Published online Mar 14, 2015. doi: 10.3748/wjg.v21.i10.3049
Peer-review started: June 3, 2014
First decision: July 21, 2014
Revised: August 29, 2014
Accepted: September 29, 2014
Article in press: September 30, 2014
Published online: March 14, 2015
Processing time: 286 Days and 21 Hours
AIM: To evaluate the prognostic value of pretreatment FDG positron emission tomography computed tomography (PET-CT) in patients with hepatocarcinoma treated by liver transplantation (LT).
METHODS: The authors retrospectively analyzed the data of 27 patients (mean age 58 ± 9 years) who underwent FDG PET-CT before LT for hepatocarcinoma. Mean follow-up was 26 ± 18 mo. The FDG PET/CT was performed according to a standard clinical protocol: 4 MBqFDG/kg body weight, uptake 60 min, low-dose non-enhanced CT. The authors measured the SUVmax and SUVmean of the tumor and the normal liver. The tumor/liver activity ratios (RSUVmax and RSUVmean) were tested as prognostic factors and compared to the following conventional prognostic factors: MILAN, CLIP, OKUDA, TNM stage, alphafoetoprotein level, portal thrombosis, size of the largest nodule, tumor differentiation, microvascular invasion, underlying cirrhosis and liver function.
RESULTS: Overall and recurrence free survivals were 80.7% and 67.4% at 3 years, and 70.6% and 67.4% at 5 years, respectively. According to a multivariate Cox model, only FDG PET/CT RSUVmax predicted recurrence free survival. Even though the MILAN criteria alone were not predictive, it is worth noting that none of the patients outside the MILAN criteria and with RSUVmax < 1.15 relapsed.
CONCLUSION: FDG PET/CT with an RSUVmax cut-off value of 1.15 is a strong prognostic factor for recurrence and death in patients with HCC treated by LT in this retrospective series. Further prospective studies should test whether this metabolic index should be systematically included in the preoperative assessment.
Core tip: Patients suffering from hepatocarcinoma are selected for liver transplantation (LT) according to the Milan criteria that were established two decades ago. The aggressiveness of the tumor has also a particular importance, but there is still no ideal way of predicting the risk of recurrence according to pretransplant tumor metabolism. This study confirms that FDG positron emission tomography computed tomography with a tumor/liver activity ratios (RSUVmax) cut-off value of 1.15 is a strong prognostic factor for recurrence and death in patients with hepatocellular cancer (HCC) treated by LT. In addition, in this series, none of the patients outside the MILAN criteria with RSUVmax < 1.15 suffered from recurrence in the follow-up. Further prospective studies should test whether this metabolic index should be systematically included in the pretransplant assessment of HCC patients.
- Citation: Detry O, Govaerts L, Deroover A, Vandermeulen M, Meurisse N, Malenga S, Bletard N, Mbendi C, Lamproye A, Honoré P, Meunier P, Delwaide J, Hustinx R. Prognostic value of 18F-FDG PET/CT in liver transplantation for hepatocarcinoma. World J Gastroenterol 2015; 21(10): 3049-3054
- URL: https://www.wjgnet.com/1007-9327/full/v21/i10/3049.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i10.3049
Hepatocellular carcinoma (HCC) is the fifth most common cancer, and the third cause of cancer related-death worldwide. HCC incidence is particularly elevated in regions where hepatitis-B virus infection is endemic[1], but is also rising in Western countries[2]. Liver transplantation (LT) has been established as the standard of care in selected candidates with underlying cirrhosis. However, the scarcity of organ donors has forced the development of strict criteria to limit LT to patients who are likely to have excellent outcomes. The universally accepted LT criteria for HCC are the Milan criteria (1 nodule ≤ 5 cm or 3 nodules ≤ 3 cm) that lead to a very low rate of post-LT recurrence[3], but many patients suffers from HCC outside the Milan criteria at the time of diagnosis. It is considered that some of these patients may benefit from LT with an acceptable risk of recurrence. This fact leads to the extension of LT criteria for HCC, as in the University of California San Francisco (UCSF)[4] criteria or up-to-seven criteria[5].
However, it is clear that size and number of tumor nodules are not sufficient to precisely predict the risk of post LT HCC recurrence, and that the aggressiveness or differentiation of HCC should be taken into consideration. Microvascular invasion, AFP levels[6], and recently captation of 18F-fluorodeoxyglucose (18FDG) at positron emission tomography (PET)[7,8] have all been proposed to evaluate the biological staging of HCC.
At the University of Liege LT program, 18FDG- PET/ computed tomography (CT) has been introduced in the pretransplant evaluation of LT candidates suffering from HCC. The objective of this study was to retrospectively analyze the value of 18FDG- PET/CT in predicting post-LT recurrence of HCC by comparison with other prognostic factors.
This study is a retrospective evaluation of the 52 patients suffering from HCC and transplanted at the University of Liege hospital transplantation center between January 2006 and December 2011. Amongst these patients, 41 underwent 18FDG- PET/ CT evaluation before transplantation. Five patients were excluded as they had a past history of unrelated neoplasia, five others as they had undergone neoadjuvant therapy (mainly chemoembolization) prior to 18FDG- PET/ CT, 3 patients were lost to follow-up, and in one patient pathology of the explanted liver showed total tumoral necrosis. Twenty-seven patients were therefoe available for complete retrospective evaluation and their basic characteristics are presented in Table 1. Amongst the 27 patients, 13 suffered from HCC within the Milan criteria according to radiology and were granted standard exception (SE) status within the patient-oriented Eurotransplant liver graft allocation, and 14 were classified outside Milan criteria and received their liver graft in a centre-oriented rescue allocation[9]. Nine patients underwent neo-adjuvant chemoembolization between PET/CT and LT. After transplant, basic immunosuppression consisted of regular triple therapy using tacrolimus, mycophenolate mofetil, and steroids that were progressively withdrawn after 4 wk.
| Characteristics | mean ± SD or n | Ranges | |
| Age (yr) | 58 ± 10 | 29-72 | |
| Gender (Male/Female) | 24/3 | ||
| Underlying liver disease | |||
| Alcohol | 8 | ||
| Viral | 15 | ||
| Other cirrhosis | 2 | ||
| Non-cirrhotic liver | 2 | ||
| AFP at transplant (ng/mL) | 199 ± 476 | 0.9-1957 | |
| Milan at listing | In/out | 13/14 | |
| Neoadjuvant treatment | Yes/no | 9/18 | |
| CHILD | A/B/C | 9/10/8 | |
| OKUDA | I/II/III | 7/15/5 | |
| CLIP | 0/1/2/3/4 | 1/7/9/6/4 | |
| Pathology | |||
| Number of nodules | 1/2/3/> 3 | 5/6/3/13 | |
| Size of largest lesion (cm) | 3.8 ± 2.2 | 1.5-1.5 | |
| Differentiation | Low/intermediate/high grade | 1/13/13 | |
| Microvascular invasion | N/Y | 17/10 | |
| pTNM (7th) | T1/T2/T3a/T3b/T4 | 4/16/5/1/1 | |
| pTNM (Yao) | T1/T2/T3a/T3b/T4a/T4b | 0/8/5/1/12/1 |
Collected clinical data included age, gender, viral status, Child-Turcotte-Pugh classification, tumor stage, tumor number, preoperative alphafoetoprotein (AFP) levels, the Okuda score[10], the Cancer of the Liver Italian Program (CLIP) score[11], histological grade, vascular invasion, recurrence and date of recurrence, and survival. Data are presented as mean ± SD and ranges. Median post transplantation follow-up was 732 d (range: 37-2016 d). Mean interval between 18FDG- PET/ CT evaluation and LT was 77 d (range: 7-363 d).
18FDG- PET/ low-dose CT were performed in a standard manner using Gemini TF 16 and Gemini Big Bore scanners (Philips, Amsterdam, The Netherlands). Patients were fasted at least 6 h before injection of 4 MBq/kg of 18FDG. Patients’ glycaemia was checked and lower than 140 mg/dL before 18FDG injection, and they received 500 mL NaCl 0.9% intravenously after 18FDG injection and before image acquisition. Static emission scanning was performed 60 min after 18FDG injection. The 18FDG- PET/CT images were first visually analyzed, then were semi-quantitatively evaluated to assess whether the 18FDG uptake in the tumor was significantly higher than the surrounding hepatic tissue. Regions of interest (ROI) were drawn over the normal liver and the tumor, and the standardized uptake values (SUV) in each ROI was measured. The ROI was drawn to encircle the highest activity of each tumor, with guidance from the CT scans that were acquired from PET/CT. The maximum SUV (SUVmax), the mean SUV (SUVmean), the ratio of tumor SUVmax to normal liver SUVmax (TSUVmax/LSUVmax), and the ratio of tumor SUVmax to normal liver SUVmean (TSUVmax/ LSUVmean) were calculated as described[12].
Data were analysed using the Prism 6.0c software for Macintosh OSX (GraphPad Software, San Diego, CA). Mean values ± SD and ranges are presented. A receiver operating curve (ROC) analysis was performed in order to define the optimal cut-off for the metabolic variables to predict the outcome. Survival rates were calculated with the Kaplan-Meier method and compared with the log-rank (Mantel-Cox) test. Parameters being predictive for global survival and recurrence-free survival were assessed in a univariate analysis. All variables with a P value less than 0.05 were then included in a multivariate analysis applying the Cox multiple backward stepwise model to identify parameters being independently predictive. A value of P < 0.05 was considered significant.
The characteristics of the 27 patients are presented in Table 1. The majority of patients were male, older than 50 years-of-age, and suffering from viral or post-alcoholic cirrhosis. Amongst patients with viral cirrhosis, 8 had past hepatitis B virus infection, 7 hepatitis C virus related liver disease and one had both.
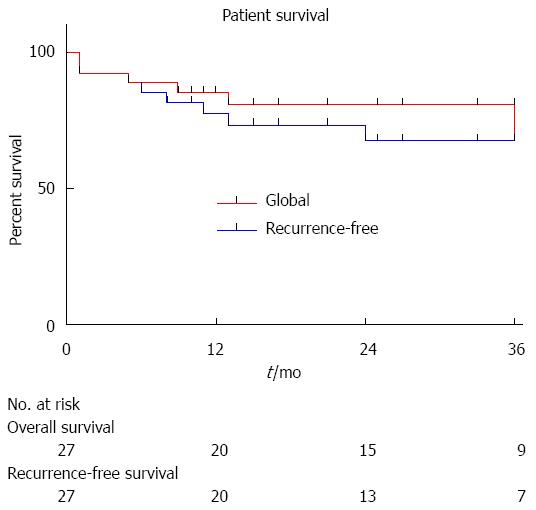
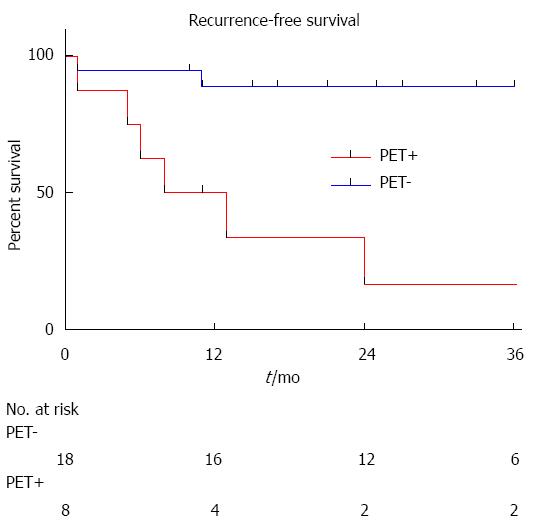
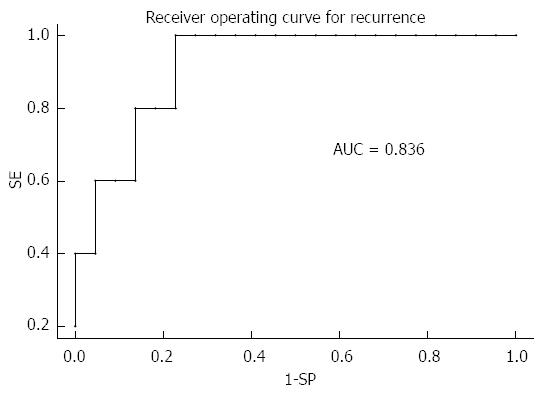
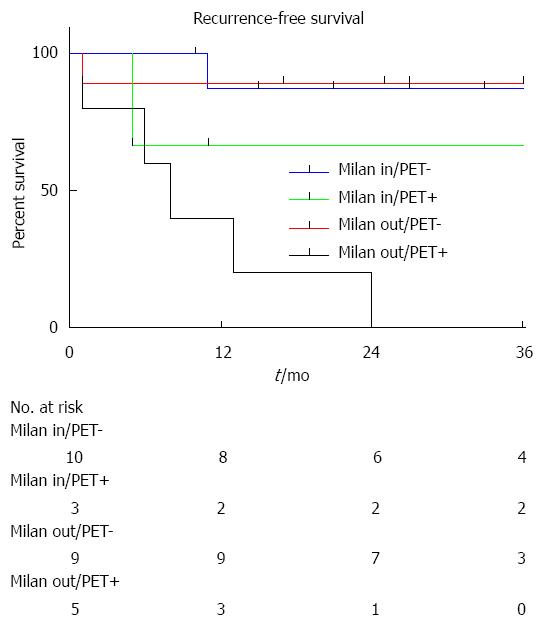
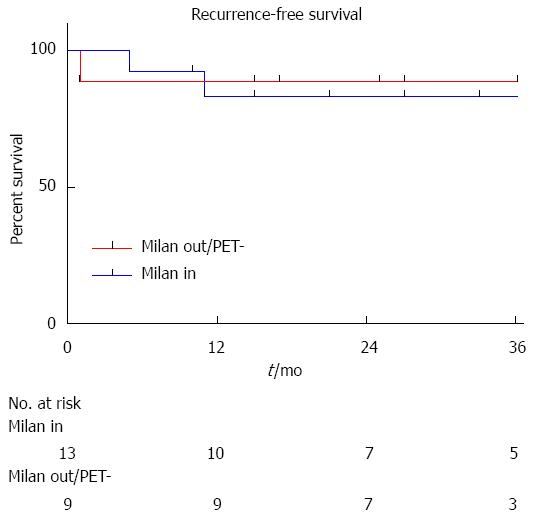
During the follow-up period, 5 patients developed HCC recurrence and 6 patients died. Within the whole series, one-, three- and five-year global patient survivals were 85.2%, 80.7% and 70.6%, respectively. One-, three- and five-year recurrence-free survivals were 77.4%, 67.4% and 67.4%, respectively (Figure 1). When comparing recurrence-free survivals according to 18FDG intake, there was a significant difference between 18FDG avid HCC compared to non-avid tumors P < 0.001) (Figure 2). The ROC curve analysis showed that 1.15 was the optimal cut-off value for predicting tumor recurrence, using the tumor to liver SUVmax activity ratios (Figure 3). When considering combination of the Milan criteria and 18FDG, there was a significantly worse survival rate for patients transplanted for 18FDG avid HCC outside the Milan criteria (Figure 4) compared to the other groups of patients (P < 0.001), with 0% recurrence-free survival at 2 years. Interestingly, there was no difference of recurrence-free survival between patients with HCC within the Milan criteria and the patients outside Milan criteria but who had 18FDG negative HCC (P = 0.782) (Figure 5).
According to univariate analysis, TSUVmax/LSUVmax, TSUVmean/ LSUVmean were prognostic factors for survival without HCC recurrence, and TSUVmax/LSUVmax, TSUVmean/ LSUVmean, the size of the largest nodule, and the CLIP classification were prognostic factors for global survival in this series (Table 2). According to multivariate analysis, only TSUVmax/LSUVmax was a prognostic factor for survival without HCC recurrence (HR = 14.38; P = 0.0176).
| Recurrence free survival | Global survival | |||
| HR | P value | HR | P value | |
| TSUVmax/LSUVmax > 1.15 | 14.4 | 0.01 | 5.62 | 0.04 |
| TSUVmean/LSUVmean > 1.15 | 14.4 | 0.01 | 5.62 | 0.04 |
| Pretransplant treatment | 1.01 | 0.99 | 0.32 | 0.30 |
| AFP | 0.99 | 0.47 | 1.00 | 0.52 |
| Milan | 3.97 | 0.21 | 1.97 | 0.43 |
| Portal vein thrombosis | 5.56 | 0.06 | 0.13 | 0.14 |
| TNM (7th) | 1.93 | 0.36 | 1.93 | 0.30 |
| Differentiation | ||||
| Low grade | 20.3 | 0.05 | 12.4 | 0.08 |
| Intermediate grade | 2.37 | 0.46 | 2.78 | 0.37 |
| High grade | 1.00 | |||
| Number of nodules | > 107 | 0.99 | > 107 | 0.99 |
| Size of the largest nodule | 1.29 | 0.05 | 1.33 | 0.009 |
| Microvascular invasion | 3.32 | 0.19 | 1.88 | 0.44 |
| OKUDA | 1.54 | 0.51 | 2.92 | 0.09 |
| CLIP | 1.26 | 0.60 | 2.39 | 0.03 |
This retrospective study confirms that 18FDG- PET with a TSUVmax/LSUVmax cut-off of 1.15 could be used as a selection criteria in the setting of LT for HCC. Particularly, HCC with a 18FDG RSUVmax < 1.15 uptake seem to have a low risk of recurrence after LT. It is therefore possible that 18FDG-PET could be used as a means of enlarging the Milan criteria for LT, allowing transplantation of HCC patients outside the Milan criteria but with an 18FDG RSUVmax < 1.15 uptake with good chances of long term recurrence free survival.
The standard care for curative management of HCC in Western countries remains surgical resection and/or LT. Compared to hepatectomy, LT has two major advantages: first LT is possible in patients with impaired liver function that would not withstand liver resection, and second, as LT removes the whole cirrhotic liver, it avoids the main cause of HCC recurrence i.e., the development of a second HCC within the diseased liver. However, LT availability is limited by the number of available (deceased or living) grafts[13], and LT carries a high risk of recurrence and death if the HCC is not limited to the removed diseased liver. For these reasons, prognostic criteria for long-term recurrence-free survival after LT have been evaluated for more than 30 years. Up to now, the Milan criteria have still been universally used as the best criteria for recurrence-free survival after LT for HCC, but these criteria are not ideal. Firstly, the Milan criteria are pretransplant radiologic criteria that were evaluated more than two decades ago, and liver imaging and particularly MRI are now revealing hypervascularized nodules that could not be detected at the time of the Milan study. In addition, the Milan criteria are very restrictive and a small proportion of patients with HCC are diagnosed within the Milan criteria; finally, it is now clear that the size and number of HCC nodules are not the only prognostic factors for recurrence, and that somehow the aggressiveness of tumors should be taken into account. Tumor differentiation and AFP levels are now evaluated as markers of tumor aggressiveness, and AFP is included in the liver graft allocation scheme in France. Differentiation is difficult to use clinically, as not all HCC are biopsied before LT, and that differentiation of HCC may vary between tumoral nodules, or even within the same nodule. In this setting, 18FDG-PET could be a useful non-invasive pretransplant tool that could evaluate the metabolism and the aggressiveness of the primary HCC tumor, and could also detect the extrahepatic spread of cancer[7,14,15].
This study confirms the experience of other groups evaluating the role of 18FDG-PET in the pretransplant evaluation of HCC patients. This series confirms that TSUVmax/LSUVmax cut-off of 1.15 is probably the best level that should be used to characterize HCC for LT. In addition, this series shows that patients with Milan out HCC with a 18FDG RSUVmax < 1.15 uptake could benefit from LT[16]. In our series, recurrence-free survival of Milan out/18FDG PET negative patients was not decreased compared to patients with Milan in HCC. If confirmed, this finding could be used as a means to enlarge LT indications for HCC, allowing LT for some Milan out patients. This series also shows that Milan out and 18FDG/PET positive HCC have a very poor prognosis after LT, as all patients suffering from these aggressive cancers died from early recurrence. Finally, we hypothesise patients with Milan in but 18FDG/PET positive HCC might be a particular group of high-risk patients who should benefit from neoadjuvant therapy.
The limitations of this study are multiple. This is a retrospective evaluation of patients who were selected for LT, and it is probable that a prospective intention-to-treat study would demonstrate more precisely the role of 18FDG-PET in the setting of LT for HCC. In addition, this series is rather small, and a larger group of patients might help to more accurately define if Milan in but 18FDG/PET positive HCC have a worse prognosis than Milan in 18FDG/PET negative HCC after transplantation. Finally, the 18FDG/PET should be compared to other criteria that extend the Milan criteria, as the UCSF criteria or the up-to-seven criteria.
In conclusion, this study confirms that 18FDG/PET could be an interesting tool in the pre-LT evaluation of HCC patients and that the TSUVmax/LSUVmax cut-off of 1.15 should be used as a means to characterize HCC. Particularly, it deserves to be evaluated in a large prospective study if patients with HCC outside Milan criteria but with negative 18FDG PET, could be at low risk of recurrence after LT.
Liver transplantation (LT) is the standard treatment of patients suffering from cirrhosis complicated with hepatocarcinoma (HCC), a cancer whose incidence is increasing. However, LT is usually applied to patients with small HCC corresponding to the so-called Milan criteria (one nodule ≤ 5 cm, ≤ 3 nodules ≤ 3 cm). However, the Milan criteria are very restrictive and only a small proportion of patients with HCC are diagnosed within the Milan criteria and might benefit of LT. Other factors than size are needed to determine if some patients outside the Milan criteria could benefit from LT.
18F-fluorodeoxyglucose/positron emission tomography (18FDG/PET) is a very useful tool in the management of many cancers. The role of 18FDG/PET in HCC is not established yet, particularly in HCC patients who could benefit from LT. Some groups advocated that 18FDG/PET could help to differentiate HCC patients with low or high risk of recurrence after transplantation.
This retrospective study confirms that patients with HCC outside the Milan criteria that have a low intake at 18FDG/PET might be good candidates for LT with a low risk of cancer recurrence.
This finding has to be confirmed by prospective studies that should prospectively determine if patients with HCC outside Milan criteria that do not have an intake at 18FDG/PET should be candidate for LT.
Hepatocarcinoma is the primary cancer of the liver that often complicates a chronic liver disease named cirrhosis. 18FDG/PET is non invasive medical exam that allows a better evaluation of cancer metabolism and evolution.
This is an interesting article describing the prognostic value of FDG PET-CT for LT recipients with HCC, even though similar researches have been conducted in recent years. The author found that FDG PET-CT could be a useful tool to select HCC patients for LT.
P- Reviewer: Jahromi A, Romagnoli R, Xia Q S- Editor: Qi Y L- Editor: A E- Editor: Ma S
| 1. | El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2881] [Cited by in RCA: 3087] [Article Influence: 220.5] [Reference Citation Analysis (0)] |
| 2. | El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745-750. [PubMed] |
| 3. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [PubMed] |
| 4. | Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1594] [Cited by in RCA: 1692] [Article Influence: 70.5] [Reference Citation Analysis (0)] |
| 5. | Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi GL. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1568] [Article Influence: 92.2] [Reference Citation Analysis (1)] |
| 6. | Duvoux C, Roudot-Thoraval F, Decaens T, Pessione F, Badran H, Piardi T, Francoz C, Compagnon P, Vanlemmens C, Dumortier J. Liver transplantation for hepatocellular carcinoma: a model including α-fetoprotein improves the performance of Milan criteria. Gastroenterology. 2012;143:986-94.e3; quiz e14-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 723] [Article Influence: 55.6] [Reference Citation Analysis (0)] |
| 7. | Pant V, Sen IB, Soin AS. Role of 18F-FDG PET CT as an independent prognostic indicator in patients with hepatocellular carcinoma. Nucl Med Commun. 2013;34:749-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Hustinx R, Detry O. Hepatobiliary disease: primary and metastatic tumors. Clinical nuclear medicine. 4th ed. London, UK: Hodder Arnold 2006; . |
| 9. | Detry O, Deroover A, Meurisse N, Hans MF, Delwaide J, Lauwick S, Kaba A, Joris J, Meurisse M, Honoré P. Donor age as a risk factor in donation after circulatory death liver transplantation in a controlled withdrawal protocol programme. Br J Surg. 2014;101:784-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, Hasegawa H, Nakajima Y, Ohnishi K. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56:918-928. [PubMed] |
| 11. | Llovet JM, Bruix J. Prospective validation of the Cancer of the Liver Italian Program (CLIP) score: a new prognostic system for patients with cirrhosis and hepatocellular carcinoma. Hepatology. 2000;32:679-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 100] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Lee JW, Paeng JC, Kang KW, Kwon HW, Suh KS, Chung JK, Lee MC, Lee DS. Prediction of tumor recurrence by 18F-FDG PET in liver transplantation for hepatocellular carcinoma. J Nucl Med. 2009;50:682-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 142] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 13. | Detry O, De Roover A, Delwaide J, Coimbra C, Kaba A, Joris J, Damas P, Meurisse M, Honoré P. Living related liver transplantation in adults: first year experience at the University of Liège. Acta Chir Belg. 2004;104:166-171. [PubMed] |
| 14. | Yang SH, Suh KS, Lee HW, Cho EH, Cho JY, Cho YB, Yi NJ, Lee KU. The role of (18)F-FDG-PET imaging for the selection of liver transplantation candidates among hepatocellular carcinoma patients. Liver Transpl. 2006;12:1655-1660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 129] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 15. | Kornberg A, Freesmeyer M, Bärthel E, Jandt K, Katenkamp K, Steenbeck J, Sappler A, Habrecht O, Gottschild D, Settmacher U. 18F-FDG-uptake of hepatocellular carcinoma on PET predicts microvascular tumor invasion in liver transplant patients. Am J Transplant. 2009;9:592-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 153] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 16. | Kornberg A, Küpper B, Tannapfel A, Büchler P, Krause B, Witt U, Gottschild D, Friess H. Patients with non-[18 F]fludeoxyglucose-avid advanced hepatocellular carcinoma on clinical staging may achieve long-term recurrence-free survival after liver transplantation. Liver Transpl. 2012;18:53-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |