Published online Jan 7, 2015. doi: 10.3748/wjg.v21.i1.214
Peer-review started: May 22, 2014
First decision: July 21, 2014
Revised: July 31, 2014
Accepted: September 12, 2014
Article in press: September 16, 2014
Published online: January 7, 2015
Processing time: 231 Days and 23.4 Hours
AIM: To investigate the value of computed tomography (CT) spectral imaging in the evaluation of intestinal hemorrhage.
METHODS: Seven blood flow rates were simulated in vitro. Energy spectral CT and mixed-energy CT scans were performed for each rate (0.5, 0.4, 0.3, 0.2, 0.1, 0.05 and 0.025 mL/min). The detection rates and the contrast-to-noise ratios (CNRs) of the contrast agent extravasation regions were compared between the two scanning methods in the arterial phase (AP) and the portal venous phase (PVP). Comparisons of the CNR values between the PVP and the AP were made for each energy level and carried out using a completely random t test. A χ2 test was used to compare the detection rates obtained from the two scanning methods.
RESULTS: The total detection rates for energy spectral CT and mixed-energy CT in the AP were 88.57% (31/35) and 65.71% (23/35), respectively, and the difference was significant (χ2 = 5.185, P = 0.023); the total detection rates in the PVP were 100.00% (35/35) and 91.4% (32/35), respectively, and the difference was not significant (χ2 = 1.393, P = 0.238). In the AP, the CNR of the contrast agent extravasation regions was 3.58 ± 2.09 on the mixed-energy CT images, but the CNRs were 8.78 ± 7.21 and 8.83 ± 6.75 at 50 and 60 keV, respectively, on the single-energy CT images, which were significantly different (3.58 ± 2.09 vs 8.78 ± 7.21, P = 0.031; 3.58 ± 2.09 vs 8.83 ± 6.75, P = 0.029). In the PVP, the differences between the CNRs at 40, 50 and 60 keV different monochromatic energy levels and the polychromatic energy images were significant (19.35 ± 10.89 vs 11.68 ± 6.38, P = 0.010; 20.82 ± 11.26 vs 11.68 ± 6.38, P = 0.001; 20.63 ± 10.07 vs 11.68 ± 6.38, P = 0.001). The CNRs at the different energy levels in the AP and the PVP were significantly different (t = -2.415, -2.380, -2.575, -2.762, -2.945, -3.157, -3.996 and -3.189).
CONCLUSION: Monochromatic energy imaging spectral CT is superior to polychromatic energy images for the detection of intestinal hemorrhage, and the detection was easier in the PVP compared with the AP.
Core tip: Recent technical advances,including monochromatic energy image spectral computed tomography (CT) with its accurate material-decomposition images and monochromatic spectral images at energy levels, are only rarely included in intestinal hemorrhage studies. This paper aimed to verify the diagnostic value of spectral CT in small bowel bleeding.
- Citation: Liu WD, Wu XW, Hu JM, Wang B, Liu B. Monochromatic energy computed tomography image for active intestinal hemorrhage: A model investigation. World J Gastroenterol 2015; 21(1): 214-220
- URL: https://www.wjgnet.com/1007-9327/full/v21/i1/214.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i1.214
Gastrointestinal (GI) bleeding, especially lower GI bleeding, is a critical and sometimes life-threatening condition. Rapid and accurate localization of the bleeding sites and early etiological determination are the focus of clinical investigations, but these factors also represent major clinical challenges. Endoscopy enables the accurate localization and diagnosis in most cases of upper GI bleeding but not in lower GI bleeding[1]. Digital subtraction angiography (DSA) has been shown to be effective in the clinical diagnosis of GI bleeding. However, detection and diagnosis often fail in cases of bleeding rates below 0.5 mL/min[2,3]. Radioisotope scanning can detect a rate below 0.1 mL/min, but accurate localization is difficult[4]. Multidetector row computed tomography (MDCT) technology has recently shown increasingly prominent advantages in the diagnosis of small bowel bleeding[5]. Recent technical advances include energy spectral CT with its advantages of enabling monochromatic energy imaging, reduced tissue attenuation, high resolution, and the detection of small changes in tissue density[6,7]. Compared with conventional MDCT, energy spectral CT is particularly sensitive regarding the detection of active bleeding in the GI tract. However, the diagnostic value of energy spectral CT in small bowel bleeding has not been previously established. Therefore, this paper aimed to verify the diagnostic value of spectral CT in small bowel bleeding.
Microsyringe: A WZ-50C6 microsyringe produced by Zhejiang University Medical Instrument Co., Ltd. (Hefei Province, China) was used, and the amount injected and the injection rates were calibrated on a regular basis. A 2.5% solution of mannitol (CT value 30-40 HU) was injected into the small bowels of pigs in vitro, and the tissues were immersed in a container of plant oil (30 cm × 15 cm × 10 cm X-ray permeable plastic container). The contrast agent was loaded into the microsyringe (in a proportion of 300 mgI/mL Omnipaque to physiological saline at 1:45, CT value 280-300 HU). Seven bleeding rates were simulated: 0. 5, 0.4, 0.3, 0.2, 0.1, 0.05 and 0.025 mL/min.
A 24G indwelling needle connected to one end of the microsyringe was used to penetrate the intestinal wall, and the needle was fixed in place. The contrast agent was injected at the seven rates mentioned above.
Single-energy spectral CT (GE Discovery HD750 CT scanner) and poly-chromatic energy CT (GE Light Speed 64 VCT) were employed. For each bleeding rate, the scans were repeated five times. Following each scan, the liquid in the intestinal canal was replaced. The gemstone spectral imaging (GSI) platform was used for image reconstruction in the standard reconstruction mode. The CT images obtained using the seven monochromatic energy images (40-100 keV with a 10 keV interval) and the polychromatic energy images (120 kVp) were analyzed. The delays used were 15 and 40 s.
The following scanning parameters in the conventional mode (polychromatic energy CT) were used: 120 kV, 300 mA, pitch 0.984, collimation 40 mm, gantry rotation time 0.8 s, slice thickness 5 mm, and eight slices scanned. The following scanning parameters in the GSI mode (helical 0.5 s, 40 mm) were used: 80/140 kV, 0.5 ms instant switching, pitch 0.984, gantry rotation time 0.8 s, slice thickness 5 mm, and eight slices scanned.
The contrast-to-noise ratios (CNRs) were determined (i.e., the CNRs of the iodine-containing region and the surrounding water-containing region in the intestinal canal). The layers that exhibited clear extravasation of the contrast agent were selected on the polychromatic energy images and the seven monochromatic energy images. Regions of interest with identical size and morphology were selected. According to the equation, CNR = (|CT1 - 2|)/SD; thus, the CNRs for the extravasation regions under the different bleeding rates were calculated. CT1 is the CT value of the region of contrast agent extravasation, CT2 is the CT value of the liquid in the intestinal canal of the same layer, and SD is the standard deviation of the CT attenuation value of the water environment in the intestinal canal of the same layer.
Image quality scoring: Based on the length of the contrast agent column and the CT value, the image quality was classified into five levels: 5 points, clearly displayed contrast agent column (length ≥ 2 cm or CT value ≥ 150 HU); 4 points, visible contrast agent column (length 1-2 cm or CT value 100-150 HU); 3 points, obscure contrast agent column (length ≤ 1 cm or CT value ≤ 100 HU); 2 points, no visible contrast agent column; and 1 point, no contrast agent imaging. Two radiologists who were blinded to the experimental parameters independently evaluated the image quality. A discussion was required to reach a consensus when their opinions were inconsistent.
SPSS 17.0 (SPSS, Inc., Chicago, United States) was used. The quantitative data are expressed as the mean ± SD. A randomized block analysis of the variance was performed on the CNRs of the images obtained under the seven energy levels and their quality scores. Each pair of energy levels was compared using the Bonferroni method. The CNRs and the image quality scores between the portal venous phase (PVP) and the arterial phase (AP) were compared for each energy level using a completely random t test. The detection rates for the energy spectral CT and the 64-slice CT were compared using the χ2 test, and P < 0.05 indicated significance.
There were differences in the detection rates for the regions of contrast agent extravasation using the two scanning methods in different phases.
Of the 35 spectral CT scans, the extravasation region was detected in 31 scans. Using 64-slice CT scanning, the extravasation region was detected in 23 scans. The detection rates of the spectral and 64-slice CT scanning were 88.57% (31/35) and 65.71% (23/35), respectively, and the difference was significant (χ2 = 5.185, P = 0.023).
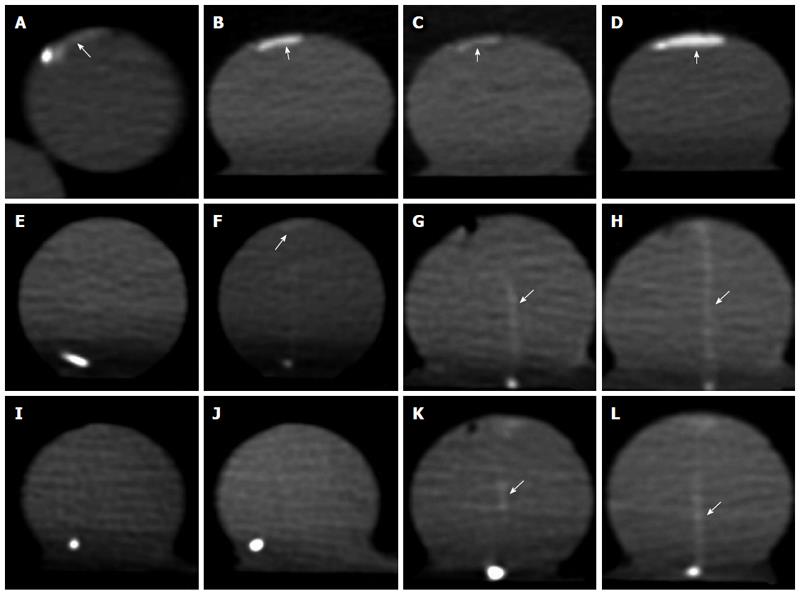
The detection rates of the spectral and 64-slice CT scanning were 100.00% (35/35) and 91.43% (32/35), respectively, and the difference was not significant (χ2 = 1.393, P = 0.238). The detection rates in the PVP and AP were significantly different when 64-slice CT scanning was used (χ2 = 6.873, P = 0.009), whereas the difference was not significant when spectral CT scanning was used (χ2 corrected = 2.386, P = 0.122) (Table 1). When the bleeding rate decreased to 0.025 mL/min, the detection rate in the AP with 64-slice CT scanning was zero, and it was 60% (3/5) in the PVP; the detection rate with spectral CT scanning was 60% (3/5)in the AP and 100% (5/5) in the PVP. When the bleeding rate was high, both 64-slice and spectral CT scanning facilitated the easy detection of the regions of extravasation. The optimal monochromatic energy images obtained using spectral CT scanning exhibited clearer extravasation regions.When the bleeding rate was 0.5 mL/min, the 60 keV images were superior compared with the 100 keV images and the polychromatic energy images (Figure 1). When the bleeding rate decreased to 0.05 and 0.025 mL/min, 64-slice CT scanning did not facilitate the detection of the extravasation region in the AP. However, the extravasation region was clearly visualized in the PVP. When spectral CT scanning at 50 keV was used, the extravasation region was clearly visualized in both the PVP and the AP (Figure 1E).
| Grouping | Detection result | Total | Detection rate | χ2 | P value | |
| Detected | Not detected | |||||
| 64-slice CT in the AP | 23 | 12 | 35 | 65.71% | 5.185 | 0.023 |
| spectral CT in the AP | 31 | 4 | 35 | 88.57% | ||
| 64-layer CT in the PVP | 32 | 3 | 35 | 91.43% | 1.3931 | 0.238 |
| spectral CT in PVP | 35 | 0 | 35 | 100.00% | ||
There were differences in the CNRs for the two scanning methods at each energy level in the PVP and the AP.
The CNRs of the extravasation region obtained with spectral CT scanning at 50 and 60 keV in the AP were 8.78 ± 7.21 and 8.83 ± 6.75, respectively; compared with the CNRs obtained with polychromatic energy CT scanning (3.58 ± 2.09), the difference was significant (P < 0.05). In the PVP, the CNRs for monochromatic energy images at 40, 50 and 60 keV were 19.35 ± 10.89, 20.82 ± 11.26 and 20.63 ± 10.07, respectively. These values were significantly different compared with polychromatic energy CT scanning (11.68 ± 6.38, P < 0.05). The CNRs for a given energy level in the AP and PVP were significantly different (t = -2.415, -2.380, -2.575, -2.762, -2.945, -3.157, -3.996 and -3.189) (P < 0.05) (Table 2).
| PVP | AP | t | P value | |
| 40 keV | 19.35 ± 10.89 | 7.69 ± 6.68 | -2.4151 | 0.036 |
| 50 keV | 20.82 ± 11.26 | 8.78 ± 7.21 | -2.380 | 0.035 |
| 60 keV | 20.63 ± 10.07 | 8.83 ± 6.75 | -2.575 | 0.024 |
| 70 keV | 15.65 ± 8.17 | 5.93 ± 4.46 | -2.762 | 0.017 |
| 80 keV | 10.46 ± 5.83 | 3.44 ± 2.41 | -2.945 | 0.012 |
| 90 keV | 7.33 ± 3.92 | 2.31 ± 1.52 | -3.157 | 0.008 |
| 100 keV | 5.83 ± 2.70 | 1.49 ± 1.01 | -3.996 | 0.004 |
| Mixed-energy image | 11.68 ± 6.38 | 3.58 ± 2.09 | -3.189 | 0.015 |
| F | 18.298 | 7.823 | ||
| P value | 0.000 | 0.000 |
There were differences in the image quality score at each energy level in the PVP and the AP using the two scanning methods.
The differences in the PVP between 40, 50 and 60 keV were not significant. However, the image quality at each of these three energy levels was significantly different compared with the polychromatic energy images (P < 0.05). The differences in the AP between 40, 50 and 60 keV were not significant, but the differences among the other energy levels were all significant (P < 0.05). The quality scores of the monochromatic energy images at 40, 50 and 60 keV were slightly higher compared with the polychromatic energy images in both phases (Table 3).
| PVP | AP | t | P value | |
| 40 keV | 4.63 ± 0.58 | 3.46 ± 1.43 | 1.9991 | 0.081 |
| 50 keV | 4.46 ± 0.65 | 3.06 ± 1.20 | 2.724 | 0.018 |
| 60 keV | 4.20 ± 0.77 | 2.97 ± 1.26 | 2.195 | 0.049 |
| 70 keV | 3.54 ± 0.91 | 2.23 ± 0.92 | 2.692 | 0.020 |
| 80 keV | 3.00 ± 0.83 | 1.97 ± 0.87 | 2.263 | 0.043 |
| 90 keV | 2.46 ± 0.55 | 1.49 ± 0.49 | 3.494 | 0.004 |
| 100 keV | 2.26 ± 0.54 | 1.09 ± 0.11 | 5.6501 | 0.001 |
| Mixed-energy imaging | 3.60 ± 1.13 | 2.46 ± 1.06 | 1.953 | 0.074 |
| F | 52.549 | 19.875 | ||
| P value | 0.000 | 0.000 |
MDCT has been shown to be effective for the visualization of small hemorrhages in the GI tract because of the sub-millimeter scanning ability and the powerful image post-processing[8-11]. When the bleeding rate is greater than 0.5 mL/min, the detection rate using MDCT can reach 93%[12]. Monochromatic energy imaging combined with the use of iodine-based contrast materials leads to an increased ability of single-energy spectral CT to detect small hemorrhages in the GI tract compared with conventional MDCT[13,14]. In this study, a GE HD750 CT scanner was used for gemstone spectral imaging to confirm its diagnostic value in cases of active bleeding in the GI tract. The results provide the basis for the clinical selection of a more simple and effective tool for the localization of small intestine bleeding sites.
The comparison of the monochromatic and polychromatic energy images demonstrated that when the bleeding rate was high, both spectral CT and MDCT scanning facilitates the easy detection of the regions of contrast agent extravasation. However, when the bleeding rate decreased, the difficulty of detecting the extravasation regions increased accordingly. The use of monochromatic energy imaging facilitated the observation of the regions of contrast agent extravasation, thereby significantly increasing the detection rates. When the bleeding rate decreased below a specific level, the extravasation region may not have been detected in the AP, but it was always successfully detected in the PVP. Dobritz et al[1] also reported that when the bleeding rate was low, the region of extravasation was not detected in some cases in the AP. Because of the increased concentration of the contrast agent in the PVP, the detection rate was substantially higher.
The detection rates in the PVP and the AP using 64-slice CT scanning were significantly different, and the detection rate was higher in the PVP. However, no difference was detected with the use of spectral CT scanning, although this finding might be related to the limited sample size. Yamaguchi et al[15] demonstrated that the detection rate obtained with MDCT during active bleeding of the GI tract could reach 80%, and the sensitivity and specificity of CT angiography were 89% and 85%, respectively[15,16]. The detection rate with DSA averaged 47% (range: 27%-77%), as reported in the literature. The sensitivity, specificity and accuracy of DSA in the detection of small bowel bleeding were 91.7%, 100% and 92%, respectively[17-19]. The detection rates using spectral CT scanning in this study were 88.57% (31/35) and 100% (35/35) in the AP and the PVP, respectively. These values were substantially higher compared with previous reports, which suggest that spectral CT is superior for the detection of active bleeding in the small intestines. It may be employed as the primary choice in clinical practice to producea higher detection rate.
The optimal monochromatic energy value for the detection of the extravasation region on monochromatic energy images: Compared with the MDCT images, the monochromatic energy images had a higher image quality and higher signal-to-noise ratios and CNRs. Single-energy spectral CT integrates single-energy imaging and iodine-based material decomposition, which provides a new prospect for the development of CT technology[20-23]. Our experiments indicated that the extravasation regions detected on monochromatic energy images varied with the specific energy level in both the AP and the PVP. The image qualities at 50 and 60 keV were substantially better compared with the polychromatic energy images. The average CNRs at 50 and 60 keV in the PVP were 1.78 and 1.76 times higher compared with the polychromatic energy images and 2.45 and 2.47 times higher compared with the AP, respectively. The extravasation regions were visualized more clearly on the monochromatic energy images at 50 and 60 keV compared with the other energy levels or on the polychromatic energy images. The CNRs in the PVP were generally higher compared with the AP. It is clear that 50-60 keV is the optimal energy level. It was demonstrated experimentally that the monochromatic energy images also had a higher quality score compared with the polychromatic energy images at 40, 50 and 60 keV. The optimal energy level provides an image that achieves a balance between tissue contrast and noise level and enables clear visualization of the lesions. Because MDCT is based on polychromatic energy imaging, the CT value of the material is likely to shift, thereby affecting the image quality[24,25].
Limitations of the present research: (1) the ideal filled state was simulated in the intestinal canal using the established model. However, under clinical conditions, the intestinal canals of some patients may be empty, which affects the detection of active bleeding. Thus, our experiments tended to overestimate the ability of CT to evaluate active bleeding; and (2) the small sample size also affected the precision of the results. Furthermore, these findings must be confirmed in in vivo studies.
Monochromatic energy imaging in spectral CT was superior compared with polychromatic energy imaging for the detection of the region of contrast agent extravasation. The optimal energy level was 50-60 keV. For patients with suspected small bowel bleeding, spectral CT at the recommended energy level of 50-60 keV is the first-line choice for diagnosis.
Rapid and accurate localization of the bleeding sites and early etiological determination are the focus of investigation, but also the major challenges. In comparison with conventional multidetector row computed tomography (MDCT), energy spectral CT is particularly sensitive to detect active bleeding in the gastrointestinal (GI). However, the diagnostic value of energy spectral CT in small bowel bleeding has not been established. Therefore, this paper aimed to verify the diagnostic value of spectral CT in small bowel bleeding.
GI bleeding, especially lower GI, is a critical and sometimes life-threatening condition. In the area of detecting hemorrhages in the GI, the hotspot is how to find the bleeding sites rapidly and accurately.
MDCT has been proved to be effective in visualizing tiny hemorrhages in the GI, owing to the sub-millimeter scanning ability and the powerful image post- processing. When the bleeding rate is over 0.5 mL/min, the detection rate by MDCT can reach 93%. The mono-chromatic energy imaging combined with the use of iodine-based materials gives single-energy spectral CT a greater ability to detect tiny hemorrhages in the GI than conventional MDCT. In this study, a GE HD750 CT scanner was used for gemstone spectral imaging to confirm its diagnostic value for cases of active bleeding in the GI. Monochromatic energy image spectral CT can detect the less than 0.05 mL/min of bleeding rate, but the digital subtraction angiography could not find it.
Monochromatic energy imaging in spectral CT showed superiority over polychromatic energy imaging in detecting GI bleeding. It plays a important role in visualizing tiny hemorrhages in the GI.
MDCT technology has shown increasingly prominent advantages recently in the diagnosis of small bowel bleeding, including energy spectral CT with its advantages of enabling monochromatic energy imaging, reduced tissue attenuation, high resolution, and the detection of tiny changes in tissue density.
This is a good descriptive study in which the authors investigated the value of computed tomography spectral imaging in evaluating the intestinal hemorrhage of 7 different bleeding rates. The results are interesting and suggest that monochromatic energy imaging in spectral CT is a good choice in detecting tiny hemorrhages in the GI.
P- Reviewer: Kurtoglu E, Luo HS, Quattrocchi CC, Syam AF, Yu B S- Editor: Gou SX L- Editor: A E- Editor: Liu XM
| 1. | Dobritz M, Engels HP, Schneider A, Wieder H, Feussner H, Rummeny EJ, Stollfuss JC. Evaluation of dual-phase multi-detector-row CT for detection of intestinal bleeding using an experimental bowel model. Eur Radiol. 2009;19:875-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Allison DJ, Hemingway AP, Cunningham DA. Angiography in gastrointestinal bleeding. Lancet. 1982;2:30-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Imdahl A, Salm R, Rückauer K, Farthmann EH. [Diagnosis and management of lower gastrointestinal hemorrhage. Retrospective analysis of 233 cases]. Langenbecks Arch Chir. 1991;376:152-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 4. | Filippone A, Cianci R, Milano A, Pace E, Neri M, Cotroneo AR. Obscure and occult gastrointestinal bleeding: comparison of different imaging modalities. Abdom Imaging. 2012;37:41-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Ernst O, Bulois P, Saint-Drenant S, Leroy C, Paris JC, Sergent G. Helical CT in acute lower gastrointestinal bleeding. Eur Radiol. 2003;13:114-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 98] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Coursey CA, Nelson RC, Boll DT, Paulson EK, Ho LM, Neville AM, Marin D, Gupta RT, Schindera ST. Dual-energy multidetector CT: how does it work, what can it tell us, and when can we use it in abdominopelvic imaging? Radiographics. 2010;30:1037-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 274] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 7. | Wang L, Liu B, Wu XW, Wang J, Zhou Y, Wang WQ, Zhu XH, Yu YQ, Li XH, Zhang S. Correlation between CT attenuation value and iodine concentration in vitro: discrepancy between gemstone spectral imaging on single-source dual-energy CT and traditional polychromatic X-ray imaging. J Med Imaging Radiat Oncol. 2012;56:379-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Horton KM, Fishman EK. The current status of multidetector row CT and three-dimensional imaging of the small bowel. Radiol Clin North Am. 2003;41:199-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 98] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Boudiaf M, Jaff A, Soyer P, Bouhnik Y, Hamzi L, Rymer R. Small-bowel diseases: prospective evaluation of multi-detector row helical CT enteroclysis in 107 consecutive patients. Radiology. 2004;233:338-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 156] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 10. | Yoon W, Jeong YY, Shin SS, Lim HS, Song SG, Jang NG, Kim JK, Kang HK. Acute massive gastrointestinal bleeding: detection and localization with arterial phase multi-detector row helical CT. Radiology. 2006;239:160-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 188] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 11. | Roy-Choudhury SH, Karandikar S. Multidetector CT of acute gastrointestinal bleeding. Radiology. 2008;246:336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Dobritz M, Engels HP, Schneider A, Bauer J, Rummeny EJ. Detection of intestinal bleeding with multi-detector row CT in an experimental setup. How many acquisitions are necessary? Eur Radiol. 2009;19:2862-2869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Johnson TR, Krauss B, Sedlmair M, Grasruck M, Bruder H, Morhard D, Fink C, Weckbach S, Lenhard M, Schmidt B. Material differentiation by dual energy CT: initial experience. Eur Radiol. 2007;17:1510-1517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1168] [Cited by in RCA: 1103] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 14. | Wu HW, Cheng JJ, Li JY, Yin Y, Hua J, Xu JR. Pulmonary embolism detection and characterization through quantitative iodine-based material decomposition images with spectral computed tomography imaging. Invest Radiol. 2012;47:85-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Yamaguchi T, Yoshikawa K. Enhanced CT for initial localization of active lower gastrointestinal bleeding. Abdom Imaging. 2003;28:634-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 50] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Wu LM, Xu JR, Yin Y, Qu XH. Usefulness of CT angiography in diagnosing acute gastrointestinal bleeding: a meta-analysis. World J Gastroenterol. 2010;16:3957-3963. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 113] [Cited by in RCA: 106] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 17. | Fang JF, Chen RJ, Wong YC, Lin BC, Hsu YB, Kao JL, Kao YC. Pooling of contrast material on computed tomography mandates aggressive management of blunt hepatic injury. Am J Surg. 1998;176:315-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 66] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Martí M, Artigas JM, Garzón G, Alvarez-Sala R, Soto JA. Acute lower intestinal bleeding: feasibility and diagnostic performance of CT angiography. Radiology. 2012;262:109-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 19. | García-Blázquez V, Vicente-Bártulos A, Olavarria-Delgado A, Plana MN, van der Winden D, Zamora J. Accuracy of CT angiography in the diagnosis of acute gastrointestinal bleeding: systematic review and meta-analysis. Eur Radiol. 2013;23:1181-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 116] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 20. | Yeh BM, Shepherd JA, Wang ZJ, Teh HS, Hartman RP, Prevrhal S. Dual-energy and low-kVp CT in the abdomen. AJR Am J Roentgenol. 2009;193:47-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 185] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 21. | Fletcher JG, Takahashi N, Hartman R, Guimaraes L, Huprich JE, Hough DM, Yu L, McCollough CH. Dual-energy and dual-source CT: is there a role in the abdomen and pelvis? Radiol Clin North Am. 2009;47:41-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 125] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 22. | Marin D, Nelson RC, Samei E, Paulson EK, Ho LM, Boll DT, DeLong DM, Yoshizumi TT, Schindera ST. Hypervascular liver tumors: low tube voltage, high tube current multidetector CT during late hepatic arterial phase for detection--initial clinical experience. Radiology. 2009;251:771-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 182] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 23. | Macari M, Spieler B, Kim D, Graser A, Megibow AJ, Babb J, Chandarana H. Dual-source dual-energy MDCT of pancreatic adenocarcinoma: initial observations with data generated at 80 kVp and at simulated weighted-average 120 kVp. AJR Am J Roentgenol. 2010;194:W27-W32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 144] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 24. | Matsumoto K, Jinzaki M, Tanami Y, Ueno A, Yamada M, Kuribayashi S. Virtual monochromatic spectral imaging with fast kilovoltage switching: improved image quality as compared with that obtained with conventional 120-kVp CT. Radiology. 2011;259:257-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 391] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 25. | Zhang D, Li X, Liu B. Objective characterization of GE discovery CT750 HD scanner: gemstone spectral imaging mode. Med Phys. 2011;38:1178-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 158] [Article Influence: 11.3] [Reference Citation Analysis (0)] |