Published online Jan 7, 2015. doi: 10.3748/wjg.v21.i1.333
Peer-review started: March 23, 2014
First decision: April 2, 2014
Revised: May 5, 2014
Accepted: July 22, 2014
Article in press: July 22, 2014
Published online: January 7, 2015
Processing time: 291 Days and 17.6 Hours
AIM: To evaluate the diagnostic and prognostic value of circulating Metastasis Associated in Colon Cancer 1 (MACC1) transcripts in plasma of gastric cancer patients.
METHODS: We provide for the first time a blood-based assay for transcript quantification of the metastasis inducer MACC1 in a prospective study of gastric cancer patient plasma. MACC1 is a strong prognostic biomarker for tumor progression and metastasis in a variety of solid cancers. We conducted a study to define the diagnostic and prognostic power of MACC1 transcripts using 76 plasma samples from gastric cancer patients, either newly diagnosed with gastric cancer, newly diagnosed with metachronous metastasis of gastric cancer, as well as follow-up patients. Findings were controlled by using plasma samples from 54 tumor-free volunteers. Plasma was separated, RNA was isolated, and levels of MACC1 as well as S100A4 transcripts were determined by quantitative RT-PCR.
RESULTS: Based on the levels of circulating MACC1 transcripts in plasma we significantly discriminated tumor-free volunteers and gastric cancer patients (P < 0.001). Levels of circulating MACC1 transcripts were increased in gastric cancer patients of each disease stage, compared to tumor-free volunteers: patients with tumors without metastasis (P = 0.005), with synchronous metastasis (P = 0.002), with metachronous metastasis (P = 0.005), and patients during follow-up (P = 0.021). Sensitivity was 0.68 (95%CI: 0.45-0.85) and specificity was 0.89 (95%CI: 0.77-0.95), respectively. Importantly, gastric cancer patients with high circulating MACC1 transcript levels in plasma demonstrated significantly shorter survival when compared with patients demonstrating low MACC1 levels (P = 0.0015). Furthermore, gastric cancer patients with high circulating transcript levels of MACC1 as well as of S100A4 in plasma demonstrated significantly shorter survival when compared with patients demonstrating low levels of both biomarkers or with only one biomarker elevated (P = 0.001).
CONCLUSION: Levels of circulating MACC1 transcripts in plasma of gastric cancer patients are of diagnostic value and are prognostic for patient survival in a prospective study.
Core tip: We provide for the first time a blood-based assay for transcript quantification of the metastasis inducer Metastasis Associated in Colon Cancer 1 (MACC1) in a prospective study of gastric cancer patients. MACC1 is a strong prognostic biomarker for tumor progression and metastasis in a variety of solid cancers. We discriminated tumor-free volunteers and gastric cancer patients (all P < 0.001, sensitivity 0.68 (95%CI: 0.45-0.85), specificity 0.89 (95%CI: 0.77-0.95) of each disease stage (P < 0.05 for each). Shorter survival correlated with high circulating MACC1 transcript levels (P = 0.0015). Thus, circulating MACC1 transcript levels in plasma of gastric cancer patients are of diagnostic value and are prognostic for patient survival.
- Citation: Burock S, Herrmann P, Wendler I, Niederstrasser M, Wernecke KD, Stein U. Circulating metastasis associated in colon cancer 1 transcripts in gastric cancer patient plasma as diagnostic and prognostic biomarker. World J Gastroenterol 2015; 21(1): 333-341
- URL: https://www.wjgnet.com/1007-9327/full/v21/i1/333.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i1.333
Gastric cancer is one of the major malignancies in the world with an estimated incidence of 140 cases per 100000 people in Europe in 2012[1]. Although there is a steady decline in gastric cancer mortality it is still the fourth most common cause of death from cancer as it is mainly diagnosed at an advanced stage. Overall survival is clearly linked to disease stage and metastasis formation. Despite the recent developments in both, chemotherapy and surgical treatment, the outcome for patients with distant metastasis is still poor with a 5-year survival rate of less than 5% compared to 60% for patients with only localised malignancies[2]. Therefore, detecting the disease already at an early stage and identifying that patient cohort with a high risk of developing metastasis is crucial and could lead to a significant better outcome.
In previous studies we have identified the previously undescribed gene Metastasis-Associated in Colon Cancer (MACC1)[3]. MACC1 induces cell proliferation, cell motility such as migration and invasion, as well as dissemination in cell culture. MACC1 regulates the transcription of genes important for the development of metastasis, such as the receptor tyrosine kinase Met. Furthermore, MACC1 induces metastasis formation in several xenografted mouse models. Following our initial publication, a variety of groups provided evidence that MACC1 contributes to fundamental biological processes such as apoptosis and Epithelial-Mesenchymal Transistion via well-known pathways such as the Hepatocyte Growth Factor (HGF)/met protooncogen (Met)/MACC1 axis. Thus, MACC1 was confirmed as decisive driver for tumorigenesis and metastasis[4].
MACC1 is an independent prognostic indicator for colon cancer metastasis formation and allows identification of high-risk subjects in early stages[3,5,6]. The role of MACC1 as prognostic biomarker for colorectal cancer patients with respect to time of progression, metastasis formation, and overall survival was meanwhile confirmed in a panel of independent studies using different techniques for MACC1 detection in tissues and blood[4]. Furthermore, the importance of MACC1 was extended by cross-entity approaches to a variety of several other solid cancers such as pancreatic, hepatocellular, hepatobiliary, lung, ovarian, breast, nasopharyngeal, esophageal, kidney, bladder, gallbladder cancers, to glioblastomas and osteosarcomas[4].
In gastric primary tumors, the presence of MACC1 correlated with peritoneal metastasis, lymph node metastasis and hepatic metastasis, and finally to a poor prognosis for the patients[7]. Wang and colleagues described an association of high MACC1 expression in the primary tumors of gastric cancer patients with more advanced disease, more frequent postoperative recurrence, more metastases, higher mortality rate, and worse survival[8]. Furthermore, a high expression of MACC1 in gastric tumors was found to be correlated with peritoneal dissemination of the tumor[9]. All these findings underline the importance of MACC1 as a biomarker for tumor progression, metastasis and survival also for gastric cancer patients.
However, molecular analyses of tumor tissues have some limitations due to a restricted availability of tumor tissue, intra-tumoral heterogeneity and the invasive character of tumor biopsies. Therefore, the establishment of blood-based tests have become more and more important. Lately we described a non-invasive, blood-based assay for circulating MACC1 transcript levels and demonstrated its diagnostic and prognostic value in the plasma of colorectal cancer patients; analyses were based on the biomarker MACC1 alone as well as in combination with the previously described metastasis gene S100A4 with respect to an improved prediction of outcome[10,11].
For gastric cancer patients, however, such a test is neither developed nor evaluated with respect to diagnose gastric cancer patients vs tumor-free volunteers or concerning the prognosis of the disease. Thus, here we aim to evaluate the diagnostic and prognostic value of circulating MACC1 transcripts in plasma of a patient cohort with gastric cancer. We hypothesize that analysing the MACC1 levels in liquid biopsies of gastric cancer patients might also be of benefit for patients as demonstrated for patients suffering from other gastrointestinal cancers such as colon and rectal cancer, also developing distant metastases[10]. Furthermore, we will combine this MACC1 data with those of circulating transcripts of the metastasis gene S100 calcium binding protein A4 (S100A4) for potential improvement of disease prognosis.
We analyzed 76 blood samples of consecutive gastric cancer patients who were treated at the Robert Rössle Cancer Hospital, Charité University Medicine Berlin, during March 2006 until March 2007 for the first or for a further time. Patients’ data for survival and the routine histopathological characterization of the tumor (including tumor infiltration, lymph node status, metastasis, grading, lymphatic vessels infiltration, blood vessels infiltration, residual tumor) were available from the tumor bank. Exclusion criteria were tumor development of another entity during history or follow-up. For more details of the patients’ characteristics see Table 1.
| Primary diagnosis | Follow-up | All cancer patients | |||
| W/o synchronous organ metastasis | With synchronous organ metastasis | With metachronous organ metastasis | |||
| Samples, n | 9 | 8 | 5 | 54 | 76 |
| AJCC/UICC I, % | 67 | 0 | 80 | 48 | 47 |
| T1-T2, N0, M0 | |||||
| T1, N1, M0 | |||||
| AJCC/UICC II, % | 11 | 0 | 0 | 28 | 21 |
| T1, N2, M0 | |||||
| T2, N1, M0 | |||||
| T3, N0, M0 | |||||
| AJCC/UICC III, % | 11 | 0 | 20 | 24 | 20 |
| T2, N2, M0 | |||||
| T3, N1, M0 | |||||
| T4, N0, M0 | |||||
| T3, N2, M0 | |||||
| AJCC/UICC IV, % | 11 | 100 | 0 | 0 | 12 |
| T4, N1-2, M0 | |||||
| Every T, N3, M0 every T, every N, M1 | |||||
| Median follow up, after blood taking (d) | 677 | 351 | 169 | 851, 5 | 803 |
| Age at blood taking, median (range) (yr) | 63 (48-77) | 59 (56-68) | 71 (39-83) | 63 (40-86) | 63 (39-86) |
| Sex, m/f | 8/1 | 7/1 | 4/1 | 38/16 | 57/19 |
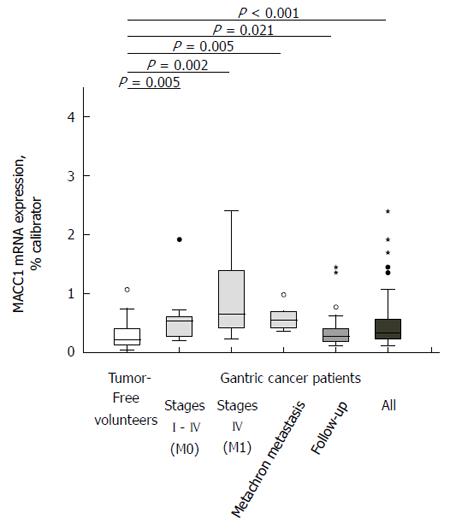
| P, vs tumor-free volunteers | P = 0.005 | P = 0.002 | P = 0.005 | P = 0.021 | P < 0.001 |
Blood samples of patients with newly diagnosed gastric cancer and blood samples of patients with newly diagnosed metachronous metastasis of gastric cancer were taken at the day of diagnosis. Blood samples from the cohort of follow-up patients were taken exclusively during the follow-up (median 1003 d after primary diagnosis) with a median follow-up period of 852 d after blood taking. Six blood samples out of 54 were from patients who developed metastasis later on (median 270 d after blood taking).
The plasma controls derived from two independent cohorts of healthy volunteers (n = 54). Median age of the tumor-free volunteers at the time of blood taking was 61 (27-87). The cohort of the tumor-free volunteers consisted of 43 male and 11 female tumor-free volunteers. Recruitment of the volunteers was supported by Klaus Sperber, Medical Practioner, Berlin, and by Ursula Plöckinger, Charité Campus Virchow Klinikum, Berlin. All volunteers were tumor-free and had no history of oncological diseases. We did not find significantly different MACC1 levels within the two cohorts of tumor-free volunteers, nor differences with respect to gender or age of the healthy individuals.
All blood specimens from patients and tumor-free volunteers were collected after written informed consent in accordance with the International Conference on Harmonisation and with the approval of the local Institutional Review Board.
Plasma separation was performed from cooled EDTA-blood at the same day within 7 h post blood taking. Procedure for plasma separation from blinded samples was as previously described[10,11]. Samples were stored at -80 °C.
Isolation of total RNA and quantitative real-time reverse transcriptase-polymerase chain reaction (qRT-PCR) were performed as previously described[10,11]. Briefly, after 30 s at 95 °C, we run 45 cycles of 10 s 95 °C, 10 s 62 °C, 10 s 72 °C, and a melting curve from 40 °C to 95 °C, using the LightCycler (DNA Master Hybridization Probes kit, Roche Diagnostics). Thereby we amplified a 136 bp MACC1-specific PCR product with the following primers and probes: forward primer 5’-TTCTTTTGATTCCTCCGGTGA-3’, reverse primer 5’-ACTCTGATGGGCATGTGCTG-3’, FITC-probe 5’-GCAGACTTCCTCAAGAAATTCTGGAAGATCTA-3’, LCRed640-probe 5’-AGTGTTTCAGAACTTCTGGACATTTTAGACGA-3’ (syntheses of primers and probes: BioTeZ and TIB MolBiol, Berlin, Germany). The calibrator cDNA derived from the cell lines SW620 (authentification of the cell line by short tandem repeat (STR) genotyping, German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany).
S100A4-specific qRT-PCR was performed as previously described. MACC1 and S100A4 mRNA expressions are given as percentage of the mRNA expression of a calibrator sample, which was set 100%. Each sample was run and calculated in duplicate, the means are depicted.
We tested differences between groups in terms of MACC1 transcript levels in plasma by using non-parametric (exact) Wilcoxon- Mann-Whitney tests (because of deviations of the distributions from normality and small samples). Samples obtained from tumor-free volunteers were compared with those from patients with primary tumors without and with synchronous organ metastases, patients with metachronous metastases, and follow-up patients. Furthermore, samples obtained from patients with tumors vs those with tumors and metastases were compared. Results are expressed as median (range) or frequencies (%). P < 0.05 was considered to be significant. All tests were conducted in the area of exploratory data analysis. Therefore, no adjustments for multiple testing have been made.
For evaluating the diagnostic value of circulating MACC1 transcripts in plasma of gastric cancer patients, we calculated sensitivity and specificity with a fourfold table for those cancer patients, who were newly diagnosed with a primary tumor without or with synchronous metastases compared to the blood samples of 54 tumor-free volunteers.
We used Kaplan Meier curves in combination with log rank test for survival analyses of newly diagnosed and of all gastric patients. Both biomarkers, MACC1 as well as S100A4, could be determined in 70 plasma samples. The respective cut-off values for the biomarkers were the median of the investigated groups (primary diagnosis or all patients). We performed all calculations with SPSS, version 21.
First, we tested on whether the levels of circulating MACC1 transcripts are different in tumor-free volunteers (n = 54) and gastric cancer patients (n = 76). We detected MACC1 transcripts in all samples analyzed. Significantly higher MACC1 levels were measured in cancer patient plasma (median 0.334 MACC1 mRNA expression/percent calibrator) compared to the tumor-free individuals (median 0.224 MACC1 mRNA expression/percent calibrator; P < 0.001; Figure 1). We observed no significant variations of MACC1 levels due to age or sex.
We then tested the levels of MACC1 of different disease stages with respect to the tumor-free volunteers, such as tumors without metastasis (median 0.531 MACC1 mRNA expression/percent calibrator; P = 0.005), with synchronous metastasis (median 0.655 MACC1 mRNA expression/percent calibrator; P = 0.002), with metachronous metastasis (median 0.561 MACC1 mRNA expression/percent calibrator; P = 0.005), and during follow-up (median 0.272 MACC1 mRNA expression/percent calibrator; P = 0.021). Interestingly, levels of MACC1 were significantly higher in each disease stage when compared with tumor-free volunteers (Figure 1).
To evaluate sensitivity and specificity of this blood-based assay for circulating MACC1 transcript levels, values were calculated with a fourfold table for patients with present gastric cancer. More precisely, blood samples of patients with newly diagnosed primary gastric cancer (with or without synchronous metastases), and patients who already underwent R0-surgery of primary gastric cancer and were newly diagnosed with metachronous metastases were compared to the tumor-free volunteers with consecutive cut-off values. The MACC1 transcript level with the highest sum of specificity and sensitivity was selected as the optimal cut-off point[12]. This resulted in a cut-off point of 0.504 MACC1 mRNA expression/% calibrator with a sensitivity of 0.68 (95%CI: 0.45-0.85) and a specificity of 0.89 (95%CI: 0.77-0.95). Thus, MACC1 transcript levels in plasma support the identification of individuals suffering of gastric cancer and might contribute to the identification of metastasis formation.
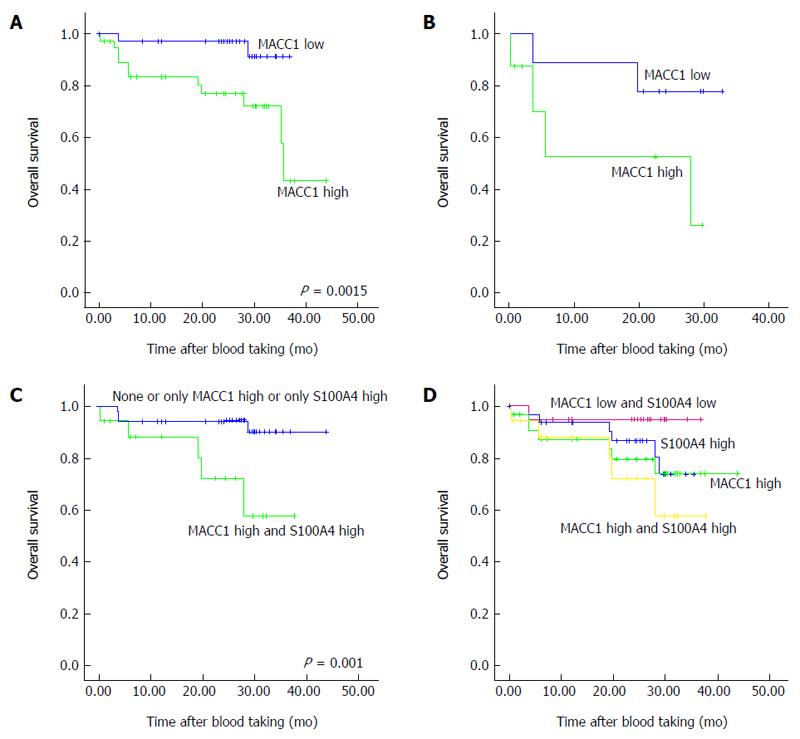
To investigate the prognostic value of high vs low MACC1 transcript levels in the plasma of gastric cancer patients within this prospective study, survival analysis were performed with Kaplan Meier curves in combination with log rank rests. The cut-off values of high vs low MACC1 transcripts levels were the median of the investigated groups, respectively. First, we analyzed the MACC1 levels of all patients (primary diagnosis of gastric cancer with and without synchronous metastasis, patients with metachronous metastasis and patients during follow up) with respect to overall survival. Patients with high MACC1 transcript levels had a statistically significant worse overall survival compared with patients with low MACC1 levels (P = 0.015; Figure 2A) with a median follow-up period of 803 d after blood taking. Additionally, also the subcohort of patients with newly diagnosed gastric cancer and high MACC1 transcript levels demonstrated a shorter overall survival compared to patients with low MACC1 levels (Figure 2B) with a median follow-up period of 618 d after blood taking.
To improve the prognosis for patient survival, we added a further metastasis gene, S100A4, to this analysis. S100A4 is known to contribute to metastasis formation for several cancers[13,14]. For gastric cancer, S100A4 is also linked to tumor progression and development of metastasis as well as to disease prognosis when determined in the primary tumor tissue[15-20]. Previously we described the diagnostic and prognostic value of S100A4 transcripts in plasma of gastrointestinal cancer patients, such as for colon, rectal and gastric cancer patients[11]. Therefore, we combined here the analyses for circulating MACC1 as well as S100A4 transcripts to test for improvement of the disease prognosis compared to analyses based exclusively on MACC1. First we compared two groups, those showing none or only one biomarker at high levels (n = 52), with patients demonstrating high levels of both biomarkers (n = 18). We demonstrated a significantly shorter overall survival for patients having both MACC1 as well as S100A4 at high levels (P = 0.01; Figure 2C). Remarkably, the combination of the biomarkers improved the prognosis compared to the analyses based on each single biomarker. This is further illustrated, when classifying the patients into 4 groups, those showing both biomarkers at low levels (MACC1 and S100A4 low), or with MACC1 high, or with S100A4 high, or in a group of patients showing high levels of both biomarkers (MACC1 and S100A4 high) (Figure 2D).
Taken together, prognosis of gastric cancer patient survival can be based on plasma levels of circulating MACC1 transcripts, and can be improved when combining the information of circulating transcripts of both the metastasis biomarkers, MACC1 and S100A4.
Here we report the diagnostic and prognostic value of circulating MACC1 transcripts in blood of patients with gastric cancer. Based on the levels of circulating MACC1 transcripts in plasma we significantly discriminate tumor-free volunteers and gastric cancer patients. Furthermore we demonstrate in this prospective study that circulating MACC1 transcript levels in plasma are prognostic for the survival of the gastric cancer patients. Finally we show, that the combination of biomarkers, here MACC1 and S100A4, improve gastric cancer patient survival prognosis. Thus, the quantitative determination of circulating transcript levels of MACC1, as well as of MACC1 and S100A4, is proven to be useful for gastric cancer patient survival prognosis.
We newly identified the gene MACC1 in colorectal cancer patients and demonstrated its prognostic value for colorectal cancer patient survival based on expression in patient tissue[3]. We showed its ability to induced cell motility in vitro and metastasis in mice. Moreover, we provided evidence for MACC1 as master regulator of further metastasis genes, for instance of the receptor tyrosine kinase Met[6]. Meanwhile, MACC1 is confirmed as biomarker by a large body of publications for prognosis of tumor progression and metastasis as well as for prediction of therapy response for a great variety of solid cancers, linked to patient survival[4].
However, in order to overcome the limitation of prognosis generation based on the analysis of only a very small number of available tissues per patient, we developed this non-invasive blood-based assay for circulating transcripts[10,11]. This assay harbors the potential to be employed for screening of the quantitation of circulating MACC1 transcripts on a routine basis. It is clinically applicable for diagnosis and prognosis of cancer patients, as shown here for patients with gastric cancer. Furthermore, it might be also used for monitoring of response to a defined treatment of cancer patients. In addition, this assay can easily be translated for the quantitative determination of further circulating transcripts which are of diagnostic, prognostic and/or predictive value for a defined disease.
In this study, we significantly classified gastric cancer patients from tumor-free volunteers, based on this assay for circulating MACC1 transcripts. Interestingly, highest levels of circulating MACC1 transcripts were found in individuals with metastases further underlining the diagnostic value of circulating MACC1 transcripts in patient plasma with respect to metastasis formation. Most importantly, these prospectively determined high levels of MACC1 transcripts correlate with shorter survival of the patients demonstrating the prognostic value of circulating MACC1 transcripts. Thus, this assay could be used for patient screening as well as for metastasis detection during follow-up to identify the patient population at high risk. This finding might imply also importance for patient stratification in future studies.
Our findings of circulating MACC1 transcripts in plasma of gastric cancer patients for diagnosis and survival prognosis are in line with findings reported previously but based on the quantification of MACC1 mRNA expression in gastric cancer tissues. Guo and colleagues showed a correlation of MACC1 in the primary gastric tumors with peritoneal metastasis, lymph node metastasis, hepatic metastasis, and finally to a poor prognosis for patients[7]. Wang and colleagues reported a correlation of high MACC1 expression in the primary tumors with more advanced disease, more frequent postoperative recurrence, more metastases, higher mortality rate, and shorter survival of the gastric cancer patients[8]. Shirahata and colleagues demonstrated a correlation of high expression of MACC1 in gastric tumors with peritoneal dissemination of the tumor[9]. All of these data point to the importance of MACC1 as a biomarker for tumor progression, metastasis and survival for gastric cancer patients. Moreover, these findings underline the relevance of the further employment of MACC1 as a biomarker to be quantitatively and repeatedly determined in gastric cancer patient blood.
Our new findings of circulating MACC1 transcripts in plasma of gastric cancer patients for diagnosis and prediction of survival are also in line with those that we reported previously for colorectal cancer[10]. Thus, the use of MACC1 as a biomarker from liquid biopsies for patient stratification might be extended from tissues to this blood-based assay allowing repeated measurements of circulating MACC1 transcript levels.
Since the quantitative determination of cell-free, circulating mRNA in blood as liquid biopsies allows real-time monitoring of disease progress, prognosis, and therapeutic response[21], the combined detection of different biomarkers in patient blood might offer additional options for improvement of diagnosis, prognosis of response prediction. Several markers, such as Carcino Embryonic Antigen, Carbohydrate Antigen 19-9 and Carbohydrate Antigen 72-4 have determined in blood of gastric cancer patients, however, with limited sensitivities (20%-30%)[22-24]. Recently the mRNA levels of KRAB-ZFP-Associated Protein 1, Tissue Inhibitor of Metalloproteinases 1, and Stanniocalcin 2 were determined in peripheral blood samples from pre-operative gastric cancer patients, patients with recurrence, and healthy volunteers and were identified as potential biomarkers for screening, diagnosis, prognosis and surveillance of gastric cancer[25]. Another recent report describes an association of increased Bone Morphogenetic Protein 8b mRNA levels in peripheral blood of gastric cancer patients with metastatic disease[26].
Here, we combined the data for circulating MACC1 levels with those of S100A4, a well-known metastasis-inducing gene. S100A4 expression was linked to tumor progression and development of metastasis and has been shown to be of prognostic value when determined in the primary gastric tumors tissue[15-20]. Moreover, Kim and colleagues demonstrated that the combination of S100A4 and p53 are useful for prediction of relapse in curatively resected stage III and IV (M0) gastric cancer[17]. We already established this blood-based assay for S100A4 and demonstrated the diagnostic and prognostic value of circulating S100A4 transcripts in colorectal cancer and in gastric cancer patients[11]. When we here combined the data of two major metastasis-inducing genes, MACC1 as master regulator of the HGF/Met signaling axis[3,6] with those of S100A4, a Wnt/β-catenin target gene[14], disease prognosis for gastric cancer patients was improved compared to those based exclusively on MACC1. We demonstrated significant differences of overall survival for patients with high expression of both markers compared to patients with a high expression of none or only one marker. Thus, a combination of biomarkers addressing different, but most relevant signaling pathways for tumor progression and metastasis might be most beneficial for improved prognosis of patient survival. In addition, it has to be demonstrated, that a combination with further types of free RNA in patient blood, such as miRNA, might result in even better disease prognosis and/or therapy response prediction.
In conclusion, survival of gastric cancer patient can be predicted based on plasma levels of circulating MACC1 transcripts, and can be improved when combining the information of circulating MACC1 transcripts with a further metastasis biomarker, S100A4. Both genes represent promising therapeutic targets and intervention strategies are desired aiming at restriction of tumor progression and metastasis development. It has to be evaluated, if this clinically applicable blood-based assay for circulating MACC1 transcripts in cancer patient plasma might represent an ideal read-out for predicting and for monitoring therapeutic response of future interventions targeting MACC1 in gastric cancer patients.
We are grateful to Ursula Plöckinger, Charité, Berlin, and to Klaus Sperber, Medical Practioner, Berlin, for support on tumor-free volunteer recruitment. We thank Janice Smith and Jutta Aumann (Max-Delbrück-Center and Charité, Berlin) for excellent technical assistance.
Gastric cancer is one of the major malignancies in the world and still the fourth most common cause of death from cancer. Patient survival is clearly linked to disease stage and metastasis formation. Biomarkers for detecting the disease already in early stage and identifying patients at high risk for metastasis are crucial.
The newly discovered gene Metastasis Associated in Colon Cancer 1 (MACC1) is highly acknowledged as prognostic and predictive biomarker for a variety of solid cancers. In gastric primary tumors, the presence of MACC1 correlated with peritoneal metastasis, lymph node metastasis and hepatic metastasis, and finally to a poor patient prognosis underlining the importance of MACC1 as biomarker for tumor progression, metastasis and survival also for gastric cancer patients.
The authors provide for the first time a blood-based assay for transcript quantification of the metastasis inducer MACC1 in a prospective study of gastric cancer patient plasma. Based on the levels of circulating MACC1 transcripts in plasma we discriminated tumor-free volunteers and gastric cancer patients. Levels of circulating MACC1 transcripts were increased in gastric cancer patients of each disease stage, compared to tumor-free volunteers. Importantly, gastric cancer patients with high circulating MACC1 transcript levels in plasma demonstrated significantly shorter survival when compared with patients demonstrating low MACC1 levels. Furthermore, gastric cancer patients with high circulating transcript levels of MACC1 as well as of S100A4 in plasma demonstrated shorter survival when compared with patients demonstrating low levels of both biomarkers or with only one biomarker elevated.
This prospective study suggests that quantitative determination of circulating transcript levels of MACC1, as well as of MACC1 and S100A4, in plasma of gastric cancer patients is proven to be useful for survival prognosis.
In previous studies we have identified the novel gene MACC1, Metastasis-Associated in Colon Cancer. MACC1 is a strong prognostic and predictive biomarker for tumor progression and metastasis in a variety of solid cancers. MACC1 is confirmed as decisive driver for tumorigenesis and metastasis. S100A4, S100 Calcium Binding Protein A4, is also linked to tumor progression and metastasis formation and has been shown to be of prognostic value for primary gastric and further tumors.
The m/s presents an interesting research study concerning the quantitation of MACC1 transcript in blood of gastric cancer patients. Quantitative results were compared in terms of cancer metastases and overall survival of patients and also in conjugation with another proposed biomarker, S100A4. The research article “circulating MACC1 transcripts in gastric cancer patient plasma as diagnostic and prognostic biomarker” by Burock et al 2015 provides a useful insight into the use of MACC1 transcripts to provide a biomarker for gastric cancer, and associated patient survival rates. The manuscript is interesting and draws upon sound clinical data sets. Overall it is a well-written manuscript which addresses an important research question.
P- Reviewer: Carter WG, Kohl M, Vynios D S- Editor: Ma N L- Editor: A E- Editor: Liu XM
| 1. | Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D, Bray F. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3526] [Cited by in RCA: 3658] [Article Influence: 304.8] [Reference Citation Analysis (2)] |
| 2. | Catalano V, Labianca R, Beretta GD, Gatta G, de Braud F, Van Cutsem E. Gastric cancer. Crit Rev Oncol Hematol. 2005;54:209-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 67] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 3. | Stein U, Walther W, Arlt F, Schwabe H, Smith J, Fichtner I, Birchmeier W, Schlag PM. MACC1, a newly identified key regulator of HGF-MET signaling, predicts colon cancer metastasis. Nat Med. 2009;15:59-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 383] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 4. | Stein U. MACC1 - a novel target for solid cancers. Expert Opin Ther Targets. 2013;17:1039-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Stein U, Dahlmann M, Walther W. MACC1 - more than metastasis? Facts and predictions about a novel gene. J Mol Med (Berl). 2010;88:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Arlt F, Stein U. Colon cancer metastasis: MACC1 and Met as metastatic pacemakers. Int J Biochem Cell Biol. 2009;41:2356-2359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 7. | Guo T, Yang J, Yao J, Zhang Y, Da M, Duan Y. Expression of MACC1 and c-Met in human gastric cancer and its clinical significance. Cancer Cell Int. 2013;13:121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Wang L, Wu Y, Lin L, Liu P, Huang H, Liao W, Zheng D, Zuo Q, Sun L, Huang N. Metastasis-associated in colon cancer-1 upregulation predicts a poor prognosis of gastric cancer, and promotes tumor cell proliferation and invasion. Int J Cancer. 2013;133:1419-1430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 9. | Shirahata A, Sakata M, Kitamura Y, Sakuraba K, Yokomizo K, Goto T, Mizukami H, Saito M, Ishibashi K, Kigawa G. MACC 1 as a marker for peritoneal-disseminated gastric carcinoma. Anticancer Res. 2010;30:3441-3444. [PubMed] |
| 10. | Stein U, Burock S, Herrmann P, Wendler I, Niederstrasser M, Wernecke KD, Schlag PM. Circulating MACC1 transcripts in colorectal cancer patient plasma predict metastasis and prognosis. PLoS One. 2012;7:e49249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Stein U, Burock S, Herrmann P, Wendler I, Niederstrasser M, Wernecke KD, Schlag PM. Diagnostic and prognostic value of metastasis inducer S100A4 transcripts in plasma of colon, rectal, and gastric cancer patients. J Mol Diagn. 2011;13:189-198. [PubMed] |
| 12. | Schäfer H. Constructing a cut-off point for a quantitative diagnostic test. Stat Med. 1989;8:1381-1391. [PubMed] |
| 13. | Mishra SK, Siddique HR, Saleem M. S100A4 calcium-binding protein is key player in tumor progression and metastasis: preclinical and clinical evidence. Cancer Metastasis Rev. 2012;31:163-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 133] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 14. | Stein U, Arlt F, Walther W, Smith J, Waldman T, Harris ED, Mertins SD, Heizmann CW, Allard D, Birchmeier W. The metastasis-associated gene S100A4 is a novel target of beta-catenin/T-cell factor signaling in colon cancer. Gastroenterology. 2006;131:1486-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 174] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 15. | Ling Z, Li R. Clinicopathological and prognostic value of S100A4 expression in gastric cancer: a meta-analysis. Int J Biol Markers. 2014;29:e99-e111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Kim MA, Lee HS, Lee HE, Kim JH, Yang HK, Kim WH. Prognostic importance of epithelial-mesenchymal transition-related protein expression in gastric carcinoma. Histopathology. 2009;54:442-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 17. | Kim YJ, Kim MA, Im SA, Kim TM, Kim DW, Yang HK, Heo DS, Lee KU, Choe KJ, Kim NK. Metastasis-associated protein S100A4 and p53 predict relapse in curatively resected stage III and IV (M0) gastric cancer. Cancer Invest. 2008;26:152-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Bai FH, Wang NJ, Wang J, Yang L, Zhang FM, Yin F, Liang J, Wu KC, Fan DM. Screening and identification of peritoneal metastasis-related genes of gastric adenocarcinoma using a cDNA microarray. Genet Mol Res. 2012;11:1682-1689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Zhao Y, Zhang T, Wang Q. S100 calcium-binding protein A4 is a novel independent prognostic factor for the poor prognosis of gastric carcinomas. Oncol Rep. 2013;30:111-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Li H, Liu Z, Xu C, Chen Y, Zhang J, Cui B, Chen X, An G, She X, Liu H. Overexpression of S100A4 is closely associated with the progression and prognosis of gastric cancer in young patients. Oncol Lett. 2013;5:1485-1490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Schwarzenbach H, Hoon DS, Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer. 2011;11:426-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1954] [Cited by in RCA: 2146] [Article Influence: 153.3] [Reference Citation Analysis (0)] |
| 22. | Marrelli D, Pinto E, De Stefano A, Farnetani M, Garosi L, Roviello F. Clinical utility of CEA, CA 19-9, and CA 72-4 in the follow-up of patients with resectable gastric cancer. Am J Surg. 2001;181:16-19. [PubMed] |
| 23. | Ishigami S, Natsugoe S, Hokita S, Che X, Tokuda K, Nakajo A, Iwashige H, Tokushige M, Watanabe T, Takao S. Clinical importance of preoperative carcinoembryonic antigen and carbohydrate antigen 19-9 levels in gastric cancer. J Clin Gastroenterol. 2001;32:41-44. [PubMed] |
| 24. | Gaspar MJ, Arribas I, Coca MC, Díez-Alonso M. Prognostic value of carcinoembryonic antigen, CA 19-9 and CA 72-4 in gastric carcinoma. Tumour Biol. 2001;22:318-322. [PubMed] |
| 25. | Wang YY, Li L, Zhao ZS, Wang HJ. Clinical utility of measuring expression levels of KAP1, TIMP1 and STC2 in peripheral blood of patients with gastric cancer. World J Surg Oncol. 2013;11:81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 26. | Mima K, Fukagawa T, Kurashige J, Takano Y, Uchi R, Ueo H, Matsumura T, Ishibashi M, Sawada G, Takahashi Y. Gene expression of bone morphogenic protein 8B in the primary site, peripheral blood and bone marrow of patients with gastric cancer. Oncol Lett. 2013;6:387-392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |