Published online Jan 7, 2015. doi: 10.3748/wjg.v21.i1.155
Peer-review started: September 10, 2014
First decision: October 14, 2014
Revised: October 29, 2014
Accepted: November 18, 2014
Article in press: December 16, 2014
Published online: January 7, 2015
Processing time: 120 Days and 5.4 Hours
AIM: To investigate the vasoactive intestinal peptides (VIP) expression in irritable bowel syndrome (IBS) and trinitrobenzene sulfonic acid (TNBS) induced colitis.
METHODS: The VIP gene expression and protein plasma levels were measured in adult participants (45.8% male) who met Rome III criteria for IBS for longer than 6 mo and in a rat model of colitis as induced by TNBS. Plasma and colons were collected from naïve and inflamed rats. Markers assessing inflammation (i.e., weight changes and myeloperoxidase levels) were assessed on days 2, 7, 14 and 28 and compared to controls. Visceral hypersensitivity of the rats was assessed with colo-rectal distension and mechanical threshold testing on hind paws. IBS patients (n = 12) were age, gender, race, and BMI-matched with healthy controls (n = 12). Peripheral whole blood and plasma from fasting participants was collected and VIP plasma levels were assayed using a VIP peptide-enzyme immunoassay. Human gene expression of VIP was analyzed using a custom PCR array.
RESULTS: TNBS induced colitis in the rats was confirmed with weight loss (13.7 ± 3.2 g) and increased myeloperoxidase activity. Visceral hypersensitivity to colo-rectal distension was increased in TNBS treated rats up to 21 d and resolved by day 28. Somatic hypersensitivity was also increased up to 14 d post TNBS induction of colitis. The expression of an inflammatory marker myeloperoxidase was significantly elevated in the intracellular granules of neutrophils in rat models following TNBS treatment compared to naïve rats. This confirmed the induction of inflammation in rats following TNBS treatment. VIP plasma concentration was significantly increased in rats following TNBS treatment as compared to naïve animals (P < 0.05). Likewise, the VIP gene expression from peripheral whole blood was significantly upregulated by 2.91-fold in IBS patients when compared to controls (P < 0.00001; 95%CI). VIP plasma protein was not significantly different when compared with controls (P = 0.193).
CONCLUSION: Alterations in VIP expression may play a role in IBS. Therefore, a better understanding of the physiology of VIP could lead to new therapeutics.
Core tip: The present study reports evidence of altered vasoactive intestinal peptide (VIP) in both humans with irritable bowel syndrome (IBS) and a rat model with induced colitis by trinitrobenzene sulfonic acid. Together these observations provide additional evidence of the role of VIP, and the potential therapeutic application in patients with IBS. The results provide a basis for future studies to elucidate the understanding of the expression, physiology, and pharmacological properties of VIP.
- Citation: Del Valle-Pinero AY, Sherwin LB, Anderson EM, Caudle RM, Henderson WA. Altered vasoactive intestinal peptides expression in irritable bowel syndrome patients and rats with trinitrobenzene sulfonic acid-induced colitis. World J Gastroenterol 2015; 21(1): 155-163
- URL: https://www.wjgnet.com/1007-9327/full/v21/i1/155.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i1.155
Irritable bowel syndrome (IBS) is a chronic gastrointestinal (GI) disorder of unknown etiology that is characterized by abdominal pain and altered bowel movements. IBS affects approximately 10%-20% of adults in the United States[1]. Despite the relatively high prevalence of this disorder, the exact pathophysiology remains unknown. Identifying the mechanism(s) responsible for IBS is a challenge, because diagnostic criteria have changed over time and the diagnosis is primarily based on the history and physical examination[2]. The diagnosis rests on the occurrence of a constellation of symptoms in the absence of other GI pathology.
Current literature suggests that the etiology of IBS is multifactorial. Bacterial, viral or parasitic infection[3], and inflammation may play a role in the etiology of IBS[4] along with altered gastrointestinal motility and visceral perception[5]. Post-infectious IBS occurs in up to 36% of patients, with symptoms that continue chronically for 8-10 years after an initial bacterial infection[3]. Those who suffer from IBS experience a variety of symptoms; changes in bowel movements are seen as one of the primary debilitating gastrointestinal symptoms. However, the most distressing associated symptom is abdominal pain[6]. Although acute symptoms usually resolve within several weeks after initial onset, bloating, diarrhea, and abdominal symptoms do not. When transient colonic inflammation occurs, the gut may continue to remain sensitized even after the inflammation has resolved[7]. In addition to GI symptoms, those with IBS report extra-intestinal symptoms (i.e., migraine, backaches, muscle pain). Moreover, IBS patients report visceral hypersensitivity to rectal distention and cutaneous hypersensitivity to heat stimuli when compared to controls without IBS[8-12]. It is evident that those who suffer from IBS experience a complex collection of symptoms.
IBS-like symptoms are often the initial presenting symptoms of patients with inflammatory bowel disease (IBD)[13,14]. In addition, patients with IBD in remission report IBS-like symptoms more frequently than healthy controls[15]. These findings further suggest that inflammation contributes to gastrointestinal symptoms experienced by those with IBS. Inflammatory markers as a potential correlate of abdominal pain have been the focus of numerous studies[16]. Many studies employing experimental animal models to characterize inflammatory cell infiltration used trinitrobenzene sulfonic acid (TNBS) to induce colitis. TNBS-induced inflammation activates the immune response thought to be involved in ulcerative colitis[7,17,18]. Visceral hypersensitivity has been shown to be increased in TNBS-treated rats when compared to saline treated rats[17]. Current research supports the use of TNBS induced colitis in rats as a model that demonstrates persistent visceral and somatic hypersensitivity similar to those who suffer from functional gastrointestinal pain. TNBS rectally infused in rats has been found to result in intense colitis involving all muscle layers of the colon. This intense colitis has been reported to mimic a post-infectious IBS. Zhou et al[7] (2008) found TNBS-induced colitis resulted in chronic colonic hypersensitivity. This hypersensitivity has been found to persist in rats even with complete resolution of histological findings of colitis, similar to individuals who suffer from post-infectious IBS. It is thought that visceral hypersensitivity, which is common in those who suffer from IBS, plays an integral role in symptom development[19].
Presently there is no universally accepted pharmacological treatment or dietary supplement to treat the full spectrum of gastrointestinal symptoms associated with IBS. Treatments are primarily focused on symptom management and often do not provide relief to the level of the patients’ expectations[20].
Vasoactive intestinal peptide (VIP) has recently been recognized as a promising therapeutic target given its role in facilitating overall gut motility and digestion. VIP, which is a member of the glucagon-secretin superfamily[21], has a broad distribution in the body, such as the heart, lung, and digestive tract[22]. VIP has many physiological effects including exocrine and endocrine secretions, neuro-protection, cell differentiation, and the regulation of immune response[23]. VIP has been targeted as a possible drug candidate for several chronic inflammatory diseases such as rheumatoid arthritis and asthma because of its potent anti-inflammatory effect[24-27]. VIP is also a potent gut muscle relaxant[28] and dysregulation in its content has been reported in several gastrointestinal disorders[29-31].
Current research suggests that motility, inflammation, and visceral hypersensitivity play an integral role in IBS. The limited pharmacologic therapies for the treatment of IBS led us to explore the role that VIP plays in IBS. The purpose of this study was to evaluate changes in the expression of VIP in both participants with IBS and in an animal model of colitis mimicking post-inflammatory visceral hypersensitivity.
Design and setting: All animal protocols were approved by the University of Florida Institutional Animal Care and Use Committee (#200801196). Male Sprague Dawley rats (300-350 g, n = 154) were maintained on a 12-h light/dark cycle and fed standard rodent chow and water ad libitum. Animals were weighed before treatments began and every day after. All reagents were purchased from Sigma-Aldrich (St. Louis, MO, United States) unless otherwise indicated.
Trinitrobenzene sulfonic acid induced colitis: Colitis was induced in rats anesthetized with 3% isoflurane in oxygen. Rats received an enema of 1 mL of TNBS (15 mg/mL) in 50% ethanol using a polyethylene catheter (18 gauge; Fisher Scientific, Pittsburgh, PA, United States) that was inserted rectally 7 cm proximal to the anus. Animals were tested for hypersensitivity and then sacrificed at 2, 7, 14, 21 or 28 d after TNBS-treatment by CO2 inhalation/decapitation. Naïve rats were also included in these studies.
Colo-rectal distension: Naïve and TNBS-treated rats underwent colo-rectal distension at 7, 14, 21 and 28 d. While anesthetized with 3% isoflurane in oxygen, a 4 cm balloon attached to a plastic tube (14 gauge; Fisher Scientific, Pittsburgh, PA, United States) was inserted rectally 2-3 cm proximal to the anus. The balloon was secured in place by taping the tube to the base of the tail, and rats were placed in a plastic restraining device. The tube was then attached to a pressure transducer (Harvard Apparatus, Holliston, MA, United States) connected to a LAB-TRAX Data Acquisition System with Transducer Amplifier (World Precision Instruments, Sarasota, FL, United States). Following recovery from anesthesia, the animals were allowed 10 min to acclimatize before behavioral testing began. Rats received phasic colon distension (0-80 mmHg) until the first contraction of the testicles, tail, or abdominal musculature occurred. These responses are considered indicative of the first nociceptive response as previously described[32,33]. The colonic distensions were repeated 3 times and the mean pressures at the nociceptive threshold were recorded for each rat. Data were then analyzed using Data-Trax 2 software (World Precision Instruments, Sarasota, FL, United States).
Mechanical threshold testing on hind paws: Mechanical hypersensitivity was measured using an electronic von Frey device (Dynamic Plantar Aesthesiometer, Ugo Basile, Italy). The experimenter was blinded to group assignment. Rats were placed on a wire mesh floor in a plastic enclosure. Rats were left to acclimate for 20 min. Then a fine filament was extended up through the mesh floor and exerted an increasing amount of pressure (max. 50 g) onto the rat’s hind paws. The mechanical threshold was defined as the force (in grams) required to induce the rat to withdraw its hind-paw. The stimulus was repeated 3 times following a 5-min rest interval, and the mean was calculated for each hind-paw. Data were reported as percentage of control (naïve) threshold response.
Myeloperoxidase activity assay: Myeloperoxidase (MPO) activity was assessed as described previously[34,35]. The descending colon was removed from naïve rats and vehicle (ethanol)-treated rats at 2 d, and TNBS-treated rats at 2, 7, 14 and 28 d. Colons were cut open along the mesenteric border and frozen in liquid nitrogen. Tissue segments weighing 100-200 mg were homogenized in potassium phosphate buffer (50 mg/mL, 50 mmol/L potassium phosphate, pH = 6.0) and centrifuged at 20000 g for 20 min. The supernatant was removed and HTAB buffer (Hexadecyltrimethylammonium Bromide: 5 g/L in 50 mmol/L potassium phosphate buffer) was added. The pellet was homogenized and centrifuged at 10000 g. The samples were sonicated, frozen in liquid nitrogen, thawed, and centrifuged at 10000 g for 10 min. This step was repeated twice. The level of MPO activity was determined from the total supernatant by adding 200 μL of O-dianisidine buffer (16.7 mg O-dianisidine dihydrochloride in 5 mmol/L phosphate buffer containing 0.005% H2O2). The change in absorbance at 450 nm was determined every 30 s over a 3-min period after adding the O-dianisidine buffer by a Synergy HT microplate reader (BioTek Instruments, Winooski, VT, United States). Values are expressed as units of MPO activity per gram of tissue sample where 1 unit of MPO is defined as that which degrades 1 μmol of hydrogen peroxide per minute.
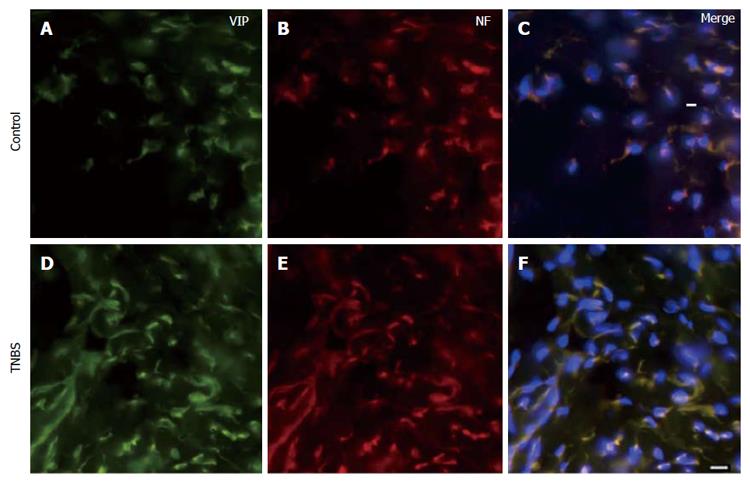
Immunohistochemistry: Descending colons were removed from naïve and TNBS-treated rats at 14 d. This time point was chosen because we previously found it to be the time at which hyperalgesia was most prominent[17]. Tissues were fixed in 4% Formalin for 24 h and preserved in 30% sucrose at 4 °C. Tissues were sectioned at 20 μm on a cryostat, serially mounted on a glass slide, and air-dried for 1 h. Slides were treated with Target Retrieval Solution, pH 6 (Dako, Carpinteria, CA, United States) for 24 h at 60 °C. All preparations were washed 3 times (10 min each) in Phosphate Buffered Saline (PBS, 10 mmol/L sodium phosphate, pH = 7.4, 0.9% NaCl). Slides were then incubated with anti-VIP and anti-Neurofilament (Santa Cruz Biotechnology, Santa Cruz, CA, United States) in 0.3% tween-20 in PBS for 24 h at 4 °C. The sections were washed 3 times in PBS (10 min each) followed by 1 h incubation in secondary antibodies. The secondary antibodies were Alexa Fluor 488 and Alexa Fluor 594 (Molecular Probes, Boston, MA, United States) in 0.3% tween-20 in PBS. Negative controls were performed by incubating samples with only secondary antibodies and omitting primary antibodies. After incubation with secondary antibodies, slides were washed 3 times (10 min each) and coverslipped with VECTASHIELD Mounting Medium with DAPI (Vector Laboratories, Burlingame, CA, United States). The sections were visualized with filters for red, green, and blue excitation. Images were photographed on a Leica DM LB2 Fluorescence microscope (Leica, Wetzlar, Germany).
Design and setting: This pilot study was composed of adult participants who met Rome III criteria for IBS for longer than 6 mo and matched healthy controls. Participants were recruited under a natural history protocol (09-NR-0064, Clinicaltrials.gov # NCT00824941) conducted at the National Institutes of Health (NIH) Hatfield Clinical Research Center. Biological samples were collected during a span of 2 outpatient visits from January 2009 to June 2010. All participants gave written consent, and the study was approved by the Institutional Review Board and the Office of Human Subjects Research at the NIH.
Sample: A cohort of 24 participants (45.8% male) included IBS participants (n = 12) and matched healthy controls (n = 12). Participants were matched for race (100% Caucasian), age (mean 28.00 ± 7.54 years, range 22 - 45 years), and body mass index (BMI, mean 25.43 ± 5.92 kg/m2), range 18.82 - 43.22 kg/m2) (Table 1). Participants with a history of organic GI disease (e.g., inflammatory bowel disease, celiac disease) or currently taking medications daily for GI symptoms (e.g., antispasmodics, laxatives) were excluded to control for potential confounding factors.
| Variable | Overall(n = 24) | IBS patients(n = 12) | Healthy controls(n = 12) | P value |
| Gender n (%) | ||||
| Male | 11 (45.8) | 5 (41.7) | 6 (50) | - |
| Female | 13 (54.2) | 7 (58.3) | 6 (50) | - |
| Race (n) | ||||
| Caucasian | 24 | 12 | 12 | - |
| Age | 28.00 ± 7.54 | 26.67 ± 7.29 | 29.33 ± 7.87 | 0.40 |
| Range (yr) | (22.00-45.00) | (14.00-44.00) | (23.00-45.00) | |
| BMI | 25.43 ± 5.92 | 25.23 ± 4.18 | 25.64 ± 7.46 | 0.87 |
| Range (kg/m²) | (18.82-43.22) | (20.19-35.07) | (18.82-43.22) |
RNA extraction: Peripheral whole blood (2.5 mL) was collected in PAXgene® RNA tubes from fasting participants via venipuncture. RNA was extracted using the PAXgene Blood miRNA Kit (Qiagen, Franklin Lakes, NJ, United States). RNA quality was verified with the Experion™ RNA Analysis Kit (Bio-Rad, Hercules, CA, United States).
Gene expression: RNA (500 ng) was reverse transcribed using the RT2 First Strand Kit (SA Biosciences by Qiagen, Frederick, MD, United States). A modified version of the RT2 Profiler™ PCR Array Human Inflammatory Response and Autoimmunity (PAHS-077A, SA Biosciences by Qiagen, Frederick, MD, United States) was used to analyze the expression of 96 wet-bench validated primer assays, including 84 key genes involved in inflammatory immune responses, 4 added custom genes (i.e., VIP), 5 housekeeping genes, and controls to check for genomic DNA contamination, RNA quality, and general PCR performance (Del Valle-Pinero et al[36]). Quantitative real-time PCR was performed using an Applied Biosystems 7900HT (Life Technologies Corporation, Grand Island, NY, United States) and QIAGEN’s RT² SYBR® Green qPCR Mastermix with ROX as passive reference dye.
Plasma from naïve rats, TNBS-treated rats, healthy participants and IBS participants were assayed for VIP protein levels using Peptide Enzyme Immunoassay Protocol (Bachem Group, San Carlos, CA). Briefly, 50 μL of samples, 25 μL of VIP antiserum, and 25 μL of biotinylated-tracer were mixed into each well of a 96-well ® with anti-rabbit secondary antibody and incubated at room temperature for 2 h. The plate was then washed 5 times with buffer, and 100 μL/well of streptavidin-conjugated horseradish peroxidase was added. The plate was incubated for 1 h. The plate was then washed 5 times with buffer and 100 μL/well of TMB solution was added. The change in absorbance at 650 nm was determined every minute over 30 min using a Bio-Tek® Synergy HT Microplate reader. The reaction was terminated by adding 100 μL 2 mol/L HCL per well and readings were taken again at 450 nm. VIP concentration (ng/mL) was calculated by comparing samples’ absorbance to a standard curve generated using the standard peptide provided with the kit.
Data were analyzed using ANOVAs. Post-hoc tests were either Dunnett’s or Bonferroni’s tests. P-values < 0.05 were considered to be significant and n values represent individual animals. GraphPad Prism 5 (GraphPad Software Inc., La Jolla, CA, United States) was used for all analyses. Quantitative PCR raw data were uploaded to SA Biosciences Web Analysis (http://www.sabiosciences.com) for statistical analysis. As previously recommended in the literature, cut off for threshold cycle (Ct) was set at 35 cycles and boundary to 2-fold[37]. Fold-change (2-∆∆Ct) was defined as the normalized gene expression (2-∆Ct) in the test group (IBS participants) divided by the normalized gene expression (2-∆Ct) in the control group. A positive fold-change value greater than 1 indicates an up-regulation; the fold-regulation is equal to the fold-change. Negative fold-change values less than 1 indicate a down-regulation; (i.e., the fold-regulation is the negative inverse of the fold-change). The results were reported as fold-regulation as it represents fold-change values in a meaningful biologic way [Arikawa, SAB Tech Article]. The clinical data (Table 1) were analyzed with t-tests (unpaired) using GraphPad Prism 5 (GraphPad Software Inc., La Jolla, CA, United States) and independent sample t-tests using SPSS 15 (SPSS Inc., Chicago, IL, United States). Statistical P-values < 0.05 were considered significant.
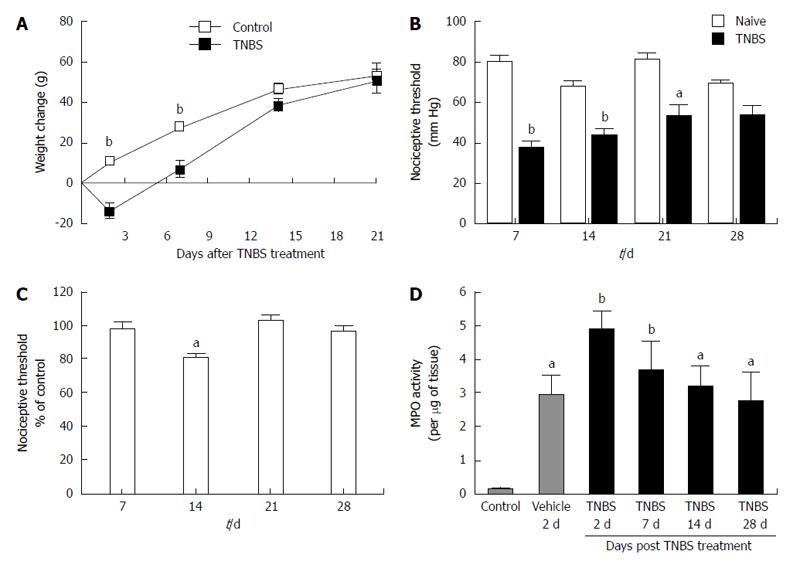
Inflammation in the TNBS-induced model of colitis has several indicators one of which is transient weight loss after treatment. Compared to controls, animals treated with TNBS lost weight (13.7 ± 3.2 g) during the first 2-7 d after the enema (Figure 1A). Treated animals stopped losing weight after day 14 and showed weights indistinguishable from controls by day 21 (Figure 1A). When compared to controls, visceral hypersensitivity to colo-rectal distension was increased in TNBS-treated rats at 7, 14, and 21 d following TNBS administration and was resolved by day 28 (Figure 1B). Somatic hypersensitivity was also increased 14 d after TNBS treatment (Figure 1C). Another marker of inflammation is an increase in the activity of MPO which is an enzyme located within granules found in the intracellular region of neutrophils that is used as an indicator of neutrophil infiltration. The levels of MPO activity were significantly elevated 2 d after vehicle (ethanol-saline, 1:1) administration and 2, 7, 14 and 28 d after TNBS treatment when compared to control (naïve) rats (Figure 1D). These results, which are comparable to Zheng et al[35] results and our previous findings[7,17], indicate that colitis was effectively induced by TNBS.
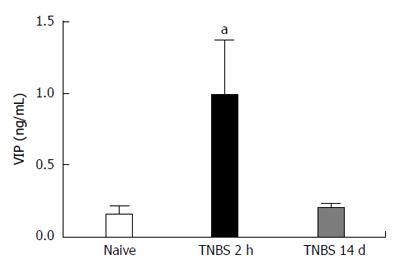
Using immunohistochemistry, we confirmed that VIP co-localizes with the neuronal marker, Neurofilament (NF), in both the naïve and the inflamed colons (Figure 2). The plasma concentration of VIP was then assessed using a peptide enzyme immunoassay (Bachem Group, San Carlos, CA) in naïve and TNBS-treated rats. VIP plasma concentration was significantly increased following TNBS enema when compared to naïve animals (P < 0.05, Figure 3).
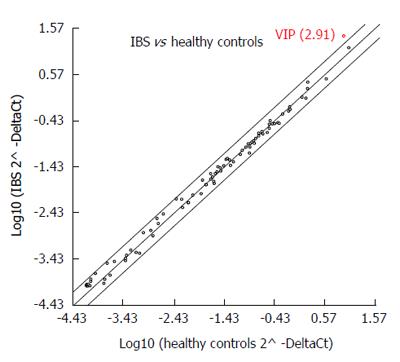
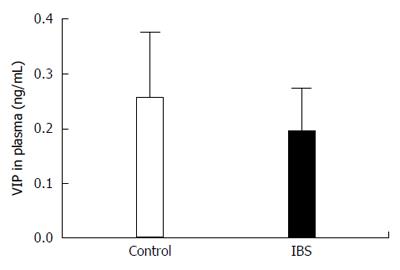
VIP gene expression was significantly upregulated (fold regulation = 2.91) in IBS patients (Group 1; n = 12) when compared to healthy controls (Control Group; n = 12) (95%CI: 0.00001-9.7) (Figure 4). Moreover, the expression level of VIP protein in the plasma of the participants with IBS (0.254 ng/mL) was not significantly different when compared with those of the control group (0.193 ng/mL, P = 0.173) (Figure 5).
Our finding of an upregulation of circulating VIP gene expression is significant and supports that of others who have shown an increased mRNA expression of VIP from rectosigmoid mucosa biopsies in patients with diarrhea-predominant IBS compared to healthy controls[38]. These finding are important as VIP may have both neuroeffective and neuroprotective roles[39], and therefore have a role not only in the development but also the continuation of chronic gastrointestinal symptoms in IBS.
The similar VIP plasma protein levels in our IBS cohort compared to the healthy controls differ from our animal findings. These results could be explained by the inherently acute state of the TNBS-induced colitis when compared to IBS patients’ chronic condition. It is plausible that IBS patients have fewer ongoing inflammatory processes or a defect in the VIP pathway, which prevents them from recovering from an inflammatory event. However, additional studies are needed with acute stimulation and repeated longitudinal measures of VIP over time to better elucidate the functional mechanisms of VIP in patients with IBS and the rat TNBS-model. Zhou et al[7] reported that 16 wk after TNBS treatment, when initial inflammation had resolved, 24% of treated rats still exhibit visceral and somatic hypersensitivity. It would be relevant to study whether this subset of animals also retains changes in VIP expression.
The TNBS-induced colitis model is not without limitations. One example is the absence of spontaneous relapse, which is a hallmark of most gastrointestinal disorders. Moreover, the reproducibility of this model is highly dependent on the TNBS dose used[40,41] .
The use of human whole blood as a correlate for colonic mucosa inflammation is an additional limitation of this study. It is important that future studies assess not only circulating VIP, but also colonic mucosa expression simultaneously. Controlled intervention studies to test the effects of VIP in experimental human studies will offer greater insight into the mechanisms and bidirectional effects of VIP. There are numerous factors, potentially interacting with each which we did not investigate including but not limited to gut hormones. Future studies require larger more representative samples in order to elucidate the underlying mechanisms in IBS and enhance the potential for developing novel treatments to aid patients living with IBS.
In conclusion, our patient and animal data show that changes in vasoactive intestinal peptide gene and protein expression may play an important role in chronic symptoms associated with gastrointestinal disorders. These data provide a starting point to better understand the expression, physiology, and pharmacological properties of VIP, which in turn may lead to the development of therapies that selectively modulate gastrointestinal function.
Vasoactive Intestinal Peptide is currently being looked at as a possible drug or therapeutic target for a number of gastro intestinal disorders. The molecule is released in response to inflammation in the gastrointestinal (GI) tract and suppresses the immune system causing a reduction in the inflammation. Altered levels of vasoactive intestinal peptides could be a factor in the development of the GI disorder. One way to test inflammatory responses in the GI tract of animal models is to artificially induce inflammation using the chemical trinitrobenzene sulfonic acid (TNBS). TNBS induced inflammation and the subsequent recovery could have an impact on the levels of vasoactive intestinal peptide in the GI tract.
Vasoactive intestinal peptide (VIP) has potent anti-inflammatory effects and facilitates gut motility and digestion. Current research suggests gastrointestinal symptoms in irritable bowel syndrome (IBS) are related to inflammatory, motility, and visceral hypersensitivity processes. Comprehensive treatment of the variety of IBS symptoms is both lacking and pressing.
Previous measures of VIP have been obtained through mucosal biopsies of the rectosigmoid. In the present study, the authors measured circulating VIP gene expression and support the mucosal findings of upregulation.
The study results suggest that VIP is a potential therapeutic target for the management of symptoms in individuals who suffer from irritable bowel syndrome.
Vasoactive intestinal peptide is a peptide hormone that is produced in the gut, pancreas and the brain. In particular to the gut, it both inhibits and stimulates secretions resulting in increased motility. IBS is a symptom-based diagnosed disorder characterized by abdominal pain or discomfort, and altered bowel habits. TNBS is an agent that induces focal inflammation and alterations in the colon when given by enema to rats with features similar to those found in chronic inflammatory diseases in humans.
This is a uniquely designed study to determine the changes in expression of VIP in an animal model of TNBS induced colitis and IBS patients. The results were very interesting.
P- Reviewer: Shimatani T S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
| 1. | Park DW, Lee OY, Shim SG, Jun DW, Lee KN, Kim HY, Lee HL, Yoon BC, Choi HS. The Differences in Prevalence and Sociodemographic Characteristics of Irritable Bowel Syndrome According to Rome II and Rome III. J Neurogastroenterol Motil. 2010;16:186-193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 2. | Mapel DW. Functional disorders of the gastrointestinal tract: Cost effectiveness review. Best Pract Res Clin Gastroenterol. 2013;27:913-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Grover M, Camilleri M, Smith K, Linden DR, Farrugia G. On the fiftieth anniversary. Postinfectious irritable bowel syndrome: mechanisms related to pathogens. Neurogastroenterol Motil. 2014;26:156-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Ahn JY, Lee KH, Choi CH, Kim JW, Lee HW, Kim JW, Kim MK, Kwon GY, Han S, Kim SE. Colonic mucosal immune activity in irritable bowel syndrome: comparison with healthy controls and patients with ulcerative colitis. Dig Dis Sci. 2014;59:1001-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Kanazawa M, Palsson OS, Thiwan SI, Turner MJ, van Tilburg MA, Gangarosa LM, Chitkara DK, Fukudo S, Drossman DA, Whitehead WE. Contributions of pain sensitivity and colonic motility to IBS symptom severity and predominant bowel habits. Am J Gastroenterol. 2008;103:2550-2561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 114] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 6. | Spiegel BM, Bolus R, Harris LA, Lucak S, Chey WD, Sayuk G, Esrailian E, Lembo A, Karsan H, Tillisch K. Characterizing abdominal pain in IBS: guidance for study inclusion criteria, outcome measurement and clinical practice. Aliment Pharmacol Ther. 2010;32:1192-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Zhou Q, Price DD, Caudle RM, Verne GN. Visceral and somatic hypersensitivity in a subset of rats following TNBS-induced colitis. Pain. 2008;134:9-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Mayer EA, Gebhart GF. Basic and clinical aspects of visceral hyperalgesia. Gastroenterology. 1994;107:271-293. [PubMed] |
| 9. | Mayer EA, Raybould HE. Role of visceral afferent mechanisms in functional bowel disorders. Gastroenterology. 1990;99:1688-1704. [PubMed] |
| 10. | Verne GN, Himes NC, Robinson ME, Gopinath KS, Briggs RW, Crosson B, Price DD. Central representation of visceral and cutaneous hypersensitivity in the irritable bowel syndrome. Pain. 2003;103:99-110. [PubMed] |
| 11. | Verne GN, Price DD. Irritable bowel syndrome as a common precipitant of central sensitization. Curr Rheumatol Rep. 2002;4:322-328. [PubMed] |
| 12. | Verne GN, Robinson ME, Price DD. Hypersensitivity to visceral and cutaneous pain in the irritable bowel syndrome. Pain. 2001;93:7-14. [PubMed] |
| 13. | Burgmann T, Clara I, Graff L, Walker J, Lix L, Rawsthorne P, McPhail C, Rogala L, Miller N, Bernstein CN. The Manitoba Inflammatory Bowel Disease Cohort Study: prolonged symptoms before diagnosis--how much is irritable bowel syndrome? Clin Gastroenterol Hepatol. 2006;4:614-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Limsui D, Pardi DS, Camilleri M, Loftus EV, Kammer PP, Tremaine WJ, Sandborn WJ. Symptomatic overlap between irritable bowel syndrome and microscopic colitis. Inflamm Bowel Dis. 2007;13:175-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 100] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 15. | Simrén M, Axelsson J, Gillberg R, Abrahamsson H, Svedlund J, Björnsson ES. Quality of life in inflammatory bowel disease in remission: the impact of IBS-like symptoms and associated psychological factors. Am J Gastroenterol. 2002;97:389-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 250] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 16. | Taylor TJ, Youssef NN, Shankar R, Kleiner DE, Henderson WA. The association of mast cells and serotonin in children with chronic abdominal pain of unknown etiology. BMC Res Notes. 2010;3:265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Zhou Q, Caudle RM, Price DD, Del Valle-Pinero AY, Verne GN. Selective up-regulation of NMDA-NR1 receptor expression in myenteric plexus after TNBS induced colitis in rats. Mol Pain. 2006;2:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Zhang Z, Zheng M, Bindas J, Schwarzenberger P, Kolls JK. Critical role of IL-17 receptor signaling in acute TNBS-induced colitis. Inflamm Bowel Dis. 2006;12:382-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 364] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 19. | Kanazawa M, Hongo M, Fukudo S. Visceral hypersensitivity in irritable bowel syndrome. J Gastroenterol Hepatol. 2011;26 Suppl 3:119-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 20. | Mayer EA, Bradesi S, Chang L, Spiegel BM, Bueller JA, Naliboff BD. Functional GI disorders: from animal models to drug development. Gut. 2008;57:384-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 119] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 21. | Said SI, Mutt V. Polypeptide with broad biological activity: isolation from small intestine. Science. 1970;169:1217-1218. [PubMed] |
| 22. | Vaudry D, Gonzalez BJ, Basille M, Yon L, Fournier A, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacol Rev. 2000;52:269-324. [PubMed] |
| 23. | Onoue S, Yamada S, Yajima T. Bioactive analogues and drug delivery systems of vasoactive intestinal peptide (VIP) for the treatment of asthma/COPD. Peptides. 2007;28:1640-1650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Delgado M. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit CBP-NF-kappaB interaction in activated microglia. Biochem Biophys Res Commun. 2002;297:1181-1185. [PubMed] |
| 25. | Delgado M, Abad C, Martinez C, Juarranz MG, Arranz A, Gomariz RP, Leceta J. Vasoactive intestinal peptide in the immune system: potential therapeutic role in inflammatory and autoimmune diseases. J Mol Med (Berl). 2002;80:16-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 120] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 26. | Said SI. Vasoactive intestinal polypeptide (VIP) in lung function and disease. Nihon Kyobu Shikkan Gakkai Zasshi. 1991;29:1525-1531. [PubMed] |
| 27. | Said SI. Vasoactive intestinal polypeptide (VIP) in asthma. Ann N Y Acad Sci. 1991;629:305-318. [PubMed] |
| 28. | Furness J. The Enteric Nervous System. Malden, MA: Blackwell Publishing 2006; . |
| 29. | Bishop AE, Polak JM, Bryant MG, Bloom SR, Hamilton S. Abnormalities of vasoactive intestinal polypeptide-containing nerves in Crohn’s disease. Gastroenterology. 1980;79:853-860. [PubMed] |
| 30. | Maunder R. Mediators of stress effects in inflammatory bowel disease: not the usual suspects. J Psychosom Res. 2000;48:569-577. [PubMed] |
| 31. | Surrenti C, Renzi D, Garcea MR, Surrenti E, Salvadori G. Colonic vasoactive intestinal polypeptide in ulcerative colitis. J Physiol Paris. 1993;87:307-311. [PubMed] |
| 32. | Ness TJ, Gebhart GF. Colorectal distension as a noxious visceral stimulus: physiologic and pharmacologic characterization of pseudaffective reflexes in the rat. Brain Res. 1988;450:153-169. [PubMed] |
| 33. | Wesselmann U, Czakanski PP, Affaitati G, Giamberardino MA. Uterine inflammation as a noxious visceral stimulus: behavioral characterization in the rat. Neurosci Lett. 1998;246:73-76. [PubMed] |
| 34. | Krauter EM, Strong DS, Brooks EM, Linden DR, Sharkey KA, Mawe GM. Changes in colonic motility and the electrophysiological properties of myenteric neurons persist following recovery from trinitrobenzene sulfonic acid colitis in the guinea pig. Neurogastroenterol Motil. 2007;19:990-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Zheng L, Gao ZQ, Wang SX. A chronic ulcerative colitis model in rats. World J Gastroenterol. 2000;6:150-152. [PubMed] |
| 36. | Del Valle-Pinero AY, Martino AC, Taylor TJ, Majors BL, Patel NS, Heitkemper MM, Henderson WA. Pro-inflammatory chemokine C-C motif ligand 16 (CCL-16) dysregulation in irritable bowel syndrome (IBS): a pilot study. Neurogastroenterol Motil. 2011;23:1092-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 37. | McCarthy DJ, Smyth GK. Testing significance relative to a fold-change threshold is a TREAT. Bioinformatics. 2009;25:765-771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 396] [Cited by in RCA: 486] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 38. | Camilleri M, Carlson P, Acosta A, Busciglio I, Nair AA, Gibbons SJ, Farrugia G, Klee EW. RNA sequencing shows transcriptomic changes in rectosigmoid mucosa in patients with irritable bowel syndrome-diarrhea: a pilot case-control study. Am J Physiol Gastrointest Liver Physiol. 2014;306:G1089-G1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 39. | Jabari S, de Oliveira EC, Brehmer A, da Silveira AB. Chagasic megacolon: enteric neurons and related structures. Histochem Cell Biol. 2014;142:235-244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 40. | Motavallian-Naeini A, Andalib S, Rabbani M, Mahzouni P, Afsharipour M, Minaiyan M. Validation and optimization of experimental colitis induction in rats using 2, 4, 6-trinitrobenzene sulfonic acid. Res Pharm Sci. 2012;7:159-169. [PubMed] |
| 41. | Stevenson CS, Marshall LA, Morgan DW. In Vivo Models of Inflamation. 2nd ed. Basel: Birkhauser 2006; 150-151. |