Published online Jan 7, 2015. doi: 10.3748/wjg.v21.i1.269
Peer-review started: July 17, 2014
First decision: August 15, 2014
Revised: September 2, 2014
Accepted: September 18, 2014
Article in press: September 19, 2014
Published online: January 7, 2015
Processing time: 174 Days and 18.1 Hours
AIM: To compare the demographics and survival rates between gallbladder adenocarcinoma (GB-adenocarcinoma) and small cell neuroendocrine carcinoma of the gallbladder (GB-NEC-SCC).
METHODS: From March 2007 to September 2012, patients who underwent resection of tumor stage T2/T3 GB cancer were enrolled for this study. Forty-two patients were included in this study, including 38 diagnosed with GB-adenocarcinoma and four diagnosed with GB-NEC-SCC. In the GB-adenocarcinoma group, a radical operation was performed in 28 patients, and ten patients underwent simple cholecystectomy. In the GB-NEC-SCC group, a radical operation was performed in three patients, and one patient underwent simple cholecystectomy. Comparative analysis of the two groups was performed, including clinicopathologic features and survival rates.
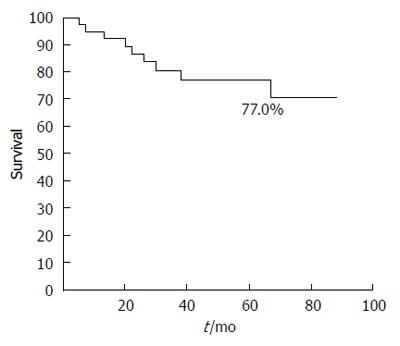
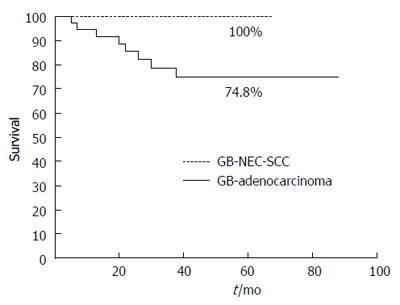
RESULTS: The median age of the patients was 68 y (range: 35-83 years) and females comprised 26/42 of the patients. GB-adenocarcinoma patients were significantly older than GB-NEC-SCC patients (67.89 ± 11.15 vs 55.75 ± 10.31 years; P = 0.029). The median tumor size in GB-adenocarcinoma patients was 2.56 ± 1.75 cm and 3.98 ± 3.74 cm in GB-NEC-SCC patients; however, there was no significant difference between the two groups. For tumors > 2 cm, T stage (T2 vs T3), lymphovascular invasion, perineural invasion, lymph node metastasis and lymph node ratio showed no significant differences between the two groups. The overall survival rate of the 42 patients at five years was 77.0%. In the GB-adenocarcinoma group, the overall five-year survival rate was 74.8%, and survival in the GB-NEC-SCC group was 100%, which was not significantly different between the two groups.
CONCLUSION: The strategy for treating patients with GB-NEC-SCC should be similar to that used for treating GB-adenocarcinoma, including radical cholecystectomy and liver resection.
Core tip: Small cell neuroendocrine carcinomas of the gallbladder are uncommon neoplasms, and therefore, little is known about their demographics and clinical course. Furthermore, the studies of the gallbladder neuroendocrine tumor and neuroendocrine carcinoma are limited to case reports with literature review. This study retrospectively compared the demographics and survival rates of gallbladder adenocarcinoma and small cell neuroendocrine carcinoma of the gallbladder, and reports the clinicopathologic features of small cell neuroendocrine carcinoma of the gallbladder, based on individual experiences.
- Citation: Yun SP, Shin N, Seo HI. Clinical outcomes of small cell neuroendocrine carcinoma and adenocarcinoma of the gallbladder. World J Gastroenterol 2015; 21(1): 269-275
- URL: https://www.wjgnet.com/1007-9327/full/v21/i1/269.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i1.269
Gallbladder (GB) cancer is an aggressive disease with characteristics of late presentation, rapid progression, and dismal results, the vast majority of which (85%-90%) are adenocarcinomas. Other histologic types of GB cancers include squamous, adenosquamous, and neuroendocrine carcinoma (NEC). Primary NECs of the gallbladder are very rare and are unknown in detail.
All neuroendocrine tumors (NET) have a malignant potential. NETs include well-differentiated NETs (classical carcinoid tumors), well-differentiated NECs (atypical carcinoids or malignant carcinoids), poorly differentiated NECs (high grade carcinoma: small-cell/large-cell types), and mixed exocrine-endocrine carcinomas. Primary NETs of the GB are particularly rare, and in the Surveillance, Epidemiology, and End Results (SEER) registry, only 278 cases have been reported between 1973 and 2005, representing 0.5% of all NETs[1,2], and 54 cases of small cell NEC of the GB (GB-NEC-SCC) were reported in another SEER-based study[3]. There have been several hypotheses about the origination of NETs of the GB. First, neuroendocrine cells usually are not seen in the normal GB and occur only in the intestinal or gastric metaplastic GB mucosa, occurring secondary to cholelithiasis and chronic cholecystitis[2]. Because of the expression of various neuroendocrine cells in this way, it is possible for NETs to occur. Secondly, heterotopic pancreatic tissue in the GB is an especially rare condition[4,5]. It is also possible that NETs may arise in ectopic pancreatic tissue.
Although classical well-differentiated NETs and GB-NEC-SCC are the most common neuroendocrine tumors of the GB, most of our knowledge about these tumors is limited and based on isolated case reports or a very small series[3]. The purpose of this study was to analyze and compare the demographics and survival rates of GB-adenocarcinoma and GB-NEC-SCC, and to report the clinicopathologic features of GB-NEC-SCC, based on individual experiences.
Patients at Pusan National University Hospital who underwent resection of tumor stage T2/T3 GB cancer between March 2007 and September 2012 were enrolled in this study. Based on the medical records, a retrospective review was performed. We excluded patients with squamous carcinoma, papillary carcinoma and mucinous carcinoma; finally, 42 patients were included. Thirty-eight patients were diagnosed with GB-adenocarcinoma and four patients were diagnosed with GB-NEC-SCC. The study was approved by the institutional review board.
We examined GB specimens taken from laparoscopic or open cholecystectomy to identify suspicious lesions. Lesions were sent to a pathologist for frozen-section biopsy. For cases reported by the pathologist as ≥ T2 GB cancer, we performed radical cholecystectomy including S4b, S5 segmentectomy of the liver. If GB cancer ≥ T2 was suspected by macroscopic appearance during exploration, we performed radical cholecystectomy and liver resection attaching GB. Patients who refused the second radical operation for T2 stage and those who were not able to receive radical operation due to severe morbidities underwent simple cholecystectomy.
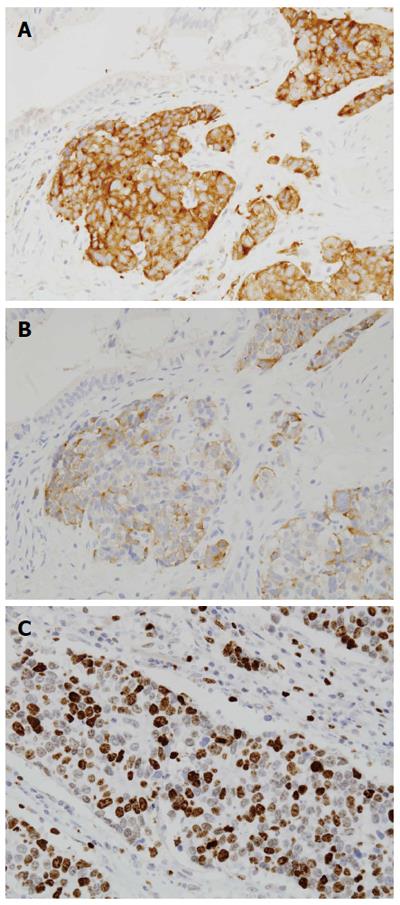
GB-NET was diagnosed according to the World Health Organization (WHO) classification published in 2010[6]. The grade of NET was classified according to the WHO classification and European Neuroendocrine Tumor Society grading system (Table 1)[6-9]. GB-NET-SCC was diagnosed if the following diagnostic criteria were fulfilled: (1) positive immunohistochemical staining of more than one protein, including chromogranin A and synaptophysin, or a cluster of differentiation 56, indicating the presence of neural cell adhesion molecules[2,10]; and (2) histopathologic presence of high-grade and small cell cytologic features, very high cellularity with hyperchromatic nuclei, absence of very small nucleoli with scant cytoplasm, high nuclear-to-cytoplasmic ratio, and a round or fusiform shape (according to the classification of SCCs as NETs by the WHO)[2,10,11].
| Grade | GET-NETs |
| Low (ENETS G1) | < 2 mitoses/10 HPF and < 3% Ki-67 index |
| Intermediate (ENETS G2) | 2-20 mitoses/10 HPF or 3%-20% Ki-67 index |
| High (ENETS G3) | > 20 mitoses/10 HPF or > 20% Ki-67 index |
Categorical variables between the GB-adenocarcinoma and GB-NEC-SCC groups were compared by the χ2 or Fisher’s exact tests. Age, tumor size and lymph node ratio were compared by Student’s t and Mann-Whitney U tests. The comparison of overall survival was analyzed by the log rank test. P values less than 0.05 were considered statistically significant. All statistical analyses were performed using the SPSS version 13.0 for Windows (SPSS Inc., Chicago, IL, United States).
The median age of the patients was 68 year (range: 35-83 year) and females comprised 26/42 of the patients (female/male ratio, 1.6:1). The age of GB-adenocarcinoma patients was significantly older than that of GB-NEC-SCC patients (P < 0.05) (Table 2). The percentage of patients over the age of 65 year was 69% (29/42), which did not differ between the groups. The median tumor size of GB-adenocarcinoma was not significantly different than GB-NEC-SCC (2.56 ± 1.75 vs 3.98 ± 3.74). When comparing tumors > 2 cm, T stage (T2 vs T3), lymphovascular invasion, perineural invasion, lymph node metastasis, and lymph node ratio were not significantly different between the two groups. Table 3 shows the pathologic features of the four patients in the GB-NEC-SCC group.
| Characteristic | GB-adenocarcinoma(n = 38) | GB-NEC-SCC(n = 4) | P value |
| Sex | 1.000 | ||
| Male | 15 | 1 | |
| female | 23 | 3 | |
| Age, yr | 67.89 ± 11.15 | 55.75 ± 10.31 | 0.029 |
| Age groups, n | 0.080 | ||
| < 65 yr | 10 | 3 | |
| ≥ 65 yr | 28 | 1 | |
| Operation type, n | 1.000 | ||
| Simple cholecystectomy | 10 | 1 | |
| Extended cholecystectomy | 28 | 3 | |
| Tumor size, cm | 2.56 ± 1.75 | 3.98 ± 3.74 | 0.788 |
| Tumor size groups, n | 0.609 | ||
| < 2 cm | 11 | 2 | |
| ≥ 2 cm | 21 | 2 | |
| Tumor stage, n | 0.410 | ||
| T2 | 34 | 3 | |
| T3 | 4 | 1 | |
| Lymphovascular invasion, n | 0.245 | ||
| - | 29 | 2 | |
| + | 8 | 2 | |
| Perineural invasion, n | 0.107 | ||
| - | 26 | 1 | |
| + | 11 | 3 | |
| Lymph node metastasis, n | 0.537 | ||
| - | 18 | 1 | |
| + | 9 | 2 | |
| Lymph node ratio | 0.15 ± 0.29 | 0.18 ± 0.28 | 0.485 |
| Recurrence, n | 1.000 | ||
| - | 28 | 3 | |
| + | 10 | 1 |
| Characteristic | Patient 1 | Patient 2 | Patient 3 | Patient 4 |
| Sex | Female | Male | Female | Female |
| Age, yr | 49 | 66 | 63 | 45 |
| Tumor size, cm | 1.9 | 1.5 | 3 | 9.5 |
| Operation | RC | RC | C | RC1 |
| Combined adenocarcinoma | - | + | - | + |
| Gross finding | Polypoid | Fungating | Fungating | Mutilobulated |
| Lymphovascular invasion | + | - | - | + |
| Perineural invasion | + | + | + | - |
| Tumor stage | T2 | T2 | T2 | T3 |
| Lymph node metastasis | 0/15 | 1/2 | 0/0 | 1/39 |
| Mitosis (/10 HPF) | 2 | 8 | 20 | 22 |
| Synaptophysin | + | + | - | - |
| Chromogranin A | + | + | - | - |
| CD56 | + | + | + | + |
| P53 | + | + | NA | NA |
| Ki-67 | 30% | 25% | 40% | 50% |
The overall survival rate of the 42 patients at five years was 77.0% (Figure 1). In the GB-adenocarcinoma group, the overall 5-year survival rate was 74.8% (radical operation, 78.4% vs simple cholecystectomy, 72.7%, P = 0.471) and in the GB-NEC-SCC group, the overall five-year survival rate was 100% (Figure 2). There was no significant difference between the two groups (P = 0.896). Immunohistochemical staining for synaptophysin, chromogranin A and Ki-67 is presented in Figure 3.
Biliary NET comprises less than 1% of all NETs[12-15]. In particular, GB-NEC-SCC is extremely rare, with only 54 cases found in a review of nearly 13981 cases of carcinoids from the SEER database[3]. Furthermore, the studies of the GB-NEC-SCC were limited to case reports with a literature review (Table 4). In this regard, a study of the comparison between GB-adenocarcinoma and GB-NEC-SCC is thought to be meaningful.
| Ref. | Age(yr) | Sex | Size (cm) | Meta or local inv | Mixed tumor | Survival (mo) | Tumor stage | Chromogranin A | Recurrence (site) | Operation type |
| Moskal et al[22], 1999 | 69 | F | NA | Regional LN meta, pancreas inv | + | 44 | T3 | NA | + (abdomen) | Radical |
| 57 | F | NA | Regional LN meta, liver inv | - | 31 | T3 | NA | + (abdomen, bone) | Radical | |
| 69 | M | NA | Regional LN meta | - | 21 | T2 | NA | + (retroperitoneum) | C | |
| 71 | F | NA | Regional LN meta peritoneum meta | + | 13 | T2 | NA | + (retroperitoneum) | C | |
| 40 | M | NA | Regional LN meta | + | 189, alive | T2 | NA | + (lung, abdomen) | C | |
| Maitra et al[21], 2001 | 85 | F | 4.0 | Liver meta | + | 13 | ≥ T2 | + | NA | Surg |
| 77 | F | 2.8 | Pancreas inv, LN meta | + | 25 | ≥ T2 | NA | NA | Surg | |
| 43 | F | 2.0 | Liver meta | + | 9 | ≥ T2 | + | NA | Surg | |
| 73 | F | 2.5 | LN meta | + | 7 | ≥ T2 | + | NA | Surg | |
| 82 | M | 1.0 | Liver meta | + | 8 | ≥ T2 | NA | NA | Surg | |
| 77 | F | 3.0 | - | + | 10 | ≥ T2 | + | NA | Surg | |
| 73 | M | 6.3 | Liver meta | - | 6 | ≥ T2 | NA | NA | Surg | |
| 71 | M | NA | - | - | 3 | T1 | NA | NA | Surg | |
| 37 | F | 8.0 | LN meta | - | 7 | ≥ T2 | NA | NA | Surg | |
| 68 | M | 2.0 | LN meta | - | 14 | ≥ T2 | + | NA | Surg | |
| 67 | F | 2.8 | - | - | NA | ≥ T2 | + | NA | Surg | |
| 78 | M | 1.8 | LN meta | - | 16 | ≥ T2 | NA | NA | Surg | |
| Lane et al[11], 2002 | 67 | M | NA | - | - | 11 | T1b | + | + (liver, LN) | C |
| Iype et al[16], 2008 | 32 | F | 2.0 | Regional LN meta, liver meta | - | 14, alive | T2 | + | + (liver, LN) | Radical |
| Current study, 2014 | 49 | F | 1.9 | - | - | 37, alive | T2 | + | - | Radical |
| 66 | M | 1.5 | Regional LN meta | + | 13, alive | T2 | + | - | Radical | |
| 63 | F | 3.0 | - | - | 67, died | T2 | - | + (liver) | C | |
| 45 | F | 9.5 | LN inv, colon inv, liver inv | + | 50, alive | T3 | - | - | Radical |
In this study, we found that GB-adenocarcinoma and GB-NEC-SCC were common in females (23/38 and ¾, respectively), consistent with findings by Albores-Saavedra et al[3]. Furthermore, GB-NEC-SCC patients were younger than GB-adenocarcinoma patients. Previous studies reported that a preoperative diagnosis of GB-NEC is very difficult because the presentation generally consists of non-specific symptoms, including right hypochondrial pain or discomfort and weight loss. Additionally, the presence of carcinoid syndrome is rare, and the majority of lesions are identified incidentally at the time of cholecystectomy performed for gallbladder stones[2,16]. Despite the development of imaging studies, preoperative differentiation between GB-adenocarcinoma and GB-NEC by ultrasound, computed tomography, or magnetic resonance imaging is still difficult. However, according to a recent study, the combination of positron emission tomography with computed tomography or magnetic resonance imaging was effective for the detection of NETs, and these modalities generally could obtain a high level of sensitivity[17,18]. In the present study, all cases of GB-NEC-SCC were diagnosed as GB-adenocarcinoma in preoperative imaging studies. Measurement of chromogranin A is known to be helpful for preoperatively diagnosing NET. Chromogranin A is elevated in 90% of gut NETs, and it correlates with the tumor burden and recurrence, and therefore, chromogranin A may be an effective biology marker in the preoperative diagnosis of NETs[19]. However, the measurement of chromogranin A to diagnose GB-NET before surgery is not cost-effective.
Though not observed in any of the patients in this study, poorly differentiated large cell GB-NECs show large cell size, low nuclear to cytoplasmic ratio, and frequent nucleoli, which are key cytologic features distinguishing them from GB-NEC-SCCs[20]. According to Iype et al[16], these NECs have a worse prognosis than GB-NEC-SCCs in the gallbladder. Grossly, GB-NEC-SCC may vary from unapparent covert lesions to largely necrotic, exophytic, or ulcerative masses[21]. In this study, two cases showed a fungating mass, one case showed a well-defined solid polypoid mass, and one case showed a well-circumscribed solid, multilobulated mass measuring 9.5 cm. Eltawil et al[2] reported that histologically, more than 90% of GB-NEC-SCC are poorly differentiated or anaplastic, with regional or distant spread at diagnosis. In this study, one patient with GB-NEC who had direct invasion of the duodenum and ascending colon (pT3) underwent hepatopancreaticoduodenectomy and right hemicolectomy. After surgery, the patient was observed without recurrence for 50 mo. A combination of GB-NEC-SCC and adenocarcinoma is quite common, and of the 36 cases of SCC reported in the literature, 28 were pure SCCs and 8 were combined with adenocarcinoma[22]. In our study, two of the cases were SCC combined with adenocarcinoma.
In general, the only curative therapeutic modality for GB cancer, which is a higher grade than T1, is radical cholecystectomy, including cholecystectomy, hepatoduodenal lymph node dissection and hepatic resection[23-25]. However, despite a more aggressive surgical approach, the majority of patients who have undergone potentially curative resection for GB cancer will develop recurrent metastatic disease. The surgical treatment for GB-NEC varies widely. For patients in whom the GB-NEC is a T in situ or T1 tumor, simple cholecystectomy is probably adequate treatment[2,23-25]. For more advanced GB-NEC, the prognosis is usually poor, but better outcomes can be obtained by aggressive radical operative therapy[26]. However, there has been no rational surgical strategy for a number of reasons, including the rarity of the disease, the lack of predictive prognostic factors, the inability to identify progression, and the limited understanding of the biology of the lesion[2]. In a previous study, cholecystectomy was performed in 12 patients, with a median survival of 4.5 mo, and radical resection was performed in two GB-NEC-SCC patients, with survivals of 4 and 20 mo[22]. In our study, among the 42 patients who underwent surgery for GB-adenocarcinoma or GB-NEC-SCC, the median survival was 38 mo. Eleven patients refused a second radical operation for T2 stage after it had been incidentally discovered after cholecystectomy. These patients’ overall five-year survivals were not significantly different from the survival of patients undergoing a radical operation. According to previous research, a significant advantage in the five-year survival rate was observed in patients with T2 stage undergoing radical operation compared to those who received only simple cholecystectomy (38%-100% vs 17%-65%)[23]. We think the reason for this opposite result is that, first, the number of patients with simple cholecystectomy was small, and secondly, favorable histopathologic features such as a tumor size < 2 cm, T2 stage, lack of lymphovascular invasion (simple cholecystectomy, 1/10 vs radical operation, 9/21) were seen in these patients.
Moskal et al[22] reported that the most common metastatic sites of GB-NEC-SCC were the nodes (88%), liver (88%), lung (23%), and peritoneum (19%). In the present study, T2 GB-NEC-SCC was confirmed in one patient after laparoscopic cholecystectomy; however, the patient refused a second radical operation. Fifty-nine months after surgery, the patient had liver metastases and died 67 mo after surgery. A radical operation was performed in three patients and these patients are still alive.
The roles of radiotherapy and chemotherapy in the management of GB-NEC-SCC are unclear. In general, NETs are insensitive to traditional radiotherapy[27]. According to several case reports, chemotherapeutic agents, including cisplatin, gemcitabine and etoposide plus 5-flurouracil, can lead to a partial response, resulting in palliation and the addition of a marginal advantage[16,28]. In the present study, after radical cholecystectomy, two patients underwent concurrent chemoradiation therapy (5-flurouracil plus etoposide or cisplatin) and they have been followed, without recurrence, for 37 mo and 50 mo, respectively.
There have only been a few reports in the literature about the prognosis of GB-NEC-SCC, and therefore, the prognosis and related factors are not generally known. Previous studies reported that an elevated Ki-67, high mitotic index and invasion of adjacent structures are likely to be predictive of a poor outcome[29,30]. According to SEER data, the one-year survival of GB-NEC-SCC was 21% and five-year survival was 0%, but GB-NETs have a similar prognosis to GB-adenocarcinoma[2]. Although the prognosis of GB-NEC-SCC is poor, past studies reported that aggressive multimodal treatment may prolong survival[2,16,22]. Therefore, it seems acceptable to adopt a similar strategy including radical cholecystectomy for GB-NEC, and doing so is likely to result in a better outcome. This study showed a similar result to previous studies in that the five-year survival rate between GB-adenocarcinoma and GB-NEC-SCC was not significantly different; however, we achieved a high survival rate for GB-NEC-SCC. We think this result was due to the performance of radical operations on these patients and the lower T stage in GB-NEC-SCC patients.
This study had some limitations. First, this was a retrospective study. Second, due to insufficient numbers of patients, we could not conduct a statistical comparison by multivariate analysis. However, it is quite important to compare GB-adenocarcinoma and GB-NEC-SCC based on curative resection using strict diagnostic criteria. Thus, the study was valuable in assessing the clinical course of patients with GB-NEC-SCCs after curative surgery.
Gallbladder (GB) cancer is an aggressive disease, and the vast majority (85%-90%) are adenocarcinomas. Small cell neuroendocrine carcinomas of the GB (GB-NEC-SCC) are extremely rare, and therefore, little is known about their demographics and clinical course.
Classical well-differentiated neuroendocrine tumor (NET) and GB-NEC-SCC are the most common NETs of the GB, most of our knowledge about these tumors is limited and based on isolated case reports or a very small series.
The authors retrospectively reviewed 42 patients with GB cancer, including four patients with GB-NEC-SCC. The authors analyzed and compared the demographics and survival rates between GB-adenocarcinoma and GB-NEC-SCC, and described the clinicopathologic features of GB-NEC-SCC. It is quite important to compare GB-adenocarcinoma and GB-NEC-SCC based on curative resection using strict diagnostic criteria. Thus, the study was valuable in assessing the clinical course of patients with GB-NEC-SCCs after curative surgery.
The study results suggest that on the basis of difficulty in distinguishing between GB-NEC and GB-adenocarcinoma preoperatively, and similar prognoses between the two groups, aggressive surgical management based on GB-adenocarcinoma may be effective for patients with GB-NECs.
NETs include well-differentiated NETs (classical carcinoid tumors), well-differentiated NEC (atypical carcinoids or malignant carcinoids), poorly differentiated NEC (high grade carcinoma: small-cell/large-cell types), and mixed exocrine-endocrine carcinoma.
This article investigates the clinical characteristics for GB-NEC-SCCs. Particularly, the demographics and survival rates are compared with GB-adenocarcinomas. This scientific paper is well written and provides some new and useful information regarding the GB-NEC-SCCs
P- Reviewer: Cao GW, Shu J, Shimizu Y S- Editor: Yu J L- Editor: AmEditor E- Editor: Wang CH
| 1. | Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063-3072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3022] [Cited by in RCA: 3245] [Article Influence: 190.9] [Reference Citation Analysis (0)] |
| 2. | Eltawil KM, Gustafsson BI, Kidd M, Modlin IM. Neuroendocrine tumors of the gallbladder: an evaluation and reassessment of management strategy. J Clin Gastroenterol. 2010;44:687-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 130] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 3. | Albores-Saavedra J, Batich K, Hossain S, Henson DE, Schwartz AM. Carcinoid tumors and small-cell carcinomas of the gallbladder and extrahepatic bile ducts: a comparative study based on 221 cases from the Surveillance, Epidemiology, and End Results Program. Ann Diagn Pathol. 2009;13:378-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 4. | Neupert G, Appel P, Braun S, Tonus C. Heterotopic pancreas in the gallbladder. Diagnosis, therapy, and course of a rare developmental anomaly of the pancreas. Chirurg. 2007;78:261-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Murakami M, Tsutsumi Y. Aberrant pancreatic tissue accompanied by heterotopic gastric mucosa in the gall-bladder. Pathol Int. 1999;49:580-582. [PubMed] |
| 6. | Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumours of the digestive system. 4th ed. Lyon: The International Agency for Research on Cancer 2010; . |
| 7. | Rindi G, Klöppel G, Alhman H, Caplin M, Couvelard A, de Herder WW, Erikssson B, Falchetti A, Falconi M, Komminoth P. TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 2006;449:395-401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1196] [Cited by in RCA: 1085] [Article Influence: 57.1] [Reference Citation Analysis (0)] |
| 8. | Rindi G, Klöppel G, Couvelard A, Komminoth P, Körner M, Lopes JM, McNicol AM, Nilsson O, Perren A, Scarpa A. TNM staging of midgut and hindgut (neuro) endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 2007;451:757-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 680] [Cited by in RCA: 653] [Article Influence: 36.3] [Reference Citation Analysis (1)] |
| 9. | Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas. 2010;39:707-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 776] [Cited by in RCA: 752] [Article Influence: 50.1] [Reference Citation Analysis (2)] |
| 10. | Jun SR, Lee JM, Han JK, Choi BI. High-grade neuroendocrine carcinomas of the gallbladder and bile duct: Report of four cases with pathological correlation. J Comput Assist Tomogr. 2006;30:604-609. [PubMed] |
| 11. | Lane JE, Walker AN, Ayers GW, Foster JL, Williams JT. Small-cell undifferentiated carcinoma of neuroendocrine type originating in the gallbladder. Curr Surg. 2002;59:495-497. [PubMed] |
| 12. | Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1848] [Cited by in RCA: 1851] [Article Influence: 84.1] [Reference Citation Analysis (1)] |
| 13. | Chamberlain RS, Blumgart LH. Carcinoid tumors of the extrahepatic bile duct. A rare cause of malignant biliary obstruction. Cancer. 1999;86:1959-1965. [PubMed] |
| 14. | Albores-Saavedra J, Henson DE, Klimstra D. Endocrine and benign mesenchymal tumors of the extrahepatic bile duct. Tumor of the Gallbladder, Extrahepatic Bile Ducts, and Ampulla of Vater. 3rd ed. Washington, DC: Armed Forces Institute of Pathology 2000; 217-221. |
| 15. | Kim J, Lee WJ, Lee SH, Lee KB, Ryu JK, Kim YT, Kim SW, Yoon YB, Hwang JH, Han HS. Clinical features of 20 patients with curatively resected biliary neuroendocrine tumours. Dig Liver Dis. 2011;43:965-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Iype S, Mirza TA, Propper DJ, Bhattacharya S, Feakins RM, Kocher HM. Neuroendocrine tumours of the gallbladder: three cases and a review of the literature. Postgrad Med J. 2009;85:213-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Koopmans KP, de Vries EG, Kema IP, Elsinga PH, Neels OC, Sluiter WJ, van der Horst-Schrivers AN, Jager PL. Staging of carcinoid tumours with 18F-DOPA PET: a prospective, diagnostic accuracy study. Lancet Oncol. 2006;7:728-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 155] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 18. | Seemann MD. Detection of metastases from gastrointestinal neuroendocrine tumors: prospective comparison of 18F-TOCA PET, triple-phase CT, and PET/CT. Technol Cancer Res Treat. 2007;6:213-220. [PubMed] |
| 19. | Modlin IM, Gustafsson BI, Moss SF, Pavel M, Tsolakis AV, Kidd M. Chromogranin A--biological function and clinical utility in neuro endocrine tumor disease. Ann Surg Oncol. 2010;17:2427-2443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 261] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 20. | Sasatomi E, Nalesnik MA, Marsh JW. Neuroendocrine carcinoma of the extrahepatic bile duct: case report and literature review. World J Gastroenterol. 2013;19:4616-4623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Maitra A, Tascilar M, Hruban RH, Offerhaus GJ, Albores-Saavedra J. Small cell carcinoma of the gallbladder: a clinicopathologic, immunohistochemical, and molecular pathology study of 12 cases. Am J Surg Pathol. 2001;25:595-601. [PubMed] |
| 22. | Moskal TL, Zhang PJ, Nava HR. Small cell carcinoma of the gallbladder. J Surg Oncol. 1999;70:54-59. [PubMed] |
| 23. | Sikora SS, Singh RK. Surgical strategies in patients with gallbladder cancer: nihilism to optimism. J Surg Oncol. 2006;93:670-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 24. | Wise PE, Shi YY, Washington MK, Chapman WC, Wright JK, Sharp KW, Pinson CW. Radical resection improves survival for patients with pT2 gallbladder carcinoma. Am Surg. 2001;67:1041-1047. [PubMed] |
| 25. | Ogura Y, Tabata M, Kawarada Y, Mizumoto R. Effect of hepatic invasion on the choice of hepatic resection for advanced carcinoma of the gallbladder: histologic analysis of 32 surgical cases. World J Surg. 1998;22:262-266; discussion 266-267. [PubMed] |
| 26. | Reid KM, Ramos-De la Medina A, Donohue JH. Diagnosis and surgical management of gallbladder cancer: a review. J Gastrointest Surg. 2007;11:671-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 156] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 27. | Modlin IM, Kidd M, Drozdov I, Siddique ZL, Gustafsson BI. Pharmacotherapy of neuroendocrine cancers. Expert Opin Pharmacother. 2008;9:2617-2626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 28. | Bhutani V, Dutta U, Nagi B, Nijhawan R, Singh K. Endocrine cell carcinoma of gall bladder. Indian J Gastroenterol. 2001;20:109-110. [PubMed] |