Published online Mar 14, 2015. doi: 10.3748/wjg.v21.i10.2988
Peer-review started: July 10, 2014
First decision: August 15, 2014
Revised: September 25, 2014
Accepted: November 18, 2014
Article in press: November 19, 2014
Published online: March 14, 2015
Processing time: 249 Days and 22.1 Hours
AIM: To explore the diagnostic value of the cross-modality fusion images provided by positron emission tomography/computed tomography (PET/CT) and contrast-enhanced CT (CECT) for pancreatic cancer (PC).
METHODS: Data from 70 patients with pancreatic lesions who underwent CECT and PET/CT examinations at our hospital from August 2010 to October 2012 were analyzed. PET/CECT for the cross-modality image fusion was obtained using TureD software. The diagnostic efficiencies of PET/CT, CECT and PET/CECT were calculated and compared with each other using a χ2 test. P < 0.05 was considered to indicate statistical significance.
RESULTS: Of the total 70 patients, 50 had PC and 20 had benign lesions. The differences in the sensitivity, negative predictive value (NPV), and accuracy between CECT and PET/CECT in detecting PC were statistically significant (P < 0.05 for each). In 15 of the 31 patients with PC who underwent a surgical operation, peripancreatic vessel invasion was verified. The differences in the sensitivity, positive predictive value, NPV, and accuracy of CECT vs PET/CT and PET/CECT vs PET/CT in diagnosing peripancreatic vessel invasion were statistically significant (P < 0.05 for each). In 19 of the 31 patients with PC who underwent a surgical operation, regional lymph node metastasis was verified by postsurgical histology. There was no statistically significant difference among the three methods in detecting regional lymph node metastasis (P > 0.05 for each). In 17 of the 50 patients with PC confirmed by histology or clinical follow-up, distant metastasis was confirmed. The differences in the sensitivity and NPV between CECT and PET/CECT in detecting distant metastasis were statistically significant (P < 0.05 for each).
CONCLUSION: Cross-modality image fusion of PET/CT and CECT is a convenient and effective method that can be used to diagnose and stage PC, compensating for the defects of PET/CT and CECT when they are conducted individually.
Core tip: Accurate pancreatic cancer (PC) diagnosis and staging are essential to choosing appropriate treatments and providing a more accurate prognosis. Combined contrast-enhanced positron emission tomography and computed tomography (PET/CT) can improve the information obtained from PET/CT or contrast-enhanced CT (CECT) alone. However, many patients with pancreatic disease have already undergone CECT examination by the time they undergo 18F-fluorodeoxyglucose PET/CT scanning. The aim of this study was to explore the value of the cross-modality fusion images provided by PET/CT and CECT in PC. We found that it is a convenient and effective method for diagnosing and staging pancreatic cancer to compensate for some of the defects of PET/CT and CECT alone.
- Citation: Zhang J, Zuo CJ, Jia NY, Wang JH, Hu SP, Yu ZF, Zheng Y, Zhang AY, Feng XY. Cross-modality PET/CT and contrast-enhanced CT imaging for pancreatic cancer. World J Gastroenterol 2015; 21(10): 2988-2996
- URL: https://www.wjgnet.com/1007-9327/full/v21/i10/2988.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i10.2988
The overall 5-year survival rate in patients with pancreatic cancer is < 5%, and even in patients with resectable disease, the 5-year survival rate is only approximately 20%[1,2]. Accurate diagnosis of pancreatic lesions and staging of pancreatic cancer are essential to choosing appropriate treatments and determining a more accurate prognosis.
Studies[3-5] have demonstrated that 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) is an important method for the diagnosis, staging, and prognostic evaluation of pancreatic cancer. However, there may be false positive or false negative results when diagnosing pancreatic cancer by 18F-FDG PET/CT[6]. Additionally, 18F-FDG PET/CT cannot be used to evaluate vascular invasion of pancreatic cancer[6,7]. However, contrast-enhanced CT (CECT) scanning is helpful for the differential diagnosis because it can reveal the blood supply to pancreatic masses, and CECT can also provide clear images of the vascular invasion of tumors[8,9]. Recent studies[10-12] have demonstrated that contrast-enhanced PET/CT examination could provide better information than PET/CT or CECT examination individually in the detection and presurgical assessment of pancreatic cancer, evaluation of the resectability of pancreatic cancer, and diagnosis of postoperative recurrence. However, many patients with pancreatic disease have already undergone CECT examination by the time they are subjected to 18F-FDG PET/CT scanning. Performing a contrast-enhanced PET/CT would increase the radiation dose patients receive as well as the risk of triggering an iodine allergy. The aim of this study was to explore the diagnostic value of the cross-modality fusion images provided by PET/CT and CECT in differentiating malignant from benign pancreatic lesions and staging pancreatic cancer.
The data of patients with pancreatic lesions who had undergone CECT and PET/CT examinations at our hospital between August 2010 and October 2012 were retrospectively analyzed.
Inclusion criteria were: (1) patients were suspected of having pancreatic cancer, as assessed by clinical or imaging examinations; and (2) the interval between the PET/CT and CECT examinations was no longer than 2 wk, and the DICOM images, including CECT scan images of the arterial phase, pancreatic parenchymal phase, and venous phase, were available. Exclusion criteria included the following: (1) treatment or invasive examinations, such as biopsy or endoscopic retrograde cholangiopancreatography (ERCP), were performed before the PET/CT and CECT examinations; and (2) there was a significant difference between the body posture during the PET/CT and CECT examinations. The study was approved by the ethics committee at our hospital.
The Siemens Biograph64 PET/CT (52 LSO crystal and 64-slice spiral CT) was used for the PET/CT. 18F-FDG (radiochemical purity > 95%) was provided by Shanghai Atomic Sinovac Pharmaceutical Co., Ltd. Subjects were instructed to fast for more than 6 h, and 3.70-5.55 MBq/kg of 18F-FDG was intravenously injected when blood glucose (BG) < 11.1 mmol/L. Then, after resting in the waiting room for 45-60 min, a body topogram scan was performed using an electric current of 35 mA at a voltage of 120 kV, a scan time of 10.5-15.6 s and a scan thickness of 0.6 mm. Then, whole-body CT scans were performed using an electric current of 170 mA at a voltage of 120 kV, with a scan time of 18.67-21.93 s and scan thickness of 3 mm. Then, whole-body PET scans were performed covering 5-6 bed positions, with an acquisition time of 2.0-2.5 min per bed position. The head scans were performed in the same order as the body scans. Image reconstruction was performed using a multi-modality workstation for postprocessing, and images in the axial, coronal, or sagittal planes and three-dimensional projection images were formed.
A cardiac 64 CT machine was used for the CECT scanning. Three-phase CECT scanning was performed. The scanning times for the arterial phase, pancreatic parenchyma phase, and delayed phase were 20 to 25 s, 40 to 50 s, and 80 to 100 s, respectively. The scanning covered the area from the top of the diaphragm to beneath the pancreas with a 3-mm thickness of the reconstruction slice.
For cross-modality image fusion of PET and CECT, multimodality Workplace TureD software was used to align the images in parallel. The original data from the CECT scanning were imported to the workplace; then, TureD software was used for the cross-modality image fusion of PET and CECT in manual and automatic modes, and 3-D images of CECT, PET, PET/CECT fusion images were provided.
The CECT images were retrospectively evaluated using the consensus of two experienced radiologists (readers A and B with 12 and 25 years of experience in CT, respectively) who had knowledge of neither the other imaging results nor the clinical data. The CECT images were analyzed using the established criteria for the assessment of the pancreatic lesions, vessel involvement (> 180o of circumferential contiguity of tumor to vessel), organ infiltration, and distant metastases[8,9,13]. LNs with a short-axis diameter greater than 1 cm were defined as malignant. Furthermore, the presence of a central unenhanced area suggesting central necrosis was considered a sign of malignancy, on the other hand the presence of peripheral low attenuation, and a fatty hilum within an LN, were considered a benign sign regardless of the node size[11,14].
The PET/CT images were retrospectively interpreted using the consensus of two experienced nuclear medicine physicians (readers C and D with 6 and 4 years of experience in PET/CT, respectively) who had knowledge of neither the other imaging results nor the clinical data.
The PET/CECT fusion images were prospectively interpreted in consensus by an experienced radiologist and an experienced nuclear medicine physician (reader E with 6 years of experience in PET/CT and reader F with 15 years of experience in CT) who had knowledge of neither the other imaging results nor the clinical data. Malignant lesions were diagnosed when abnormal focal FDG uptake was observed on the PET images, corresponding to an abnormal mass on the CT or CECT. If typical manifestations in CECT strongly supported a conclusion of benign or malignant, the lesions were diagnosed according to the CECT. LNs with increased glucose uptake were considered positive for metastatic spread, even if they were smaller than 1 cm in short-axis diameter. Conversely, LNs with no detectable tracer uptake were deemed negative for metastatic spread, even if they were larger than 1 cm in short-axis diameter. Maximum standard uptake values (SUV) were measured on all suspected lesions on the PET images. The maximum SUV was defined as the ratio of activity per milliliter of tissue to the activity in the injected dose, corrected for the decay and for the patient’s body weight. The regions of interest with a diameter of 1 cm were placed on the area of the lesion with the highest FDG uptake, and when there was no high uptake in the normal pancreas, the regions of interest were placed on the region of the suspected lesion based on the previous imaging procedures. The results for the histopathology or follow-up (≥ 6 mo) of the clinical imaging examinations were chosen as the final diagnosis.
Data were analyzed using SPSS 17.0 for Windows (SPSS Inc.). We performed patient-based analyses of PET/CECT fusion image results based on the consensus verdict in general, and compared these with the analyses of PET/CT and enhanced CT. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), accuracy, and Kappa value were calculated to evaluate the consistency among each of these three methods and the final diagnosis using standard statistical formulae. Differences between the imaging modalities were tested using a χ2 test. P < 0.05 was considered statistically significant.
In the present study, we included 70 patients imaged between August 2010 and October 2012. Forty-five patients were male, and 25 were female. The median age was 57 years, ranging from 13 to 81 years. Fifty patients presented with a malignant lesion; 31 cases were confirmed by postoperative pathology and 19 by endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA). For the 20 patients who presented with benign lesions, 13 were confirmed by postoperative pathology and 7 by clinical follow-up with imaging examinations (5 cases with chronic pancreatitis, 2 cases with autoimmune pancreatitis) (Table 1). The mean SUVmax of the benign pancreatic lesions was 5.06, with a range of 1.10 to 29.10, while the mean SUVmax of the malignant pancreatic lesions was 7.86, with a range of 1.60 to 17.60. Satisfying the fusion effect was accomplished using the TureD software for all 70 patients included in the study.
| Characteristics | |
| Sex | |
| Male | 45 (64.3 ) |
| Female | 25 (35.7 ) |
| Median age | 57 (13-81) |
| Final diagnosis | |
| Malignancy | 50 (71.4) |
| Duct adenocarcinoma | 37 |
| Cystadenocarcinoma | 4 |
| Adenosquamous Carcinoma | 3 |
| Metastatic tumor | 1 |
| Lymphoma | 2 |
| Neuroendocrine carcinoma | 3 |
| Benign | 20 (28.6) |
| Chronic pancreatitis | 8 |
| Tubercle | 1 |
| Autoimmune pancreatitis | 2 |
| Cystadenoma/IPMN | 7 |
| Neuroendocrine neoplasm | 2 |
The sensitivity, specificity, PPV, NPV, accuracy and Kappa value of the three methods in differentiating benign from malignant pancreatic lesions were 82.0%, 65.0%, 85.4%, 59.1%, 77.1% and 0.465 (CECT); 92.0%, 65.0%, 86.8%, 76.5%, 84.2%, and 0.597 (PET/CT); and 96.0%, 90.0%, 96.0%, 90.0%, 94.3%, and 0.860% (PET/CECT fusion images), respectively. The differences in the sensitivity, NPV, and accuracy between CECT and PET/CECT in differentiating benign from malignant pancreatic lesions were statistically significant (P < 0.05 for each) (Table 2).
| SEN | SPE | PPV | NPV | ACC | Kappa | |
| CECT | 82.0% | 65.0% | 85.4% | 59.1% | 77.1% | 0.456 |
| PET/CT | 92.0% | 65.0% | 86.8% | 76.5% | 84.2% | 0.597 |
| PET/CECT | 96.0% | 90.0% | 96.0% | 90.0% | 94.3% | 0.860 |
| PET/CT vs CECT | χ2 = 2.210 | χ2 = 0.000 | χ2 = 0.04 | χ2 = 1.303 | χ2 = 1.147 | |
| P = 0.137 | P = 1.000 | P = 1.303 | P = 0.254 | P = 0.284 | ||
| PET/CECT vs PET/CT | χ2 = 0.709 | χ2 = 3.584 | χ2 = 2.735 | χ2 = 1.238 | χ2 = 3.659 | |
| P = 0.400 | P = 0.058 | P = 0.098 | P = 0.266 | P = 0.056 | ||
| PET/CECT vs CECT | χ2 = 5.005 | χ2 = 3.584 | χ2 = 3.289 | χ2 = 5.177 | χ2 = 8.400 | |
| P = 0.025 | P = 0.580 | P = 0.070 | P = 0.023* | P = 0.004 |
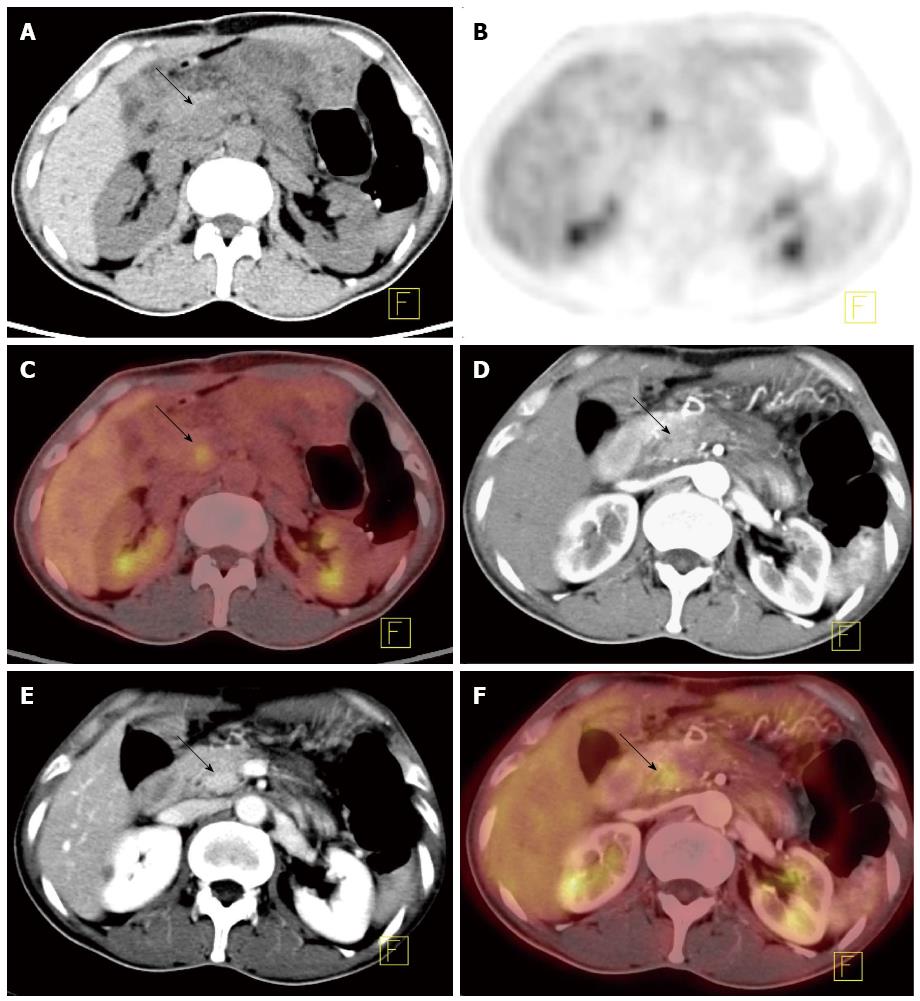
The Kappa value was calculated to evaluate the consistency between each of these three methods and the final diagnosis. Moderate consistency was found between the final diagnosis and CECT (κ = 0.456; P = 0.001 < 0.05) or PET/CT (κ = 0.597; P = 0.001 < 0.05), and there was excellent consistency between the gold standard and PET/CECT fusion image (κ = 0.860; P = 0.001 < 0.05) (Figure 1).
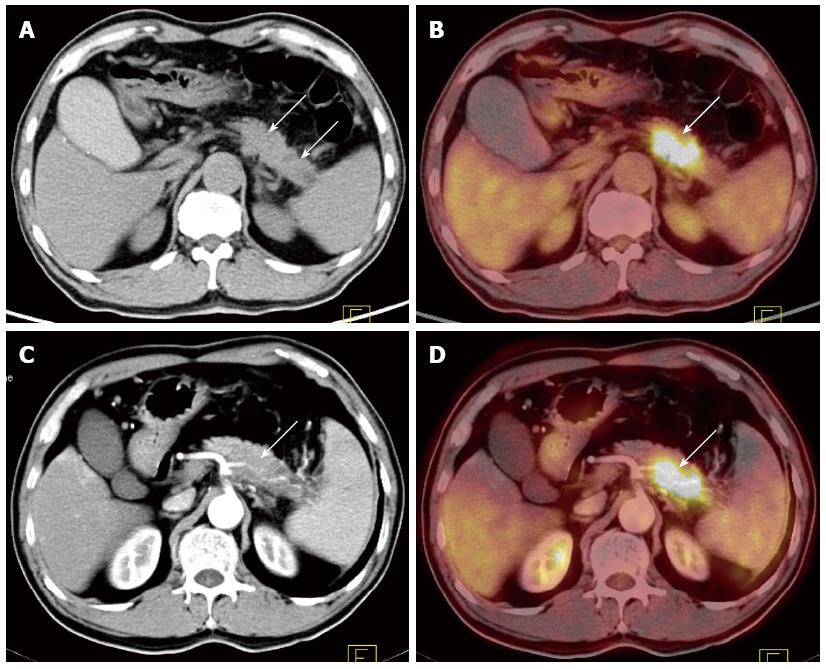
Peripancreatic vessel invasion: Thirty-one of the 50 malignant pancreatic cases underwent resection. Peripancreatic vessel invasion was verified (including invasion of the coeliac trunk artery, superior mesenteric artery, splenic artery, splenic vein, and portal vein) in 15 of the 31 patients who were confirmed as having a malignant tumor after surgery (Table 3). The sensitivity, specificity, PPV, NPV, and accuracy of the methods in diagnosing peripancreatic vessel invasion were 93.3%, 93.7%, 93.3%, 93.8%, and 93.5% (CECT); 26.7%, 75%, 50.0%, 52.2%, and 51.6% (PET/CT); and 93.3%, 93.7%, 93.3%, 93.8%, and 93.5% (PET/CECT fusion images), respectively. The differences in the sensitivity, PPV, NPV, and accuracy between CECT and PET/CT and between PET/CECT and PET/CT in diagnosing peripancreatic vessel invasion were statistically significant (P < 0.05 for each) (Figure 2).
| Peripancreatic vessel invasion | Regional lymph node metastasis | Distant metastasis | |||||||||||||
| SEN | SPE | PPV | NPV | ACC | SEN | SPE | PPV | NPV | ACC | SEN | SPE | PPV | NPV | ACC | |
| CECT | 14 (93.3) | 15 (93.8) | 14 (93.3) | 15 (93.8) | 29 (93.5) | 12 (63.2) | 11 (91.7) | 12 (92.3) | 11 (61.1) | 23 (74.2) | 10 (58.8) | 33 (100) | 10 (100) | 33 (82.5) | 43 (86.0) |
| Routine PET/CT | 4 (26.7) | 12 (75.0) | 4 (50) | 12 (52.2) | 16 (51.6) | 15 (78.9) | 10 (83.3) | 15 (88.2) | 10 (71.4) | 25 (80.6) | 14 (82.4) | 30 (90.9) | 14 (82.4) | 30 (90.9) | 44 (88) |
| PET/CECT | 14 (93.3) | 15 (93.8) | 14 (93.3) | 15 (93.8) | 29 (93.5) | 17 (89.5) | 11 (91.7) | 17 (94.4) | 11 (84.6) | 28 (90.3) | 16 (94.1) | 32 (97.0) | 16 (94.1) | 32 (97.0) | 48 (96.0) |
| PET/CECT vs PET/CT | χ2 = 13.889 P = 0.001 | χ2 = 2.133 P = 0.144 | χ2 = 5.759 P = 0.016 | χ2 = 7.657 P = 0.006 | χ2 = 13.697 P = 0.001 | χ2 = 0.792 P = 0.374 | χ2 = 0.381 P = 0.537 | χ2 = 0.430 P = 0.512 | χ2 = 0.678 P = 0.410 | χ2 = 1.170 P = 0.279 | χ2 = 1.133 P = 0.287 | χ2 = 1.065 P = 0.302 | χ2 = 1.133 P = 0.287 | χ2 = 1.065 P = 0.302 | χ2 = 2.174 P = 0.140 |
| PET/CECT vs CECT | χ2 = 0.000 P = 1.000 | χ2 = 0.000 P = 1.000 | χ2 = 0.000 P = 1.000 | χ2 = 0.000 P = 1.000 | χ2 = 0.000 P = 1.000 | χ2 = 3.640 P = 0.056 | χ2 = 0.000 P = 1.000 | χ2 = 0.057 P = 0.811 | χ2 = 2.024 P = 0.155 | χ2 = 2.763 P = 0.096 | χ2 = 5.885 P = 0.015 | χ2 = 1.015 P = 0.314 | χ2 = 0.611 P = 0.434 | χ2 = 3.880 P = 0.049 | χ2 = 3.053 P = 0.081 |
Regional lymph node metastasis: Regional lymph node metastasis was verified histologically in 19 of the 31 patients who were confirmed as having a malignant tumor after surgery. Patient-based analysis showed that in detecting regional lymph node metastasis, the sensitivity, specificity, PPV, NPV, and accuracy of the methods were 63.2%, 91.7%, 92.3%, 61.1%, and 74.2% (CECT); 78.9%, 83.3%, 88.2%, 71.4% and 80.6% (PET/CT); and 89.5%, 91.7%, 94.4%, 84.6%, and 90.3% (PET/CECT fusion images), respectively. There were no statistically significant differences among the three methods in detecting regional lymph node metastasis (P > 0.05 for each).
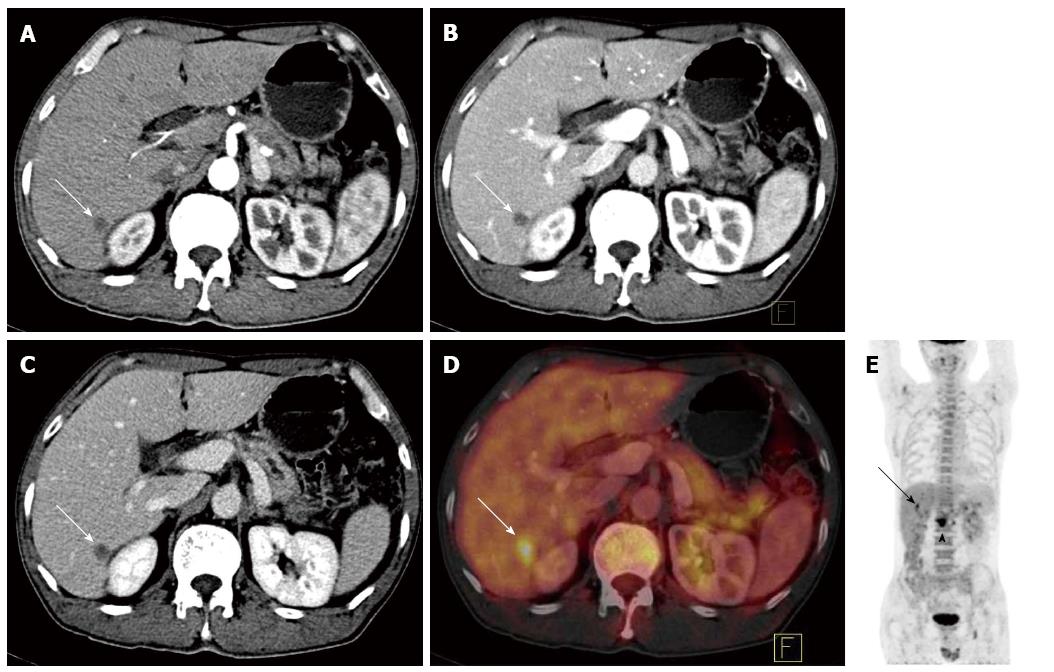
Distant metastases: Distant metastasis was confirmed after biopsy or clinical follow-up for at least 6 mo in 17 of the 50 cases with a malignant tumor. The sensitivity, specificity, NPV, PPV, and accuracy of the methods in detecting distant metastasis were 58.8%, 100%, 100%, 82.5%, and 86% (CECT); 82.4%, 91.0%, 82.4%, 90.9%, and 88% (PET/CT); and 94.1%, 97.0%, 94.1%, 97.0% and 96.0% (PET/CECT fusion images), respectively. The differences in the sensitivity and NPV between CECT and PET/CECT in detecting distant metastasis were statistically significant (P < 0.05 for each) (Figure 3).
In the present study, Multimodality Workplace TureD software (Siemens Ltd.) was used to fuse the images obtained by 18F-FDG PET and CECT. Automatic fusion was performed with TureD according to the main anatomical landmarks; manual and fine adjustments were also performed. Fusion images allow for more accurate positioning and easier viewing by the clinician without the strong subjectivities or significant differences in interpretation among different clinicians that occur with non-fused PET/CT and CECT imaging. Moreover, 18F-FDG PET/CECT fusion images can provide information not only on the metabolism and enhancement pattern of a lesion but also on the relationship between the lesion and adjacent vessels without increasing the medical cost to patients compared to conventional PET/CT.
CECT is the most common imaging method for diagnosing and staging PC. The role of PET/CT scanning remains unclear, with the NCCN suggesting that a PET/CT scan may be considered after the formal pancreatic CT protocol has been undertaken in “high-risk” patients to detect extra-pancreatic metastases. The accuracy of common imaging methods, such as CT and magnetic resonance imaging, in diagnosing pancreatic lesions depends heavily on the size of the lesion. However, this is not the case for 18F-FDG PET/CT, the accuracy of which depends more on the glucose metabolism of the lesion than on the size of the lesion. In recent years, 18F-FDG PET/CT has been shown to be more accurate than other imaging methods in diagnosing pancreatic cancer[15], differentiating malignant from benign cystic neoplasms[16], and diagnosing autoimmune pancreatitis[17]. In our study, for diagnosing malignant pancreatic tumors, the sensitivity, NPV, and accuracy of the PET/CECT fusion images were significantly higher than those of CECT (P < 0.05 for each), but there were no statistically significant differences between the PET/CECT fusion images and PET/CT. In the present study, the PET/CECT fusion images excluded 5 of the 7 false positive cases detected in the PET/CT images (including 3 patients with chronic pancreatitis, 2 cases of islet cell tumors), and 5 of the 7 false positive cases present in the CECT images (including 1 case of cystadenoma, 2 cases of chronic pancreatitis, and 2 cases of autoimmune pancreatitis). However, 2 cases of focal-mass-forming pancreatitis produced a false positive in all three methods. PET/CECT fusion images also excluded 2 out of 4 false negative cases displayed in the PET/CT images (1 case of pancreatic head cancer accompanied by acute pancreatitis and 1 case of pancreatic head cancer with a pseudocyst), as well as 7 of 9 false negative cases displayed in the CECT images (3 cases of small pancreatic ductal adenocarcinoma, 3 cases of cystadenocarcinoma, and 1 case of lymphoma). However, 2 cases of small ductal adenocarcinoma were false negatives for all three methods. Notably, PET/CECT correctly diagnosed all 11 cystic lesion patients (4 cases of cystadenocarcinoma and 7 cases of cystadenoma/IPMN), demonstrating its high application value in cytomas. Buchs et al[12]’s preliminary data obtained from 45 patients showed that the contrast enhanced PET/CT seemed to be superior to the unenhanced version. However, despite the complementary effect of PET/CT and CECT, there were still false positive and false negative cases with PET/CECT, especially in small pancreatic cancer and focal-mass-forming pancreatitis. To overcome these deficits, EUS may be helpful, and the use of other PET tracers that could be specific for cancer needs to be investigated.
CECT and multidetector computed tomographic angiography can clearly display tumor invasion into adjacent vessels or organs[8,9,18], but PET or PET/CT without CECT cannot clearly display it. In the present study, the differences in the sensitivity, PPV, NPV, and accuracy between PET/CT and CECT and PET/CECT in diagnosing peripancreatic vessel invasion were statistically significant (P < 0.05 for each). In Wakabayashi et al[7]’s study, the sensitivities of PET and CECT in detecting adjacent artery invasions were 22.2% and 100%, respectively. In another study, Strobel et al[10] also reported that enhanced PET/CT allowed for correctly diagnosing arterial infiltration in all 5 patients who were examined (100%), while PET and unenhanced PET/CT failed to allow for the detection of arterial infiltration in all 5 cases (0%). By displaying the enhanced vessels in CECT, 18F-FDG PET/CECT fusion images may provide more information on the relationships between the tumors and adjacent vessels, making up for the defect in PET/CT.
Although CECT clearly shows the anatomy, which can help in detecting and diagnosing lymph nodes, the identification of metastatic LNs by CECT is mainly based on measuring the node size. In our series, PET/CT and PET/CECT showed better sensitivity NPV, PPV and accuracy for detecting metastatic LNs than CECT, and PET/CECT and CECT showed better specificity than PET/CT. Sironi et al[19] reported that although PET and PET/CT can sometimes detect metastatic LNs smaller than 1 cm, the sensitivity of these modalities is insufficient because of their low spatial resolution. In the present study, the sensitivities of CECT, PET/CT and PET/CECT fusion images in diagnosing lymph node metastasis were 63.2%, 78.9%, and 89.5%, respectively, and there was no statistically significant difference among the three methods in detecting regional lymph node metastasis (P > 0.05 for each). Our findings were consistent with those of Kitajima et al[11]. In their study, the sensitivities of CECT, PET/CT, and PET/CECT in diagnosing abdominal lymph node metastasis were 62.5%, 75%, and 87.5% when evaluating the recurrence of pancreatic cancer. Thus, with PET/CT, CECT and PET/CECT, it is very difficult to depict and diagnose small peripancreatic lymph node metastases, especially when the peripancreatic lymph node metastasis is fused with the tumor.
PET/CT can detect distant metastasis more accurately than conventional imaging techniques. In our study, PET/CT excluded 4 false negative metastasis cases displayed in CECT (including 1 case of hepatic metastasis, 1 case of peritoneal metastasis, and 2 cases of distant lymph node metastasis); however, the PET/CECT fusion images excluded 2 false negative cases of hepatic metastasis displayed in PET/CT as well as 2 false positive cases displayed in PET/CT (1 case of intrahepatic cholangitis, which was misdiagnosed as hepatic metastasis, and 1 case of physiologic intestinal uptake of the tracer, which was misdiagnosed as peritoneal metastasis). Nevertheless, 1 case of peritoneal metastasis was not diagnosed in the PET/CECT fusion images, and 1 case of peritoneal inflammation was misdiagnosed as peritoneal metastasis in the PET/CECT fusion images. In the study performed by Kitajima et al[11], approximately 50% of hepatic metastasis cases and 33.3% of peritoneal metastasis cases remained undiagnosed. In the present study, the differences in the sensitivity and NPV between CECT and PET/CECT in detecting distant metastasis were statistically significant (P < 0.05 for each). However, even with PET/CECT fusion images, there were false negative and positive cases in diagnosing peritoneal metastasis. PET/CECT fusion images could diagnose the vast majority of distant metastases of pancreatic cancer, but neither PET/CECT nor PET/CT is a reliable imaging method in the preoperative assessment of the extent of peritoneal involvement, especially for predicting small bowel involvement[20] and diffuse metastasis without nodule formation; laparotomy remains the gold standard in diagnosing peritoneal metastasis.
There were some limitations to our study. First, the sample was relatively small, and we failed to demonstrate some areas of statistical significance between the PET/CT and PET/CECT fusion images. Second, only 63% of the patients had undergone an operation; 27% of the diagnoses were based on an FNA biopsy and 10% were made based on the clinical follow-up. In agreement with others[19], we think that it is unethical to perform extensive sampling in cases with strong suspicion of either benign or disseminated disease.
In summary, the cross-modality image fusion of PET and CECT is a convenient and effective method that can be used to compensate for some of defects of PET/CT and CECT individually in differentiating the diagnosis of pancreatic lesions or stage assessments of pancreatic cancer. However, several insufficiencies remain that need to be further investigated, including those associated with the diagnosis of peripancreatic lymph node metastasis, peritoneal metastasis, focal-mass-forming pancreatitis, or some small pancreatic cancer.
Pancreatic cancer is one of the most lethal cancers. Accurate diagnosis and staging of pancreatic cancer (PC) are essential to choosing appropriate treatments and creating a more accurate prognosis. Contrast-enhanced computed tomography (CECT) has been the most common imaging method for diagnosing and staging PC. In recent years, 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) has been shown to be more accurate than other imaging methods in diagnosing pancreatic cancer. However, there may be false positive or false negative results when diagnosing pancreatic cancer by 18F-FDG PET/CT. Additionally, 18F-FDG PET/CT cannot be used to accurately evaluate the vascular invasion of pancreatic cancer.
Recently, several studies have demonstrated that contrast-enhanced PET/CT could provide better information than PET/CT or CECT in the detection and presurgical assessment of pancreatic cancer, evaluation of the resectability of pancreatic cancer, and diagnosis of postoperative recurrence.
However, many patients with pancreatic disease have already undergone CECT examination by the time they are subjected to 18F-FDG PET/CT scanning. Performing another round of CECT would increase the radiation dose the patients receive and the risk of iodine allergy. To overcome these disadvantages, we fused cross-modality images provided by PET/CT and CECT and explored the diagnostic value of the fusion images (PET/CECT) in diagnosing and staging pancreatic cancer.
The study results suggest that the cross-modality image fusion of PET and CECT is a convenient and effective method that can be used to compensate for some defects of using PET/CT and CECT individually in the differential diagnosis of pancreatic lesions or stage assessments of pancreatic cancer.
18F-FDG is 2-18-fluoro-2-deoxy-D-glucose, which is proposed as a sugar analog to detect glucose metabolism in the human body. PET/CT is integrated positron emission tomography/computed tomography in which a full-ring detector clinical PET scanner and multidetector row helical CT scanner are combined; this technology has made it possible to acquire both metabolic and anatomic imaging data using a single device in a single diagnostic session, and it provides precise anatomic localization of suspicious areas of abnormal FDG uptake.
The submitted manuscript is a retrospective review of the diagnostic accuracy for various imaging modalities in patients with pancreatic lesions and suspected cancer. Although most guidelines and clinicians utilize CT/magnetic resonance imaging and endoscopic ultrasound initially, there is evidence that the use of PET/CT may be beneficial. There have been many studies looking at PET/CT for the diagnosis and staging of pancreatic cancer, but the use of computer software for fusing images already obtained appears to be relatively novel. The data collection and analysis appear to be sound.
P- Reviewer: Kapischke M, Muscarella P, Ramia JM S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Ma S
| 1. | Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8283] [Cited by in RCA: 8224] [Article Influence: 483.8] [Reference Citation Analysis (0)] |
| 2. | Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363:1049-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1481] [Cited by in RCA: 1543] [Article Influence: 73.5] [Reference Citation Analysis (0)] |
| 3. | Kim MJ, Lee KH, Lee KT, Lee JK, Ku BH, Oh CR, Heo JS, Choi SH, Choi DW. The value of positron emission tomography/computed tomography for evaluating metastatic disease in patients with pancreatic cancer. Pancreas. 2012;41:897-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Saito M, Ishihara T, Tada M, Tsuyuguchi T, Mikata R, Sakai Y, Tawada K, Sugiyama H, Kurosawa J, Otsuka M. Use of F-18 fluorodeoxyglucose positron emission tomography with dual-phase imaging to identify intraductal papillary mucinous neoplasm. Clin Gastroenterol Hepatol. 2013;11:181-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Lee JW, Kang CM, Choi HJ, Lee WJ, Song SY, Lee JH, Lee JD. Prognostic Value of Metabolic Tumor Volume and Total Lesion Glycolysis on Preoperative 18F-FDG PET/CT in Patients with Pancreatic Cancer. J Nucl Med. 2014;55:898-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 166] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 6. | Belhocine T, Spaepen K, Dusart M, Castaigne C, Muylle K, Bourgeois P, Bourgeois D, Dierickx L, Flamen P. 18FDG PET in oncology: the best and the worst (Review). Int J Oncol. 2006;28:1249-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Wakabayashi H, Nishiyama Y, Otani T, Sano T, Yachida S, Okano K, Izuishi K, Suzuki Y. Role of 18F-fluorodeoxyglucose positron emission tomography imaging in surgery for pancreatic cancer. World J Gastroenterol. 2008;14:64-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Vargas R, Nino-Murcia M, Trueblood W, Jeffrey RB. MDCT in Pancreatic adenocarcinoma: prediction of vascular invasion and resectability using a multiphasic technique with curved planar reformations. AJR Am J Roentgenol. 2004;182:419-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 155] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 9. | Zamboni GA, Kruskal JB, Vollmer CM, Baptista J, Callery MP, Raptopoulos VD. Pancreatic adenocarcinoma: value of multidetector CT angiography in preoperative evaluation. Radiology. 2007;245:770-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 98] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 10. | Strobel K, Heinrich S, Bhure U, Soyka J, Veit-Haibach P, Pestalozzi BC, Clavien PA, Hany TF. Contrast-enhanced 18F-FDG PET/CT: 1-stop-shop imaging for assessing the resectability of pancreatic cancer. J Nucl Med. 2008;49:1408-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 100] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 11. | Kitajima K, Murakami K, Yamasaki E, Kaji Y, Shimoda M, Kubota K, Suganuma N, Sugimura K. Performance of integrated FDG-PET/contrast-enhanced CT in the diagnosis of recurrent pancreatic cancer: comparison with integrated FDG-PET/non-contrast-enhanced CT and enhanced CT. Mol Imaging Biol. 2010;12:452-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Buchs NC, Bühler L, Bucher P, Willi JP, Frossard JL, Roth AD, Addeo P, Rosset A, Terraz S, Becker CD. Value of contrast-enhanced 18F-fluorodeoxyglucose positron emission tomography/computed tomography in detection and presurgical assessment of pancreatic cancer: a prospective study. J Gastroenterol Hepatol. 2011;26:657-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Yoon SH, Lee JM, Cho JY, Lee KB, Kim JE, Moon SK, Kim SJ, Baek JH, Kim SH, Kim SH. Small (≤ 20 mm) pancreatic adenocarcinomas: analysis of enhancement patterns and secondary signs with multiphasic multidetector CT. Radiology. 2011;259:442-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 182] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 14. | Kitajima K, Murakami K, Yamasaki E, Domeki Y, Kaji Y, Fukasawa I, Inaba N, Suganuma N, Sugimura K. Performance of integrated FDG-PET/contrast-enhanced CT in the diagnosis of recurrent ovarian cancer: comparison with integrated FDG-PET/non-contrast-enhanced CT and enhanced CT. Eur J Nucl Med Mol Imaging. 2008;35:1439-1448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 15. | Okano K, Kakinoki K, Akamoto S, Hagiike M, Usuki H, Yamamoto Y, Nishiyama Y, Suzuki Y. 18F-fluorodeoxyglucose positron emission tomography in the diagnosis of small pancreatic cancer. World J Gastroenterol. 2011;17:231-235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Zhang Y, Frampton AE, Martin JL, Kyriakides C, Bong JJ, Habib NA, Vlavianos P, Jiao LR. 18F-fluorodeoxyglucose positron emission tomography in management of pancreatic cystic tumors. Nucl Med Biol. 2012;39:982-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Zhang J, Shao C, Wang J, Cheng C, Zuo C, Sun G, Cui B, Dong A, Liu Q, Kong L. Autoimmune pancreatitis: whole-body 18F-FDG PET/CT findings. Abdom Imaging. 2013;38:543-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Kaneko OF, Lee DM, Wong J, Kadell BM, Reber HA, Lu DS, Raman SS. Performance of multidetector computed tomographic angiography in determining surgical resectability of pancreatic head adenocarcinoma. J Comput Assist Tomogr. 2010;34:732-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Sironi S, Buda A, Picchio M, Perego P, Moreni R, Pellegrino A, Colombo M, Mangioni C, Messa C, Fazio F. Lymph node metastasis in patients with clinical early-stage cervical cancer: detection with integrated FDG PET/CT. Radiology. 2006;238:272-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 214] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 20. | Dromain C, Leboulleux S, Auperin A, Goere D, Malka D, Lumbroso J, Schumberger M, Sigal R, Elias D. Staging of peritoneal carcinomatosis: enhanced CT vs. PET/CT. Abdom Imaging. 2008;33:87-93. [PubMed] |