliferation and induced G1-phase cell cycle arrest and apoptosis in human HCC cells. Moreover, PDSS2 reduced epithelial-mesenchymal transition in HCC.
- Citation: Huang W, Gao F, Li K, Wang W, Lai YR, Tang SH, Yang DH. Decaprenyl diphosphate synthase subunit 2 as a prognosis factor in hepatocellular carcinoma. World J Gastroenterol 2015; 21(10): 3055-3065
- URL: https://www.wjgnet.com/1007-9327/full/v21/i10/3055.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i10.3055
Hepatocellular carcinoma (HCC) is one of the most common aggressive tumors worldwide[1]. Most cases of HCC are secondary to either chronic liver disease from hepatitis B or C virus infection or cirrhosis due to alcohol, obesity, cholestasis, and autoimmune disorders[2-5]. Treatment of HCC remains highly challenging because of the poor prognosis and its potential for recurrence and metastasis even after surgical resection[6-8]. It is important for us to understand the molecular changes associated with HCC occurrence, recurrence and metastasis. Among these changes, the activation of oncogenes and inactivation of tumor suppressor genes may play important roles in tumor formation and development[9-13].
Decaprenyl diphosphate synthase subunit 2 (PDSS2), known as a candidate tumor suppressor protein in non-small cell lung cancer and gastric cancer, plays a significant role in regulating cell proliferation, cell cycle distribution, apoptosis and maintenance of normal tissue homeostasis[14,15]. However, the role of PDSS2 in the pathogenesis of HCC has not been elucidated. Therefore, the aim of this study was to investigate whether PDSS2 is related to the development and progression of human HCC.
Approval from the Jinan University Institute Research Ethics Committee was obtained, and written informed consent was provided by each human subject.
Human HCC HepG2 cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum in a humidified incubator with 5% CO2 at 37 °C.
The biopsies of 33 hepatic cancer patients and 33 non-cancerous tissues were collected from the Department of Pathology at The First Affiliated Hospital of Jinan University of Guangzhou City in China between 2009 and 2012. None of the patients received preoperative radiotherapy or chemotherapy.
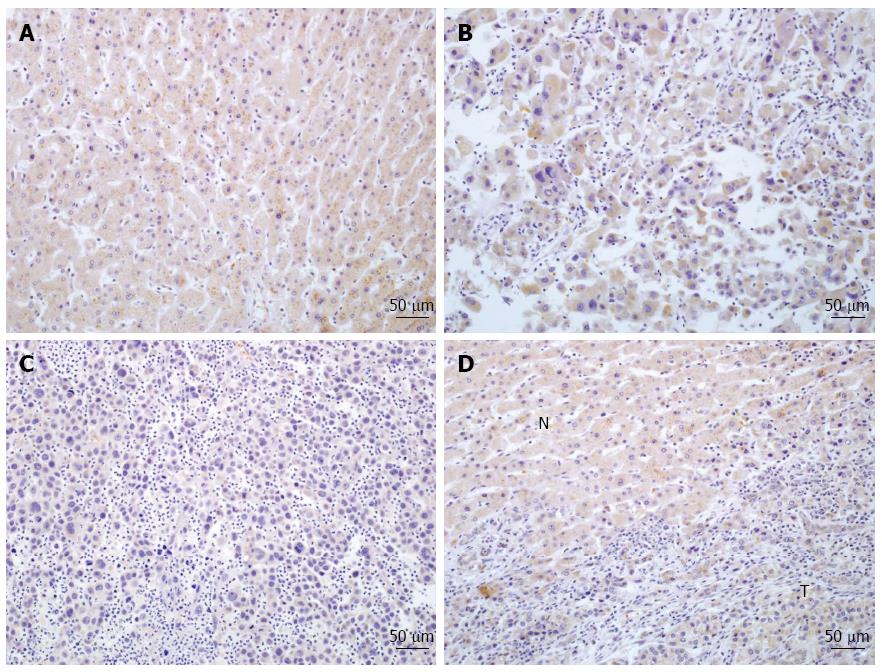
After deparaffinization and rehydration, tissue microarray sections were subjected to high pressure for 2 min for antigenic retrieval. The slides were incubated overnight at 4 °C with PDSS2 antibodies (1:500 dilution; Abcam, Cambridge, MA, United States). The sections were then incubated with diaminobenzidine for 2 min. In every run, the primary antibodies were substituted with PBS for the negative controls. For the evaluation of the immunohistochemistry results, the proportion of stained tumor cells was evaluated using four grades: 0, no positive cells; 1, < 10% positive cells; 2, 10%-50% positive cells; and 3, > 50% positive cells. Similarly, the scoring criteria for the staining intensity were: 0, no staining; 1, weak staining; 2, modest staining; and 3, strong staining. The final score was calculated by multiplying the tumor staining area by the intensity score (0, 1, 2, 3, 4, 6, and 9). According to this method of assessment, staining scores ≤ 4 and ≥ 6 were regarded as tumors with low and high expression, respectively.
For the mRNA analyses, the total RNA was extracted using Trizol Reagent (Takara, Otsu, Japan) and reverse transcribed using PrimeScript RT reagent Kit (Takara) according to the protocols provided by the manufacturer. The qRT-PCR was performed on a Stratagene Mx3005P qRT-PCR system using the SYBR Green qRT-PCR master mix (Takara). The primers used for the amplification of the indicated genes are listed in Table 1. All of the samples were normalized to the internal controls (glyceraldehyde-3-phosphate dehydrogenase), and the fold changes were calculated by relative quantification (2-ΔΔCt).
| Gene | Forward primer (5'-3') | Reverse primer (5'-3') |
| E-cadherin | TGCCCAGAAAATGAAAAAGG | GTGTATGTGGCAATGCGTTC |
| N-cadherin | ACAGTGGCCACCTACAAAGG | CCGAGATGGGGTTGATAATG |
| Vimentin | GAGAACTTTGCCGTTGAAGC | GCTTCCTGTAGGTGGCAATC |
| Fibronectin | CAGTGGGAGACCTCGAGAAG | TCCCTCGGAACATCAGAAAC |
| Cyclin A2 | TGCTGGAGCTGCCTTTCATT | TGAAGGTCCATGATACAAGGCT |
| Cyclin D1 | CAGGCGGCTCTTTTTCAC | CCCTCGGTGTCCTACTTCAA |
| Cyclin D2 | CTGTGTGCCACCGACTTTAAGTT | GATGGCTGCTCCCACACTTC |
| Cyclin D3 | TGGATGCTGGAGGTATGTG | CGTGGTCGGTGTAGATGC |
| PDSS2 | GGACTATGCTAAGTTGCGAGAA | GGTCACAGCAAACACAATGT |
| Confirm FN = fibronectin |
Protein lysates were separated by 10%-15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, electrophoretically transferred to a polyvinylidene difluoride membrane (Millipore of Merck KGaA, Billerica, MA, United States), and incubated with the following antibodies: PDSS2, and E-cadherin, N-cadherin, vimentin, and fibronectin (Santa Cruz Biotechnology, Dallas, TX, United States). The membrane was then incubated with horseradish peroxidase-labeled goat-anti-mouse or rabbit IgG, and the proteins were detected using a high sensitivity chemiluminescence imaging system (Biorad Laboratories Inc., Hercules, CA, United States). Glyceraldehyde-3-phosphate dehydrogenase was used as the protein-loading control.
Cells (1 × 104) were plated onto 96-well plates in 80% growth medium and allowed to adhere overnight. At different time points (4 h and 1, 2 and 3 d), the culture medium was removed and replaced with culture medium containing CellTiter 96 Aqueous One Solution Reagent (Promega Corp., Madison, WI, United States). After incubation at 37 °C for 4 h, spectrometric absorbance at 490 nm was measured using a microplate photometer (Thermo Fisher Scientific, Waltham, MA, United States).
For cell cycle analysis, cells were plated in 6-well plates at a density of 2 × 105 cells per well and transfected with miRNAs. At 48 h post-transfection, the cell cycle distribution was analyzed by propidium iodide (PI) staining and flow cytometry.
Cells were plated in 6-well plates at a density of 400 cells per well and grown for 2 wk. The cells were then washed twice with PBS, fixed with methanol/acetic acid (3:1, v/v), and stained with hematoxylin. Then, the number of colonies was counted.
An Annexin V-FITC Apoptosis Detection Kit (Keygen Biotech, Nanjing, China) was used for quantification of apoptosis. Cells were spun down to remove supernatant and resuspended in 100 μL of binding buffer. Then, 5 μL of Annexin V-FITC and 5 μL PI were added into the solution. After 15 min of incubation, 400 μL of Annexin V binding buffer was added. The Annexin V-FITC and PI stained cells were analyzed by the FL1 and FL2 channels.
For the cell migration assay, 1 × 105 cells in 100 μL RPMI 1640 medium without newborn calf serum were seeded on a fibronectin-coated polycarbonate membrane insert in a Transwell apparatus (Corning Inc., Corning, NY, United States). In the lower chamber, 500 μL RPMI 1640 with 10% calf serum was added as a chemoattractant. After the cells were incubated for 20-24 h at 37 °C in a 5% CO2 atmosphere, the insert was washed with PBS, and cells on the top surface of the insert were removed with a cotton swab. Cells adhering to the lower surface were fixed with methanol, stained with hematoxylin and counted under a microscope in five predetermined fields (200× magnification). All assays were independently repeated at least three times. For the cell invasion assay, the Transwell membranes were precoated with 24 μg/μL Matrigel (Becton Dickinson and Co., Franklin Lakes, NJ, United States) and the cells adhering to the lower surface were counted in the same manner.
Cells in each well were scratched using the tip of a sterile 10 μL pipette (width: approximately 1 mm). The plates were washed twice with PBS in order to remove the detached cells, and incubated at 37 °C in 5% CO2. Wound closure was monitored at various time points by observation under a microscope, and the degree of cell migration was quantified by the ratio of gap distance at 48 h to that at 0 h. The experiment was done in triplicate.
One-way analyses of variance were conducted for comparisons among groups using the SPSS 13.0 software (SPSS Inc., Chicago, IL, United States). The data are presented as the mean ± SE of at least three independent experiments unless otherwise; P < 0.05 was considered as statistically significant.
Immunohistochemistry was used to determine the expression and subcellular localization of PDSS2 protein in 33 archived paraffin-embedded HCC samples and 33 matched histologically normal or non-tumoral adjacent tissue. Decreased cytoplasmic expression of PDSS2 was observed in HCC samples compared to non-cancerous tissues, and the expression level of PDSS2 was significantly lower in poorly differentiated cancer samples than in well-differentiated tumor tissues (P < 0.05) (Figure 1, Table 2).
| Characteristics | n | PDSS2 expression | P value | |
| Low | High | |||
| Group | ||||
| HCC | 33 | 13 | 20 | 0.026 |
| Non-cancerous tissue | 33 | 5 | 28 | |
| Gender | ||||
| Male | 5 | 2 | 3 | 0.6691 |
| Female | 28 | 11 | 17 | |
| Age, yr | ||||
| ≥ 45 | 21 | 7 | 14 | 0.282 |
| < 45 | 12 | 6 | 6 | |
| AFP expression | ||||
| Normal | 18 | 5 | 13 | 0.221 |
| High level | 15 | 8 | 7 | |
| ALT expression | ||||
| Normal | 17 | 7 | 10 | 0.556 |
| High level | 16 | 6 | 10 | |
| HbsAg | ||||
| Positive | 21 | 9 | 12 | 0.436 |
| Negative | 12 | 4 | 8 | |
| Tumor size | ||||
| ≥ 5 cm | 20 | 5 | 15 | 0.004 |
| < 5 cm | 13 | 8 | 5 | |
| Differentiation | ||||
| Well | 19 | 4 | 15 | 0.015 |
| Poor | 14 | 9 | 5 | |
| Clinical stage | ||||
| I + II | 18 | 3 | 15 | 0.005 |
| III + IV | 15 | 10 | 5 | |
The relationship between clinicopathologic characteristics and PDSS2 expression in individuals with HCC is summarized in Table 2. PDSS2 expression levels were not associated with patient age, gender, or hepatitis B surface antigen, alpha-fetoprotein or alanine aminotransferase expression. However, the expression level of PDSS2 was inversely correlated with tumor size and clinical stage (I-II vs III-IV) in HCC patients (Ps < 0.05).
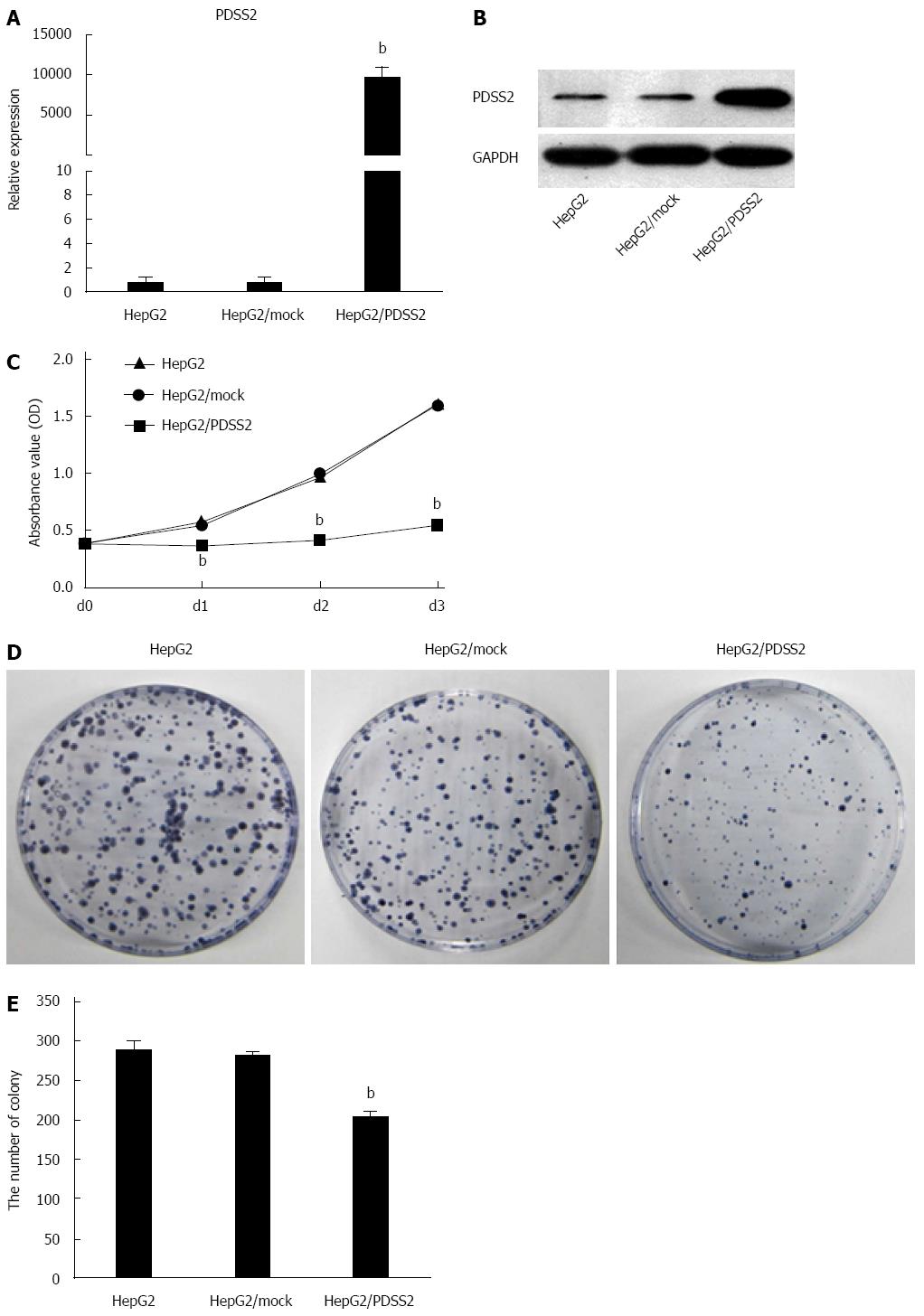
To examine its biologic function, PDSS2 was overexpressed in HCC HepG2 cells; increased mRNA and protein expression was confirmed by qRT-PCR and Western blot (Figure 2A and B). Analysis of proliferation rates showed that PDSS2 overexpression reduced the growth rate of HepG2 cells compared to control cells over a three-day period (P < 0.05) (Figure 2C). Similarly, PDSS2-expressing cells formed a significantly decreased number of colonies compared to the control cells over a two-week period (P < 0.05) (Figure 2D and E).
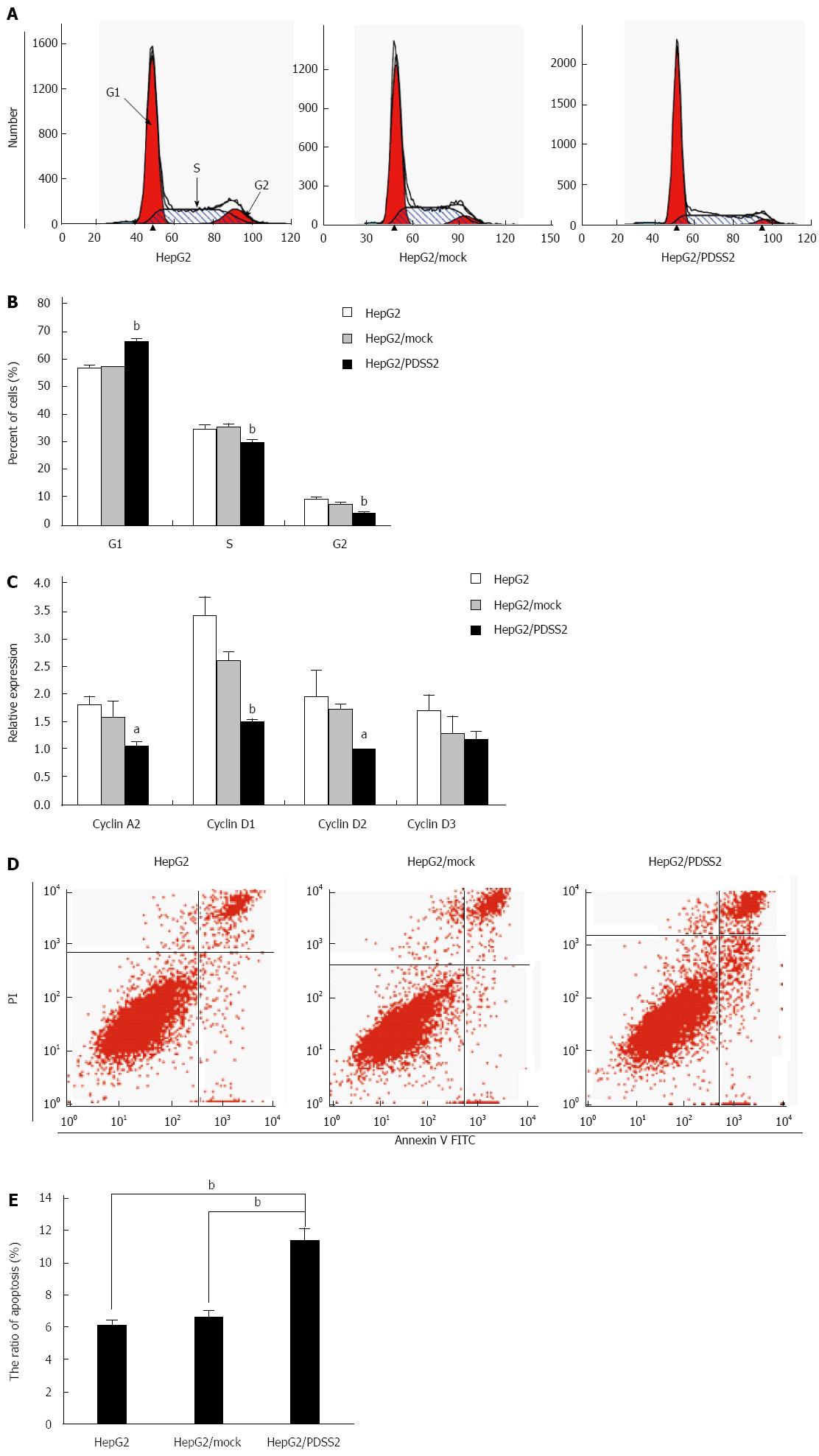
To explore the effect of PDSS2 on cell cycle, HepG2 cells were transiently transfected with PDSS2 and cell cycle distribution was examined. As shown in Figure 3A and B, compared with blank HepG2 cells and control cells transfected with pcDNA3.1 (mock), HepG2 cells transfected with PDSS2 displayed an increased percentage of cells in G1 phase and fewer cells in S phase. To reveal whether cell-cycle regulators were involved in the growth inhibition of PDSS2, we analyzed mRNA levels of four cell-cycle regulators in PDSS2-expressing HepG2. The levels of cyclins A2, D1, D2, and D3 were decreased after PDSS2 overexpression (Figure 3C). In addition, PDSS2-overexpressing HepG2 cells demonstrated an increased rate of apoptosis (11.44% ± 0.69% vs 6.72% ± 0.35% and 6.22% ± 0.21% in controls).
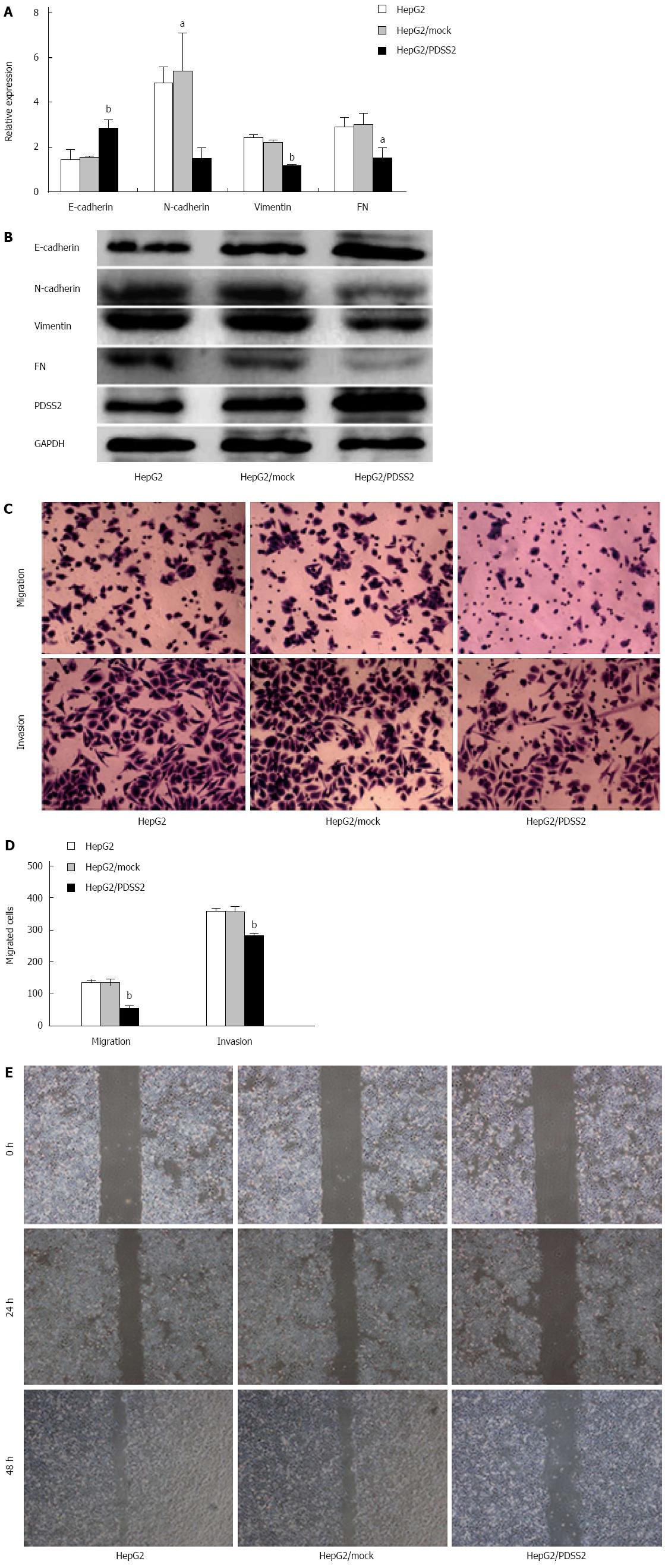
In order to determine whether PDSS2 reduces epithelial-mesenchymal transition, the expressions of an epithelial marker (E-cadherin) and mesenchymal markers (N-cadherin, vimentin, and fibronectin) were measured. PDSS2 overexpression resulted in an upregulation of E-cadherin and downregulation of N-cadherin, vimentin, and fibronectin mRNA and protein (Figure 4A and B).
Migration and invasion assays were conducted to examine the effect of PDSS2 overexpression. PDSS2-expressing HepG2 cells exhibited significantly decreased mobility compared with control cells (Ps < 0.05) (Figure 4C and D). The result was confirmed using a scratch migration assay (Figure 4E).
HCC is often diagnosed at an advanced stage when most potentially curative therapies, such as surgical resection, transplantation or percutaneous and transarterial interventions, are of limited efficacy[16]. In addition, HCC is insensitive to systemic chemotherapy and radiotherapy[17,18]. There is a critical need to explore new therapeutic approaches in HCC, and gene therapy has emerged as a viable alternative.
PDSS2 is an enzyme that synthesizes the prenyl side-chain of coenzyme Q, an essential electron carrier in the respiratory chain. Homozygous mutations in the gene encoding PDSS2 lead to severe neuromuscular disease, Leigh syndrome and nephrotic syndrome[19]. An increasing body of evidence suggests that PDSS2 is a tumor suppressor gene in certain cancers, such as melanoma, gastric cancer and non-small cell lung cancer[13,14,20]. Our study shows that PDSS2 is frequently downregulated in primary HCC, and the expression of PDSS2 is markedly lower in poorly differentiated compared to well-differentiated tumor tissues. Furthermore, the expression of PDSS2 was inversely correlated with clinical stage. These findings indicate that reduced PDSS2 expression is negatively associated with the status of HCC progression, and suggest that PDSS2 has a suppressive role of in HCC tumorigenesis (i.e., loss of expression of PDSS2 may promote HCC initiation and progression). PDSS2 could therefore be used as a prognostic biomarker for HCC, pending further verification in a larger cohort of clinical patients.
Overexpression of PDSS2 in HepG2 cells decreased in vitro cell proliferation, which was consistent with our previous investigation. Moreover, the results show that PDSS2 induces G1-phase cell cycle arrest and apoptosis in human HCC cells.
Metastasis is a basic biologic characteristic of malignant tumors, and reports have suggested that epithelial-mesenchymal transition endows cells with migratory and invasive properties, and prevents apoptosis and senescence[21-26]. The results of our current study indicate a pivotal role for PDSS2 in the progression of HCC through the reduction of the epithelial-mesenchymal transition, which remains to be fully characterized.
The complex regulatory machineries associated with PDSS2 during anticancer activity have not been fully elucidated. Hence, further investigations of the underlying signaling network that regulates these PDSS2-associated pathways through bioinformatics predictions, use of various PDSS2 mutants, and different pathway inhibitors will provide important insights into the precise role of PDSS2 in HCC.
Hepatocellular carcinoma (HCC) is one of the most common aggressive tumors worldwide. The activation of oncogenes and inactivation of tumor suppressor genes may play important roles in tumor formation and development. Decaprenyl diphosphate synthase subunit 2 (PDSS2), known as a candidate tumor suppressor protein in non-small cell lung cancer and gastric cancer, plays a significant role in regulating cell proliferation, cell cycle distribution, apoptosis and maintenance of normal tissue homeostasis.
The roles of PDSS2 in the pathogenesis of HCC are not well elucidated, which prompted us to investigate whether PDSS2 is related to the development and progression of human HCC.
The authors found that PDSS2 was frequently downregulated in primary HCC, and the expression level of PDSS2 was markedly lower in poorly differentiated cancer samples than in well-differentiated tumor tissues. Furthermore, the expression of PDSS2 was inversely correlated with clinical stage. These results indicate that reduced PDSS2 expression is negatively associated with the status of HCC progression. Overexpression of PDSS2 in HepG2 cells decreased cell proliferation, and induced G1-phase cell cycle arrest and apoptosis in human HCC cells. Moreover, the results of our current study indicate a pivotal role for PDSS2 in the progression of HCC through the reduction of epithelial-mesenchymal transition.
This study demonstrates that decreased PDSS2 expression is an unfavorable prognostic factor for HCC, and PDSS2 has potent anticancer activity in HCC tissues and HepG2 cells.
Epithelial-mesenchymal transition or transformation is a phenomenon in embryonic development, and has been gradually accepted as a potential mechanism underlying cancer progression and metastasis.
This article is aimed to elucidate the involvement of decaprenyl diphosphate synthase subunit 2 in the development and progression of HCC.
P- Reviewer: Romero MR, Tanaka T S- Editor: Qi Y L- Editor: AmEditor E- Editor: Ma S
| 1. | McGlynn KA, London WT. The global epidemiology of hepatocellular carcinoma: present and future. Clin Liver Dis. 2011;15:223-243, vii-x. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 360] [Cited by in RCA: 375] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 2. | Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35-S50. [PubMed] |
| 3. | Venook AP, Papandreou C, Furuse J, de Guevara LL. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist. 2010;15 Suppl 4:5-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 626] [Cited by in RCA: 737] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 4. | McMahon BJ. The natural history of chronic hepatitis B virus infection. Hepatology. 2009;49:S45-S55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 556] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 5. | Cao GW. Clinical relevance and public health significance of hepatitis B virus genomic variations. World J Gastroenterol. 2009;15:5761-5769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 102] [Cited by in RCA: 116] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 6. | Olsen SK, Brown RS, Siegel AB. Hepatocellular carcinoma: review of current treatment with a focus on targeted molecular therapies. Therap Adv Gastroenterol. 2010;3:55-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Abou-Alfa GK. Hepatocellular carcinoma: molecular biology and therapy. Semin Oncol. 2006;33:S79-S83. [PubMed] |
| 8. | Shariff MI, Cox IJ, Gomaa AI, Khan SA, Gedroyc W, Taylor-Robinson SD. Hepatocellular carcinoma: current trends in worldwide epidemiology, risk factors, diagnosis and therapeutics. Expert Rev Gastroenterol Hepatol. 2009;3:353-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 226] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 9. | Imbeaud S, Ladeiro Y, Zucman-Rossi J. Identification of novel oncogenes and tumor suppressors in hepatocellular carcinoma. Semin Liver Dis. 2010;30:75-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Lee JS, Thorgeirsson SS. Comparative and integrative functional genomics of HCC. Oncogene. 2006;25:3801-3809. [PubMed] |
| 11. | Ma NF, Hu L, Fung JM, Xie D, Zheng BJ, Chen L, Tang DJ, Fu L, Wu Z, Chen M. Isolation and characterization of a novel oncogene, amplified in liver cancer 1, within a commonly amplified region at 1q21 in hepatocellular carcinoma. Hepatology. 2008;47:503-510. [PubMed] |
| 12. | Li Z, Huang X, Zhan H, Zeng Z, Li C, Spitsbergen JM, Meierjohann S, Schartl M, Gong Z. Inducible and repressable oncogene-addicted hepatocellular carcinoma in Tet-on xmrk transgenic zebrafish. J Hepatol. 2012;56:419-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 13. | Shanbhogue AK, Prasad SR, Takahashi N, Vikram R, Sahani DV. Recent advances in cytogenetics and molecular biology of adult hepatocellular tumors: implications for imaging and management. Radiology. 2011;258:673-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Chen P, Yu J, Knecht J, Chen Q. Decrease of PDSS2 expression, a novel tumor suppressor, in non-small cell lung cancer. Cancer Epidemiol. 2013;37:166-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Chen P, Zhao SH, Chu YL, Xu K, Zhu L, Wu Y, Song J, Cao CX, Xue X, Niu YY. Anticancer activity of PDSS2, prenyl diphosphate synthase, subunit 2, in gastric cancer tissue and the SGC7901 cell line. Anticancer Drugs. 2009;20:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Pei Y, Zhang T, Renault V, Zhang X. An overview of hepatocellular carcinoma study by omics-based methods. Acta Biochim Biophys Sin (Shanghai). 2009;41:1-15. [PubMed] |
| 17. | Avila MA, Berasain C, Sangro B, Prieto J. New therapies for hepatocellular carcinoma. Oncogene. 2006;25:3866-3884. [PubMed] |
| 18. | Herskovic A, Martz K, al-Sarraf M, Leichman L, Brindle J, Vaitkevicius V, Cooper J, Byhardt R, Davis L, Emami B. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med. 1992;326:1593-1598. [PubMed] |
| 19. | Peng M, Falk MJ, Haase VH, King R, Polyak E, Selak M, Yudkoff M, Hancock WW, Meade R, Saiki R. Primary coenzyme Q deficiency in Pdss2 mutant mice causes isolated renal disease. PLoS Genet. 2008;4:e1000061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 102] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 20. | Fung JM, Smith R, Brown MA, Lau SH, Xie D, Lau GK, Guan XY. Identification and characterization of a novel melanoma tumor suppressor gene on human chromosome 6q21. Clin Cancer Res. 2009;15:797-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Osada T, Sakamoto M, Ino Y, Iwamatsu A, Matsuno Y, Muto T, Hirohashi S. E-cadherin is involved in the intrahepatic metastasis of hepatocellular carcinoma. Hepatology. 1996;24:1460-1467. [PubMed] |
| 22. | Miyoshi A, Kitajima Y, Sumi K, Sato K, Hagiwara A, Koga Y, Miyazaki K. Snail and SIP1 increase cancer invasion by upregulating MMP family in hepatocellular carcinoma cells. Br J Cancer. 2004;90:1265-1273. [PubMed] |
| 23. | Zheng F, Liao YJ, Cai MY, Liu YH, Liu TH, Chen SP, Bian XW, Guan XY, Lin MC, Zeng YX. The putative tumour suppressor microRNA-124 modulates hepatocellular carcinoma cell aggressiveness by repressing ROCK2 and EZH2. Gut. 2012;61:278-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 321] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 24. | Na DC, Lee JE, Yoo JE, Oh BK, Choi GH, Park YN. Invasion and EMT-associated genes are up-regulated in B viral hepatocellular carcinoma with high expression of CD133-human and cell culture study. Exp Mol Pathol. 2011;90:66-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Fabregat I. Dysregulation of apoptosis in hepatocellular carcinoma cells. World J Gastroenterol. 2009;15:513-520. [PubMed] |
| 26. | Yu H, Shen H, Zhang Y, Zhong F, Liu Y, Qin L, Yang P. CAV1 promotes HCC cell progression and metastasis through Wnt/β-catenin pathway. PLoS One. 2014;9:e106451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |