Published online Jan 7, 2015. doi: 10.3748/wjg.v21.i1.94
Peer-review started: April 11, 2014
First decision: April 28, 2014
Revised: May 12, 2014
Accepted: June 26, 2014
Article in press: June 26, 2014
Published online: January 7, 2015
Processing time: 271 Days and 8.9 Hours
In addition to surgical procedures, radiofrequency ablation is commonly used for the treatment of hepatocellular carcinomas (HCCs) of limited size and number. Transcatheter arterial chemoembolization (TACE), using iodized poppy seed oil, Lipiodol and anticancer drugs, has been actively performed for the treatment of unresectable HCC, particularly in Asian countries. Recently, Sorafenib become available for advanced HCCs when the liver is still sufficiently functional. Sorafenib is an oral multikinase inhibitor with antiproliferative and antiangiogenic effects. However, the effect of sorafenib seems to be inadequate to control the progression of HCC. Radiation therapy (RT) for HCC has a potential role across all stages of HCC. However, RT is generally not considered an option in HCC consensus documents or national guidelines, primarily because of insufficient supporting evidence. However, the method of RT has much improved because of advances in technology. Moreover, combined treatment of RT plus other treatments (TACE, sorafenib and chemotherapy etc.) has become one of the alternative therapies for HCC. Therefore, we should understand the various kinds of RT available for HCC. In this review, we focus on various kinds of external beam radiation therapy.
Core tip: Radiation therapy (RT) for hepatocellular carcinoma (HCC) has a potential role across all stages of HCC. However, RT is generally not considered an option in HCC consensus documents or national guidelines, primarily because of insufficient supporting evidence. However, the method of RT has much improved because of advances in technology. Therefore, we should understand the various kinds of RT available for HCC. In this review, we focus on various kinds of external beam radiation therapy.
- Citation: Kondo Y, Kimura O, Shimosegawa T. Radiation therapy has been shown to be adaptable for various stages of hepatocellular carcinoma. World J Gastroenterol 2015; 21(1): 94-101
- URL: https://www.wjgnet.com/1007-9327/full/v21/i1/94.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i1.94
Hepatocellular carcinoma (HCC) is the third highest cause of cancer-related death worldwide[1]. Risk factors for the incidence of HCC include hepatitis B, hepatitis C, alcoholic hepatitis, non- alcoholic fatty liver disease (NAFLD), and liver cirrhosis from any cause. Among these causes, chronic infection of hepatitis B or hepatitis C virus (HBV/HCV) can strongly affect the incidence of HCC[2]. Therefore, the age of HCC patients might be affected by the cause of HCC. The average age of HCC patients in Japan is higher than that in other Asian-pacific regions due to the incidence of HCV-related HCC[3]. In Asia and Africa, approximately 80% of cases of HCC are due to infection with HBV[4]. There are relatively many HCV-related HCC patients in South America (particularly Brazil)[5].
HCC tends to remain within the liver, although multi-focality and vascular invasion are common[6]. A balance between the remaining liver function and tumor factors influences the selection of various kinds HCC treatment[7]. Surgical resection of HCC, an option for a limited number of HCCs, results in 5-year survival rates of 60%-70%[8]. Liver transplantation (LT) can cure both the HCC and underlying liver disease. A meta-analysis suggested that LT provides increased survival and lower recurrence rates than surgical resection for HCC patients[9]. In addition to surgical procedures, radiofrequency ablation (RFA) is commonly used for the treatment of HCCs of limited size and number[8]. Transcatheter arterial chemoembolization (TACE), using iodized poppy seed oil, Lipiodol and anticancer drugs, has been actively performed for the treatment of unresectable HCC, particularly in Asian countries[10]. However, HCCs remain viable in and around the tumor-capsule, which is supplied by both arterial and portal blood, and these cells are often responsible for late recurrence after TACE treatment[11]. Recently, Sorafenib become available for advanced HCCs with sufficient remaining liver function[12]. Sorafenib is an oral multikinase inhibitor with antiproliferative and antiangiogenic effects. It has been shown to inhibit the activity of serine/threonine kinase c-Raf (Raf-1) and B-Raf; the mitogen-activated protein kinases MEK and ERK; vascular endothelial growth factor receptors (VEGFR)-1, 2, and 3; platelet-derived growth factor receptors (PDGFR)-α and β; cytokine receptor c-KIT; the receptor tyrosine kinases Flt-3 and RET; and the Janus kinase/signal transducer and activator of transcription pathway. The intracellular signaling pathway Raf/MEK/ERK and the extracellular receptors VEGFR and PDGFR should be inhibited by sorafenib[13]. However, the effect of sorafenib seems to be inadequate to control the progression of HCC. In addition to sorafenib, hepatic arterial infusion chemotherapy (HAIC) has been used for advanced HCC[14].
Radiation therapy (RT) for HCC has a potential role across all stages of HCC. However, RT is generally not considered an option in HCC consensus documents or national guidelines, primarily because of insufficient supporting evidence[15,16]. However, methods of RT have greatly improved because of advances in technology. Moreover, combined treatment of RT plus other treatments (TACE, sorafenib and chemotherapy etc.) has become one of the alternative therapies for HCC. Therefore, we should understand the various kinds of RT available for treating HCC. In this review, we focused on various kinds of external beam radiation therapy.
Many groups reported that RT combined with locoregional therapy such as TACE could be effective for the control of HCC (Table 1). Several general approaches with such combinations are used[17-21]. One of them, RT specifically targeting the tumor thrombus, can be used before TACE[17,22]. In 1989, Takagi et al[23] reported a 29% response rate in 7 patients irradiated for HCC with portal venous thrombosis. A group of 42 patients treated only for tumor thrombosis were compared with control patients treated with TACE without RT. RT combined with TACE had higher response rates (43% vs 14%) and overall survival (medial, 11.7 mo vs 4.7 mo)[22]. In another comparative study, significantly improved survival was reported in 16 patients treated with RT combined with TACE compared with 29 patients treated with TACE alone (33% vs 7% 1-year survival)[24]. Since RT could be effective for the treatment of tumor thrombus, RT combined with TACE should be used for the treatment of HCC with tumor thrombus.
| Ref. | Year | Country | Patient numberTACE + RT vs TACE | Child-Pugh (CP-A:CP-B) | Tumor thrombus | Tumor size | Response rate | Over all survival |
| Koo et al[22] | 2010 | South Korea | 42 vs 29 | 26:16 vs 17:12 | All patients had inferior vena cava tumor thrombus | 10 ± 4.0 vs 12 ±3.8 cm | 42.9% vs 13.8% | 1 yr OS 47.7% vs 17.2% |
| Zhang et al[24] | 2009 | China | 16 vs 29 | 13:3 19:10 | Stenosis: occlusion | < 10 cm : ≥ 10 cm | N/A | 1 yr OS 32.5% vs 6.9% |
| 14:2 vs 21:9 | 13:3 vs 21:8 | |||||||
| Shim et al[25] | 2005 | South Korea | 38 vs 35 | 33:5 vs 32:3 | yes: no | 10.2 vs 9.5cm | 65.8% vs N/A | 2 yr OS 36.8% vs 14.3% |
| 12:26 vs 10:25 | ||||||||
| Zeng et al[20] | 2004 | China | 54 vs 149 | Non | 76% vs 31% | 1 yr OS 71.5% vs 59.6% |
As another approach, RT could be used as a consolidative treatment after TACE. A systematic review of 17 trials found that patients treated with RT combined with TACE had improved survival compared to patients treated with TACE alone. Moreover, serious adverse events were not increased, with the exception of an elevation of the total bilirubin level. Another group reported the use of local radiotherapy as a complement to incomplete transcatheter arterial chemoembolization in locally advanced hepatocellular carcinoma[25]. In 73 patients treated with incomplete TACE, TACE was repeatedly performed in 35 patients, and the remaining 38 patients were also treated with local RT. The 2-year survival rate was significantly higher in the RT combined with TACE group than in the TACE alone group (36.8% vs 14.3%, P = 0.001)[25]. Another group reported the efficacy of 3-dimensinal conformal RT for patients with unresectable HCC after incomplete TACE. The overall survival rate was 72.0% at 1 year and 45.6% at 2 years[26]. In addition to a comparison between RT combined with TACE and TACE only alone, the feasibility of RT combined with TACE in comparison with sorafenib for advanced HCC has been investigated. The overall survival of the RT combined with TACE group was longer compared to the sorafenib treatment in locally advanced HCC patients without distant metastasis[27]. Moreover, another group reported a comparison between surgical resection and conformal RT combined with TACE for resectable HCC with portal vein tumor thrombus. In this report, conformal RT combined with TACE yielded better survival than surgical resection for HCC with portal vein thrombosis[28].
In addition to RT combined with TACE, a combination therapy of RT and sorafenib has been considered, recently[29-31]. Yu et al[31] reported that sorafenib enhanced the radiosensitivity of human HCC cell lines in a schedule-dependent manner. Sorafenib induced DNA damage and suppressed the DNA repair capacity, decreased radiation-activated NF-kappaB and increased the radiation-induced apoptosis. Moreover, RT combined with sorafenib was carried out in 31 patients[29]. The median overall survival was 7.8 mo (95%CI: 3.0-12.6)[29]. The combined treatment of sorafenib and RT was feasible and induced substantial tumor responses in the target lesions.
The efficacy of RT combined with HAIC was reported by several groups[32,33]. A group investigated the efficacy of HAIC using 5-FU and systemic interferon (IFN)-alpha for advanced HCC with venous tumor thrombosis (VTT) in the hepatic vein trunk (Vv2) or inferior vena cava (Vv3)[32]. HAIC-5-FU/IFN, despite being ineffective, could be useful in combination with radiotherapy for VTT to improve the prognosis. Another group evaluated the efficacy of intra-arterial 5-FU and subcutaneous interferon combined with image-guided RT in advanced HCC with PVTT. The overall median survival was significantly longer in the combination group (12.0 mo, 95%CI: 9.3-17.6 mo) than in the non-combination group (9.1 mo, 95%CI: 5.5-11.1 mo) (P = 0.041)[33].
With the progress of radiation therapy, it was found to be effective for HCC[34]. It provides a better curative effect by irradiating the tumor more precisely and reducing damage of the normal part of the liver. Therefore, recently, stereotactic body radiotherapy (SBRT) has become widely used because of its stronger curative effect. SBRT was used to treat intracranial targets from the 1950s[35,36]. In recent years, progress in the fields of image-guided radiotherapy and computer science has enabled its use for extracranial targets[36]. SBRT for HCC was made possible by establishing a method for tracing the regions of the body that fluctuate with the respiratory movement[37]. SBRT is suitable for non-resectable small HCCs as a noninvasive treatment. Although RFA treatment is very effective, it can sometimes be problematic because the puncture route taken could result in damage to nearby organs. In addition, if blood vessels exist near the HCC, the blood flow can reduce the thermal effects of RFA[38]. Therefore, medical devices used for laparoscopy or artificial hydrothorax can be used to perform RFA. However, technical skill is required to treat HCC properly. SBRT is now considered as alternative to RFA. Recent studies indicate that the results of RFA are equal to those of surgery[39,40]. There are few articles that compared the surgery with SBRT directly. On the other hand, SBRT is not described in the Barcelona Clinic Liver Cancer system[41] nor in the algorithm created by the Japan Society of Hepatology[15]. However, in recent years many good results have been reported for SBRT. The curative effects and side effects of SBRT should be the focus of discussions about its suitability as an alternative to RFA. The articles concerning SBRT reviewed here had different protocols. There are many reports about the effectiveness and safety of SBRT (Table 2). Generally, SBRT is considered for lesions smaller than 5 cm in diameter. The treatment dose range was 6-15 Gy per day and the total dose was 18-50 Gy in 1 to 10 fractions[36,42-51]. The daily irradiation dose is very high, so appropriate planning at the irradiation center is required.
| Ref. | Patient number | Tumor | Back ground | Median tumor volume | Median dose (Gy) | Fraction | Follow up (mo) | Local control | Overall survival | Adverse effectAcute toxicity grade≥3Late toxicity grade≥3 |
| Andolino et al[42] | 60 | HCC | LC (CP-A: 36, CP-B: 24) | 3.2 cm | 44 | 3 | 27 | 2 yr local control 90% | 2 yr OS 67% | 0% |
| N/A | ||||||||||
| Choi et al[44] | 20 | HCC | LC (CP-A: 15, CP-B: 5), PVTT: 4 | 2-6.5 (3.8 cm) | 50 | 5-10 | 3-55 | 1 yr OS 70% | 0% | |
| -23 | 2 yr sOS 43.1% | N/A | ||||||||
| Goodman et al[45] | 26 | meta | meta: 19, CCC: 5, HCC: 4 | 0.8-146.6 (32.6 cc) | 22.5-46.6 | 1 | 2-55 | 1 yr OS 64.3% | 0% | |
| CCC | -34.8 | -17 | 2 yr OS 50.4% | N/A | ||||||
| HCC | ||||||||||
| Huang et al[46] | 36 | HCC | LC (CP-A: 28, CP-B: 7, CP-C: 1) | 1.1-12.3 (4.4 cm) | 25-48 | 4-5 | 14 | 1 yr in-field failure free rate 87.6% | 2 yr OS 72.6% | 2.8% (1: gastric ulcer) |
| -37 | 2 yr in-field failure free rate 75.1% | N/A | ||||||||
| Kwon et al[47] | 42 | HCC | LC (CP-A: 38, CP-B: 4, CP-C: 0) | 15.4-81.8 (15.4 cc) | 30-39 | 3 | 8.4-49.1 | 1 yr in-field progression-free survival rate 72.0% | 1 yr OS 72% | 0% |
| -28.7 | 3 yr in-field progression-free survival rate 68.0% | 3 yr OS 67.5% | 1% (1:liver failure) | |||||||
| Louis et al[36] | 25 | HCC | LC (CP-A: 22, CP-B: 3) | 1.8-10.0 (4.5 cm) | 45 | 3 | 1-24 | 1 yr local control rate 95% | 1 yr OS 79% | 4% (1: hepatic/epgastric pain) |
| -12.7 | 2 yr OS 52% | 10% (1: gastro duodenal ulcer, | ||||||||
| 1: hepatic toxicity) | ||||||||||
| Méndez Romero et al[48] | 25 | meta | meta: 17, HCC: 8 | 0.5-7.2 | 25-37.5 | 3-5 | 0.5-31 | 1 yr local control rate 94% | 1 yr OS 82% | 16% (1: pateient died in a hepatic tixicity and bleeding from varices. |
| HCC | (3.2 cm) | -12.9 | 2 yr local control rate 82% | 2 yr OS 54% | 3: hepatic toxicity.) | |||||
| 0% | ||||||||||
| Takeda et al[50] | 16 | HCC | LC (CP-A: 14, CP-B: 2) | 3.4-72 (13.6 cm3) | 35-50 | 5-7 (6) | 8.1-33.1 | 1 yr local control rate 90% < | All patients were alive | 0% |
| -20.4 | 0% | |||||||||
| Tse et al[51] | 41 | HCC | HCC (CP-A: 31), CCC (10) | 9-1913 | 24-54 | 6 | 10.8-39.2 (17.6) | 1 yr in-field local control rate 65% | 1 yr OS 51% | 43% (13: hepatic toxicity, 1: hemato toxicity, 1: lethargy, 3 : nausea) |
| CCC | (173 mL) | -36 | 6% (1: GI bleeding, 1: bowel obstruction) |
SBRT is effective for HCC, with especially a 1 year local control rate of more than 90%[36,43,48,50]. However, the complete response rate was 20%-60%[43,44,47], lower than that of RFA. SBRT cannot completely replace RFA. However, it is reported that the complete response rate is higher for smaller HCCs. Kwon et al[47] described that the progression-free survival rate changes at 32 cc of tumor volume.
Although it has been described that SBRT did not produce major side effects. The side effects of SBRT include acute toxicities and late toxicities. Acute toxicities are elevations in liver enzymes, thrombocytopenia, leukopenia and nausea. Many of these are grade 1 to grade 3[43,44,52]. Late toxicities occur in the first six months and are rare. Late toxicities include decompensation and duodenal ulcer. A few patients have died of liver failure by SBRT[47,48]. If the patient’s original liver function can be classified as Child-PugB, caution is required. SBRT is a concentrated irradiation treatment, but it may be lethal when employed at unsuitable hepatic lesions in patients with poor liver function. Concerning the liver function, it is reported that the CP value generally rises after the treatment. Takeda et al[50] reported that patients with sufficient liver function don’t experience any major problems and show improved liver function at one year after SBRT.
SBRT has few side effects and good disease control potency. In order to enhance the therapeutic effect, SBRT after TACE has been performed[35,36,44,46,48,50]. The combination of RT and TACE has been shown to be more effective only when compared to RT alone[38]. SBRT is a very effective therapy and has the possibility of expanded use with further technological improvements.
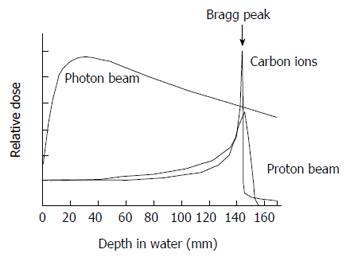
Clinically, proton beam and heavy iron therapies are described as particle beam therapies[53]. Heavy ion therapy uses a carbon ion beam. The particle beam therapy is produced by irradiating carbon nuclei or high energy protons using a cyclotron accelerator or synchrotron accelerator[54]. It requires a relatively large facility because the accelerator itself is quite huge. Therefore, facilities for particle beam therapy are limited in number and its use is limited clinical trials. In general, X-rays are reduced gradually in the human body. On the other hand, an accelerated particle beam emits maximum energy just before it is stopped. It makes a peak of steep energy called the Bragg-Peak[55] (Figure 1). Therefore, particle beam therapy creates maximum energy at the depth of a lesion.
Using particle beams for clinical applications was first proposed by Wilson in 1946[56]. In the early 1950, proton and helium ion beams were studied at Lawrence Berkeley National Laboratory (LBNL) in the United State[57]. LBNL paved the way for heavy ion therapy in the 1970’s. In 1994, the first Phase I and II heavy ion treatment took place at Heavy Ion Medical Accelerator in Chiba, Japan. Twenty-four HCC patients in Phase I and II were treated with total doses ranging from 49.5 GyE to 79.5 GyE given in 15 fixed fractions and 5 wk[58]. No sever side effects appeared and the overall tumor response rate was 71%. The local control and overall survival rate was 92% and 92%, 81% and 50%, and 81% and 25% at 1, 3, and 5 years. The heavy ion beam is similar to normal radiation therapy but it can expect to have a strong effect for deep lesions. Heavy ion beam therapy was effective for HCC in the porta hepatis[59]. This report showed that the 5-year overall survival, local control rate, and toxicities were not significantly different between the porta hepatis and control groups.
Carbon ion beams have the advantage of a higher cytotoxic effect than proton beams. Proton beams can be irradiated from any direction, and they can also be applied for cases in which other treatments are not suitable. Proton beam therapy yielded good results when the dose of radioactivity and the cautery range for the lesion in contact with the gastrointestinal tract and a porta hepatis region were properly regulated[60-62].
Particle beam therapy is an effective treatment that can overcome some of the normal radiation therapy. It is a major disadvantage that treatment facilities are limited. Further clinical applications are expected in the future since such facilities have been increasing.
Intrahepatic lesions of HCC are controlled by treatment surgery, RFA, TACE and RT. Sometimes, lesions in the liver can be controlled, but extrahepatic lesions develop. In HCCs, intrahepatic lesions more strongly affect the prognosis compared with extrahepatic lesions. In a report by Uchino et al[63], extrahepatic metastases were related directly to death in 23 patients (7.6%) of 342 patients. Seventeen patients died from respiratory failure due to pulmonic metastasis, five patients from brain hemorrhages due to cerebral metastasis, and one patient from hemorrhages due to fractures from bone metastasis. Bone metastasis has little influence as a direct cause of death. However, bone fractures due to bone metastasis reduce the performance status, and treatment is important to prevent a decrease in quality of life. Habermehl et al[64] reported that the response rate to palliative radiotherapy for bone metastasis from CCC (11 patients, 19 lesions) and HCC (30 patients, 48 lesions) was 77%. It did not contribute to the overall survival for advanced cancer, but such radiation therapy contributed to the amelioration of pain (63 lesion, 94%), neurologic complications (9 lesions, 13%) and unstable lesions with a risk of pathologic fracture (6 lesions, 9%).In addition, Jiang et al[65] carried out palliative irradiation for lung metastases with symptoms (stuffy chest,hematosputum and cough). In 92.3% of the patients, significant symptoms were completely or partially relieved. Other groups reported the effect of RT on lymph node metastasis[66] and adrenal gland metastasis[67]. The curative effect of RT for distant metastasis of HCC is sufficiently meaningful. In the future, the primary tumor will be controlled, and RT should provide useful treatment oligometastases[68] and local recurrences.
P- Reviewer: Niibe Y, Storto G S- Editor: Ma YJ L- Editor: A E- Editor: Liu XM
| 1. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. [PubMed] |
| 2. | Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol. 2013;47 Suppl:S2-S6. [PubMed] |
| 3. | Kim MN, Kim BK, Han KH. Hepatocellular carcinoma in patients with chronic hepatitis C virus infection in the Asia-Pacific region. J Gastroenterol. 2013;48:681-688. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 4. | Trinchet JC. Hepatocellular carcinoma in 2014: current situation and future prospects. Diagn Interv Imaging. 2014;95:705-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Carrilho FJ, Kikuchi L, Branco F, Goncalves CS, Mattos AA. Clinical and epidemiological aspects of hepatocellular carcinoma in Brazil. Clinics (Sao Paulo). 2010;65:1285-1290. [PubMed] |
| 6. | Herrero JI, Sangro B, Quiroga J, Pardo F, Herraiz M, Cienfuegos JA, Prieto J. Influence of tumor characteristics on the outcome of liver transplantation among patients with liver cirrhosis and hepatocellular carcinoma. Liver Transpl. 2001;7:631-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 162] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 7. | Llovet JM. Updated treatment approach to hepatocellular carcinoma. J Gastroenterol. 2005;40:225-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 390] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 8. | Wang Y, Luo Q, Li Y, Deng S, Wei S, Li X. Radiofrequency ablation versus hepatic resection for small hepatocellular carcinomas: a meta-analysis of randomized and nonrandomized controlled trials. PLoS One. 2014;9:e84484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 9. | Zheng Z, Liang W, Milgrom DP, Zheng Z, Schroder PM, Kong NS, Yang C, Guo Z, He X. Liver transplantation versus liver resection in the treatment of hepatocellular carcinoma: a meta-analysis of observational studies. Transplantation. 2014;97:227-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Yamashita T, Kaneko S. Treatment strategies for hepatocellular carcinoma in Japan. Hepatol Res. 2013;43:44-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Yu YQ, Xu DB, Zhou XD, Lu JZ, Tang ZY, Mack P. Experience with liver resection after hepatic arterial chemoembolization for hepatocellular carcinoma. Cancer. 1993;71:62-65. [PubMed] |
| 12. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10237] [Article Influence: 602.2] [Reference Citation Analysis (2)] |
| 13. | Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3854] [Cited by in RCA: 4638] [Article Influence: 272.8] [Reference Citation Analysis (0)] |
| 14. | Ando E, Tanaka M, Yamashita F, Kuromatsu R, Yutani S, Fukumori K, Sumie S, Yano Y, Okuda K, Sata M. Hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis: analysis of 48 cases. Cancer. 2002;95:588-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 259] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 15. | Kudo M, Izumi N, Kokudo N, Matsui O, Sakamoto M, Nakashima O, Kojiro M, Makuuchi M. Management of hepatocellular carcinoma in Japan: Consensus-Based Clinical Practice Guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis. 2011;29:339-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 663] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 16. | Song P, Tobe RG, Inagaki Y, Kokudo N, Hasegawa K, Sugawara Y, Tang W. The management of hepatocellular carcinoma around the world: a comparison of guidelines from 2001 to 2011. Liver Int. 2012;32:1053-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 17. | Koom WS, Seong J, Han KH, Lee do Y, Lee JT. Is local radiotherapy still valuable for patients with multiple intrahepatic hepatocellular carcinomas? Int J Radiat Oncol Biol Phys. 2010;77:1433-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Li B, Yu J, Wang L, Li C, Zhou T, Zhai L, Xing L. Study of local three-dimensional conformal radiotherapy combined with transcatheter arterial chemoembolization for patients with stage III hepatocellular carcinoma. Am J Clin Oncol. 2003;26:e92-e99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Seong J, Park HC, Han KH, Chon CY. Clinical results and prognostic factors in radiotherapy for unresectable hepatocellular carcinoma: a retrospective study of 158 patients. Int J Radiat Oncol Biol Phys. 2003;55:329-336. [PubMed] |
| 20. | Zeng ZC, Tang ZY, Fan J, Zhou J, Qin LX, Ye SL, Sun HC, Wang BL, Yu Y, Wang JH. A comparison of chemoembolization combination with and without radiotherapy for unresectable hepatocellular carcinoma. Cancer J. 2004;10:307-316. [PubMed] |
| 21. | Zhou ZH, Liu LM, Chen WW, Men ZQ, Lin JH, Chen Z, Zhang XJ, Jiang GL. Combined therapy of transcatheter arterial chemoembolisation and three-dimensional conformal radiotherapy for hepatocellular carcinoma. Br J Radiol. 2007;80:194-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Koo JE, Kim JH, Lim YS, Park SJ, Won HJ, Sung KB, Suh DJ. Combination of transarterial chemoembolization and three-dimensional conformal radiotherapy for hepatocellular carcinoma with inferior vena cava tumor thrombus. Int J Radiat Oncol Biol Phys. 2010;78:180-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 23. | Takagi H, Takayama H, Yamada S, Uehara M, Ojima T, Saitoh S, Katakai S, Yamada T, Abe T, Sakurai S. [Radiation therapy of hepatocellular carcinoma]. Nihon Shokakibyo Gakkai Zasshi. 1989;86:237-245. [PubMed] |
| 24. | Zhang XB, Wang JH, Yan ZP, Qian S, Du SS, Zeng ZC. Hepatocellular carcinoma with main portal vein tumor thrombus: treatment with 3-dimensional conformal radiotherapy after portal vein stenting and transarterial chemoembolization. Cancer. 2009;115:1245-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 25. | Shim SJ, Seong J, Han KH, Chon CY, Suh CO, Lee JT. Local radiotherapy as a complement to incomplete transcatheter arterial chemoembolization in locally advanced hepatocellular carcinoma. Liver Int. 2005;25:1189-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 96] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 26. | Oh D, Lim do H, Park HC, Paik SW, Koh KC, Lee JH, Choi MS, Yoo BC, Lim HK, Lee WJ. Early three-dimensional conformal radiotherapy for patients with unresectable hepatocellular carcinoma after incomplete transcatheter arterial chemoembolization: a prospective evaluation of efficacy and toxicity. Am J Clin Oncol. 2010;33:370-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Cho JY, Paik YH, Park HC, Yu JI, Sohn W, Gwak GY, Choi MS, Lee JH, Koh KC, Paik SW. The feasibility of combined transcatheter arterial chemoembolization and radiotherapy for advanced hepatocellular carcinoma. Liver Int. 2014;34:795-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 28. | Tang QH, Li AJ, Yang GM, Lai EC, Zhou WP, Jiang ZH, Lau WY, Wu MC. Surgical resection versus conformal radiotherapy combined with TACE for resectable hepatocellular carcinoma with portal vein tumor thrombus: a comparative study. World J Surg. 2013;37:1362-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 29. | Cha J, Seong J, Lee IJ, Kim JW, Han KH. Feasibility of sorafenib combined with local radiotherapy in advanced hepatocellular carcinoma. Yonsei Med J. 2013;54:1178-1185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Wild AT, Gandhi N, Chettiar ST, Aziz K, Gajula RP, Williams RD, Kumar R, Taparra K, Zeng J, Cades JA. Concurrent versus sequential sorafenib therapy in combination with radiation for hepatocellular carcinoma. PLoS One. 2013;8:e65726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Yu W, Gu K, Yu Z, Yuan D, He M, Ma N, Lai S, Zhao J, Ren Z, Zhang X. Sorafenib potentiates irradiation effect in hepatocellular carcinoma in vitro and in vivo. Cancer Lett. 2013;329:109-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 32. | Murakami E, Aikata H, Miyaki D, Nagaoki Y, Katamura Y, Kawaoka T, Takaki S, Hiramatsu A, Waki K, Takahashi S. Hepatic arterial infusion chemotherapy using 5-fluorouracil and systemic interferon-α for advanced hepatocellular carcinoma in combination with or without three-dimensional conformal radiotherapy to venous tumor thrombosis in hepatic vein or inferior vena cava. Hepatol Res. 2012;42:442-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Chuma M, Taguchi H, Yamamoto Y, Shimizu S, Nakanishi M, Ogawa K, Sho T, Horimoto H, Kobayashi T, Nakai M. Efficacy of therapy for advanced hepatocellular carcinoma: intra-arterial 5-fluorouracil and subcutaneous interferon with image-guided radiation. J Gastroenterol Hepatol. 2011;26:1123-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 34. | Wigg AJ, Palumbo K, Wigg DR. Radiotherapy for hepatocellular carcinoma: systematic review of radiobiology and modeling projections indicate reconsideration of its use. J Gastroenterol Hepatol. 2010;25:664-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 35. | Choi BO, Choi IB, Jang HS, Kang YN, Jang JS, Bae SH, Yoon SK, Chai GY, Kang KM. Stereotactic body radiation therapy with or without transarterial chemoembolization for patients with primary hepatocellular carcinoma: preliminary analysis. BMC Cancer. 2008;8:351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 36. | Louis C, Dewas S, Mirabel X, Lacornerie T, Adenis A, Bonodeau F, Lartigau E. Stereotactic radiotherapy of hepatocellular carcinoma: preliminary results. Technol Cancer Res Treat. 2010;9:479-487. [PubMed] |
| 37. | Blomgren H, Lax I, Göranson H, Kræpelien T, Nilsson B, Näslund I, Svanström R, Tilikidis A. Radiosurgery for Tumors in the Body: Clinical Experience Using a New Method. J Radiosurgery. 1998;1:63-74. [RCA] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 144] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 38. | Seong J, Park HC, Han KH, Lee DY, Lee JT, Chon CY, Moon YM, Suh CO. Local radiotherapy for unresectable hepatocellular carcinoma patients who failed with transcatheter arterial chemoembolization. Int J Radiat Oncol Biol Phys. 2000;47:1331-1335. [PubMed] |
| 39. | Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, Lin XJ, Lau WY. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1100] [Cited by in RCA: 1101] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 40. | Feng K, Yan J, Li X, Xia F, Ma K, Wang S, Bie P, Dong J. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol. 2012;57:794-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 594] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 41. | Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2645] [Cited by in RCA: 2866] [Article Influence: 110.2] [Reference Citation Analysis (1)] |
| 42. | Andolino DL, Johnson CS, Maluccio M, Kwo P, Tector AJ, Zook J, Johnstone PA, Cardenes HR. Stereotactic body radiotherapy for primary hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2011;81:e447-e453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 320] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 43. | Bujold A, Dawson LA. Stereotactic radiation therapy and selective internal radiation therapy for hepatocellular carcinoma. Cancer Radiother. 2011;15:54-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 44. | Choi BO, Jang HS, Kang KM, Lee SW, Kang YN, Chai GY, Choi IB. Fractionated stereotactic radiotherapy in patients with primary hepatocellular carcinoma. Jpn J Clin Oncol. 2006;36:154-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 45. | Goodman KA, Wiegner EA, Maturen KE, Zhang Z, Mo Q, Yang G, Gibbs IC, Fisher GA, Koong AC. Dose-escalation study of single-fraction stereotactic body radiotherapy for liver malignancies. Int J Radiat Oncol Biol Phys. 2010;78:486-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 213] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 46. | Huang WY, Jen YM, Lee MS, Chang LP, Chen CM, Ko KH, Lin KT, Lin JC, Chao HL, Lin CS. Stereotactic body radiation therapy in recurrent hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2012;84:355-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 146] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 47. | Kwon JH, Bae SH, Kim JY, Choi BO, Jang HS, Jang JW, Choi JY, Yoon SK, Chung KW. Long-term effect of stereotactic body radiation therapy for primary hepatocellular carcinoma ineligible for local ablation therapy or surgical resection. Stereotactic radiotherapy for liver cancer. BMC Cancer. 2010;10:475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 195] [Cited by in RCA: 197] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 48. | Méndez Romero A, Wunderink W, Hussain SM, De Pooter JA, Heijmen BJ, Nowak PC, Nuyttens JJ, Brandwijk RP, Verhoef C, Ijzermans JN. Stereotactic body radiation therapy for primary and metastatic liver tumors: A single institution phase i-ii study. Acta Oncol. 2006;45:831-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 352] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 49. | Son SH, Choi BO, Ryu MR, Kang YN, Jang JS, Bae SH, Yoon SK, Choi IB, Kang KM, Jang HS. Stereotactic body radiotherapy for patients with unresectable primary hepatocellular carcinoma: dose-volumetric parameters predicting the hepatic complication. Int J Radiat Oncol Biol Phys. 2010;78:1073-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 108] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 50. | Takeda A, Takahashi M, Kunieda E, Takeda T, Sanuki N, Koike Y, Atsukawa K, Ohashi T, Saito H, Shigematsu N. Hypofractionated stereotactic radiotherapy with and without transarterial chemoembolization for small hepatocellular carcinoma not eligible for other ablation therapies: Preliminary results for efficacy and toxicity. Hepatol Res. 2008;38:60-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 51. | Tse RV, Hawkins M, Lockwood G, Kim JJ, Cummings B, Knox J, Sherman M, Dawson LA. Phase I study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol. 2008;26:657-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 410] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 52. | Lo SS, Dawson LA, Kim EY, Mayr NA, Wang JZ, Huang Z, Cardenes HR. Stereotactic body radiation therapy for hepatocellular carcinoma. Discov Med. 2010;9:404-410. [PubMed] |
| 53. | Tsujii H, Mizoe J, Kamada T, Baba M, Tsuji H, Kato H, Kato S, Yamada S, Yasuda S, Ohno T. Clinical Results of Carbon Ion Radiotherapy at NIRS. J Radiat Res. 2007;48 Suppl A:A1-A13. [PubMed] |
| 54. | Sato K, Yamada S, Ogawa H, Kawachi K, Araki N, Itano A, Kanazawa M, Kitagawa A, Kohno T, Kumada M. Performance of Himac. Nucl Phys A. 1995;588:C229-C234. [DOI] [Full Text] |
| 55. | Fokas E, Kraft G, An H, Engenhart-Cabillic R. Ion beam radiobiology and cancer: time to update ourselves. Biochim Biophys Acta. 2009;1796:216-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 56. | Wilson RR. Radiological use of fast protons. Radiology. 1946;47:487-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 825] [Cited by in RCA: 575] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 57. | Linstadt DE, Castro JR, Phillips TL. Neon ion radiotherapy: results of the phase I/II clinical trial. Int J Radiat Oncol Biol Phys. 1991;20:761-769. [PubMed] |
| 58. | Kato H, Tsujii H, Miyamoto T, Mizoe JE, Kamada T, Tsuji H, Yamada S, Kandatsu S, Yoshikawa K, Obata T. Results of the first prospective study of carbon ion radiotherapy for hepatocellular carcinoma with liver cirrhosis. Int J Radiat Oncol Biol Phys. 2004;59:1468-1476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 127] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 59. | Imada H, Kato H, Yasuda S, Yamada S, Yanagi T, Kishimoto R, Kandatsu S, Mizoe JE, Kamada T, Yokosuka O. Comparison of efficacy and toxicity of short-course carbon ion radiotherapy for hepatocellular carcinoma depending on their proximity to the porta hepatis. Radiother Oncol. 2010;96:231-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 60. | Mizumoto M, Okumura T, Hashimoto T, Fukuda K, Oshiro Y, Fukumitsu N, Abei M, Kawaguchi A, Hayashi Y, Ookawa A. Proton beam therapy for hepatocellular carcinoma: a comparison of three treatment protocols. Int J Radiat Oncol Biol Phys. 2011;81:1039-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 124] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 61. | Bush DA, Kayali Z, Grove R, Slater JD. The safety and efficacy of high-dose proton beam radiotherapy for hepatocellular carcinoma: a phase 2 prospective trial. Cancer. 2011;117:3053-3059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 138] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 62. | Fukumitsu N, Sugahara S, Nakayama H, Fukuda K, Mizumoto M, Abei M, Shoda J, Thono E, Tsuboi K, Tokuuye K. A prospective study of hypofractionated proton beam therapy for patients with hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2009;74:831-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 163] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 63. | Uchino K, Tateishi R, Shiina S, Kanda M, Masuzaki R, Kondo Y, Goto T, Omata M, Yoshida H, Koike K. Hepatocellular carcinoma with extrahepatic metastasis: clinical features and prognostic factors. Cancer. 2011;117:4475-4483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 326] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 64. | Habermehl D, Haase K, Rieken S, Debus J, Combs SE. Defining the role of palliative radiotherapy in bone metastasis from primary liver cancer: an analysis of survival and treatment efficacy. Tumori. 2011;97:609-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 65. | Jiang W, Zeng ZC, Zhang JY, Fan J, Zeng MS, Zhou J. Palliative radiation therapy for pulmonary metastases from hepatocellular carcinoma. Clin Exp Metastasis. 2012;29:197-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 66. | Yoon SM, Kim JH, Choi EK, Ahn SD, Lee SW, Yi BY, Chung YW, Lee YS, Seo DJ. Radioresponse of hepatocellular carcinoma-treatment of lymph node metastasis. Cancer Res Treat. 2004;36:79-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 67. | Zeng ZC, Tang ZY, Fan J, Zhou J, Qin LX, Ye SL, Sun HC, Wang BL, Zhang JY, Yu Y. Radiation therapy for adrenal gland metastases from hepatocellular carcinoma. Jpn J Clin Oncol. 2005;35:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 68. | Niibe Y, Hayakawa K. Oligometastases and oligo-recurrence: the new era of cancer therapy. Jpn J Clin Oncol. 2010;40:107-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 217] [Cited by in RCA: 273] [Article Influence: 18.2] [Reference Citation Analysis (0)] |