Published online Mar 7, 2014. doi: 10.3748/wjg.v20.i9.2279
Revised: December 25, 2013
Accepted: January 3, 2014
Published online: March 7, 2014
Processing time: 128 Days and 19.1 Hours
There is growing evidence that metabolic alterations play an important role in cancer development and progression. The metabolism of cancer cells is reprogrammed in order to support their rapid proliferation. Elevated fatty acid synthesis is one of the most important aberrations of cancer cell metabolism. An enhancement of fatty acids synthesis is required both for carcinogenesis and cancer cell survival, as inhibition of key lipogenic enzymes slows down the growth of tumor cells and impairs their survival. Based on the data that serum fatty acid synthase (FASN), also known as oncoantigen 519, is elevated in patients with certain types of cancer, its serum level was proposed as a marker of neoplasia. This review aims to demonstrate the changes in lipid metabolism and other metabolic processes associated with lipid metabolism in pancreatic ductal adenocarcinoma (PDAC), the most common pancreatic neoplasm, characterized by high mortality. We also addressed the influence of some oncogenic factors and tumor suppressors on pancreatic cancer cell metabolism. Additionally the review discusses the potential role of elevated lipid synthesis in diagnosis and treatment of pancreatic cancer. In particular, FASN is a viable candidate for indicator of pathologic state, marker of neoplasia, as well as, pharmacological treatment target in pancreatic cancer. Recent research showed that, in addition to lipogenesis, certain cancer cells can use fatty acids from circulation, derived from diet (chylomicrons), synthesized in liver, or released from adipose tissue for their growth. Thus, the interactions between de novo lipogenesis and uptake of fatty acids from circulation by PDAC cells require further investigation.
Core tip: Metabolic alterations associated with mutation in oncogenes and tumor suppressor genes play an important role in cancer development and progression. One of the most important aberrations of metabolism in cancer cells is an elevated synthesis of lipids, which are building blocks for cell membrane formation during cell proliferation and signalling molecules. This review aims to demonstrate the changes in lipid metabolism in pancreatic ductal adenocarcinoma, the most common pancreatic neoplasm, with very high mortality. The potential role of elevated lipid synthesis in diagnosis, prognosis and therapy of pancreatic cancer is also discussed.
- Citation: Swierczynski J, Hebanowska A, Sledzinski T. Role of abnormal lipid metabolism in development, progression, diagnosis and therapy of pancreatic cancer. World J Gastroenterol 2014; 20(9): 2279-2303
- URL: https://www.wjgnet.com/1007-9327/full/v20/i9/2279.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i9.2279
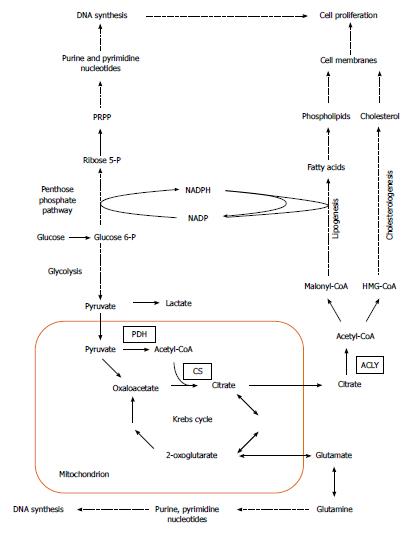
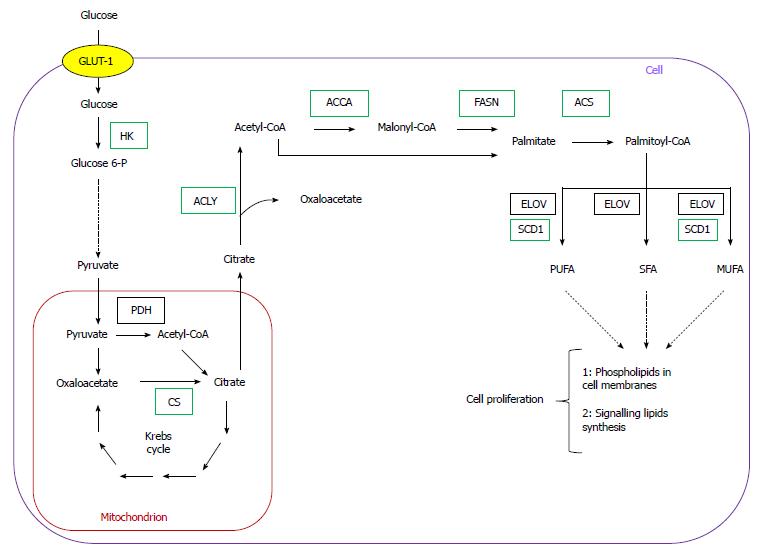
Cancer development is generally attributed to the accumulation of genetic alterations, which leads to activation of cellular oncogenes and inactivation of tumor suppressor genes. Apart from mutations, epigenetic modulation, numerical and structural abnormalities in chromosomes, and aneuploidy are commonly observed in cancer cells, and may play a critical role in tumorigenesis[1]. In addition, carcinogenesis involves significant changes in cellular metabolism, especially in carbohydrate, lipid, nucleic acid, and amino acid metabolism (Figure 1).
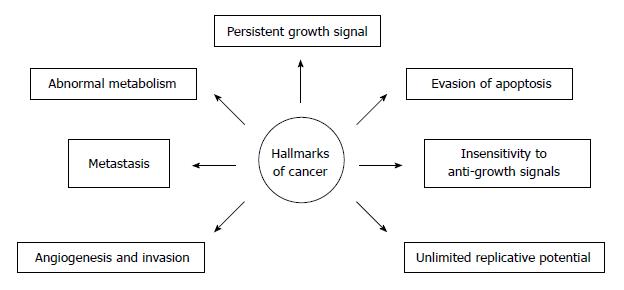
The metabolism of cancer cells is reprogrammed in order to support their rapid proliferation. Nowadays, metabolic alteration, also referred to as metabolic transformation, should be added to six classic hallmarks of cancer cells proposed by Hanahan and Weinberg[2], Tennant et al[3] and illustrated on Figure 2. Over eight decades ago, Warburg revealed that an elevated rate of glycolysis under aerobic conditions, a phenomenon commonly known as the Warburg effect, is a distinctive feature of many human and animal tumors[4]. In the majority of cancers, glucose is converted mostly to lactate, and, therefore, only 2 moles of ATP per 1 mole of glucose are synthesized. In contrast, most non-cancer cells containing mitochondria, produce CO2 and H2O from glucose, and 38 moles of ATP are synthesized per 1 mole of glucose, under aerobic conditions.
Over the last two decades, several authors reported overexpression of genes encoding lipogenic enzymes in many human cancers (Table 1)[5-12]. This phenomenon is usually associated with an increased glucose carbon incorporation into lipids[13-16]. The possible pathways for the conversion of glucose into phospholipids and cholesterol, required for membrane formation in cancer cells, are illustrated on Figure 1. Pyruvate formed from glucose during active aerobic glycolysis, is either converted to lactate by lactate dehydrogenase (LDH), or can enter into mitochondria, where it is decarboxylated to acetyl-CoA by pyruvate dehydrogenase (PDH). Then, by means of reactions of citrate synthase (CS), present in mitochondria, and ATP citrate lyase (ACLY), present in cytosol, cytosolic acetyl-CoA, a key substrate for lipid biosynthesis is formed (Figure 1). Elevated activities of both enzymes (CS and ACLY) are observed in some malignancies, and the inhibition of ACLY is known to lead to cessation of tumor growth[17-21]. Interestingly, some tumors display a diminished flux of glucose carbon through PDH-catalyzed reaction, due to increased PDHK (pyruvate dehydrogenase kinase) activity, under the influence either hypoxia or oncogenic factors. This points to the possible use of carbon source other than glucose, for lipid synthesis[22-25].
| Enzyme name | Neoplasm type | Experimental model | Ref. |
| Fatty acid synthase (FASN) | Pancreatic cancer | Human tumor tissue, cell line | [96,104,105] |
| Breast carcinoma | Human tumor tissue | [5,9,166] | |
| Prostate cancer | Human tumor tissue | [167] | |
| Melanoma | Human tumor tissue | [168] | |
| Nephroblastoma | Human tumor tissue | [169] | |
| Renal cancer | Cell line | [170] | |
| Endometrial carcinoma | Human tumor tissue | [12,171] | |
| Colon cancer | Human tumor tissue | [11,172] | |
| Ovarian neoplasms squamous cell | Human tumor tissue | [10,173] | |
| Carcinoma of the lung head and neck squamous | Human tumor tissue | [174] | |
| Cell carcinoma squamous cell | Human tumor tissue | [175] | |
| Carcinoma of the tongue | Human tumor tissue | [176] | |
| ATP citrate lyase (ACLY) | Small cell lung cancer | Cell line | [251] |
| Bladder cancer | Human tumor tissue | [7] | |
| Breast cancer | Cell line | [252] | |
| Gastric cancer | Human tumor tissue, cell line | [253] | |
| Colon cancer | Human tumor tissue | [254] | |
| Prostate cancer | Human tumor tissue | [254] | |
| Hepatocellular carcinoma | Human tumor tissue | [255] | |
| Acetyl-CoA carboxylase (ACCA) | Prostate cancer | Human tumor tissue | [6] |
| Hepatocellular carcinoma | Human tumor tissue | [255] | |
| Breast carcinoma | Human tumor tissue | [256] | |
| Stearoyl-CoA desaturase (SCD1) | Pancreatic cancer | SCD1 indices in patients serum | [128] |
| Clear cell renal cell carcinoma | Human tumor tissue | [200] | |
| Acetyl-CoA synthetase (ACS) | Colon adenocarcinoma | Human tumor tissue | [257] |
| Malignant glioma | Cell line | [258] | |
| Citrate synthase (CS) | Pancreatic cancer | Human tumor tissue | [19] |
| Renal cell carcinoma | Human tumor tissue | [20] |
Through conversion to fructose 6-phosphate, glucose also serves as a substrate for hexosamine phosphate synthesis (according to reaction: fructose 6-phosphate + glutamine → glucosamine 6 phosphate + glutamate), required for biosynthesis of glycoproteins and glycosaminoglycans. Glucose may also be converted to penthose phosphate on pentose phosphate pathway (PPP), and then to phosphoribosyl pyrophosphate (PRPP), a precursor of purine and pyrimidine nucleotides necessary for DNA synthesis (Figure 1). PPP generates NADPH, which is required for many processes, including lipid biosynthesis (Figure 1). The activity of glucose 6-phosphate dehydrogenase (G6PDH), a rate limiting enzyme of PPP, is elevated in certain cancers, including human pancreatic cancer (PC)[19,26]. Glutamine for hexosamine and nucleotide synthesis may originate from citrate produced in mitochondria. Citrate is converted by Krebs cycle to 2-oxoglutarate, a precursor of glutamate (Figure 1), and later to glutamine. However, glutamine is not synthesized on that pathway in many cancer cells, but is rather taken up from the circulation, where it is one of the most abundant amino acids[27].
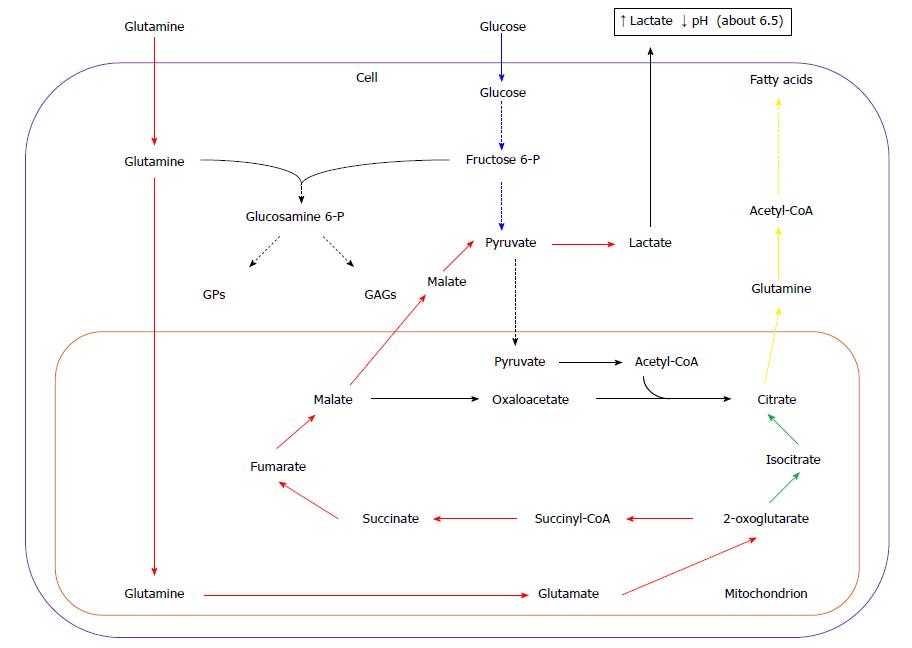
Glucose and glutamine are two main sources of energy and carbon for most cancer cells[28-30]. Some data suggest that glucose accounts mainly for lipid, purine, and pyrimidine nucleotide synthesis, whereas glutamine is contributing to: (1) anaplerotic re-feeding of Krebs cycle; (2) amino acid synthesis; and (3) providing nitrogen necessary for purine and pyrimidine nucleotide synthesis[14], however, there is also evidence of glutamine participation (as carbon donor) in lipid biosynthesis[31]. High expression of glutaminase-encoding gene was revealed during the S phase of the cell cycle in some cancer cell lines (i.e. HeLa cells), along with the low expression in G2/M phase[32]. Upon cellular uptake, glutamine is transported to mitochondria, and then converted to ammonia and glutamate by mitochondrial glutaminase. Then glutamate is deaminated to 2-oxoglutarate by glutamate dehydrogenase. In mitochondria, 2-oxoglutarate is further metabolized by Krebs cycle to malate (Figure 3). Part of the malate is released to cytosol, converted to pyruvate by NADP-linked malic enzyme (ME), and, finally, to lactate by LDH, similarly to pyruvate formed from glucose during glycolysis (Figure 3). The conversion of glutamine to lactate is called glutaminolysis analogically to glycolysis (Figure 3). The increased synthesis of lactic acid by cancer cells leads to the decrease in pH of tumor micro-environment, which promotes angiogenesis, invasion, and metastasis, and suppresses the anticancer immune response through diminished cytotoxic T-cell function[33] (Figure 3).
In a variety of tumors, pyruvate formed during active glutaminolysis is converted into acetyl-CoA by PDH (instead of being converted to lactate by LDH), and later to citrate, supplying carbons for lipid synthesis (Figure 3)[34,35]. Conversion of glutamine to citrate may be also the result of reductive carboxylation of 2-oxoglutarate derived from glutamine, catalyzed by two isoforms of NADP+-dependent isocitrate dehydrogenase - mitochondrial (IDH2), and/or cytosolic (IDH1) (Figure 1)[36-40]. In some cancer cell lines 10%-25% of fatty acids carbons are derived from glutamine under normoxia, and up to 80% under hypoxia[14,36,37]. Wise et al[38] suggest that IDH2 is mainly contributing to conversion of glutamine to lipids. However, other data show that in A549 (adenocarcinoma of human alveolar basal epithelial cells), and in renal carcinoma cells (RCC) cell lines IDH1 is more important[36]. In melanoma or osteosarcoma cell lines both IDH isoforms equally participate in 2-oxoglutarate reduction[37,40].
Continuous loss of citrate from mitochondria to cytosol requires replenishment of Krebs cycle intermediates. Glutamine serves as a key substrate for Krebs cycle intermediates in many cancer cells, and is critical for cell proliferation. A proliferating cell dies upon glutamine (but not glucose) withdrawal from the medium[41].
Fatty acid (FA) biosynthesis remains at a low level in most non-cancerogenic tissues, except liver and adipose tissue. The two latter lipogenic tissues convert the excess of carbohydrates to triacylglycerols[42-49]. Conversely FAs synthesized in cancer cells are esterified mainly to phospholipids required for membrane formation, which promotes cellular replication (Figure 1). Overall, coordinated enhancement of glucose, lipid, and amino acid metabolism, leading to increased synthesis of membrane lipids, nucleotides, and amino acids supports rapid proliferation of cancer cells (Figure 1).
Proliferation and metabolism of cancer cells share common regulatory pathways[50-53]. MYC, proto-oncogene and major regulator of transcription in growing cells, controls several metabolic processes such as: (1) glycolysis and glutaminolysis; (2) nucleotide biosynthesis; and (3) lipid biosynthesis, and mitochondrial biogenesis[53]. Furthermore, MYC stimulates glutamine uptake and metabolism[54,55]. Tumor suppressor protein, p53, is involved in regulation of bioenergetic homeostasis and lipid metabolism in both normal and cancer cells[51,56-58]. p53 induces the expression mitochondrial glutaminase-encoding gene, increasing energy production from glutaminolysis[59,60]. Mutant p53 increases lipid synthesis, via sterol regulatory element-binding protein 1c (SREBP1c), and promotes ovarian cancer metastasis[52]. Certain oncoproteins such as: Akt, Ras, and Src, also stimulate glycolysis in transformed cells[50]. Regulation of glutamine metabolism by Rho GTPases and Ras was also proposed[61]. The oncogenes and tumor suppressor genes whose products participate in regulation of carbohydrate, lipid, nucleotide and amino acid metabolism are presented in Table 2.
| Oncogene/tumor suppressor | Metabolic pathway | Enzyme | Ref. |
| MYC | Glucose transport | GLUT1 | [53-55] |
| Glycolysis | Hexokinase 2 | ||
| Phosphohexose isomerase | |||
| Phosphofructokinase 1 | |||
| Aldolase A | |||
| 3-phosphoglyceraldehyde dehydrogenase | |||
| Phosphoglycerate kinase | |||
| Phosphoglycerate mutase | |||
| Enolase 1 | |||
| Pyruvate kinase 2 | |||
| Lactate dehydrogenase A | |||
| Regulation of PDH | Pyruvate dehydrogenase kinase 1 | ||
| Glutamine transport | Glutamine transporters ASCT2 and SN2 | ||
| Glutaminolysis | Glutaminase 1 | ||
| Serine hydroxymethyltransferase | |||
| Pyrimidine synthesis | |||
| Aminoacids metabolism | CAD | ||
| Ornithine decarboxylase | |||
| Lipogenesis | Fatty acid synthase | ||
| p53 | Glucose transport | GLUT1 | [51,56-60] |
| Glycolysis | Hexokinase 2 | ||
| Fructose-2,6-bisphosphatase | |||
| Phosphoglycerate mutase | |||
| Oxidative phosphorylation | Cyrochrome c oxidase | ||
| Glutaminolysis | Glutaminase 2 | ||
| Pentose Phosphate Pathway | Glucose-6-phosphate dehydrogenase | ||
| Regulation of PDH | Pyruvate dehydrogenase kinase 1 | ||
| Krebs cycle | Aconitase | ||
| KRAS | Glucose transport | GLUT1 | [61,73,94] |
| Glycolysis | Hexokinase 2 | ||
| Phosphofructokinase 1 | |||
| Lactate dehydrogenase A | |||
| Pentose phosphate pathway | Transketolase | ||
| Hexosamine synthesis | Phosphohexose aminotransferase | ||
| Glutaminolysis | Glutamate dehydrogenase | ||
| Aspartate transaminase | |||
| Akt/PTEN | Glucose transport | GLUT1 | [50,113-115] |
| Lipogenesis | FASN |
Also mutations of some genes can contribute to abnormal cellular metabolism, which in turn can affect oncogenic signaling pathways. For example mutation in gene encoding IDH1/2 is associated with deregulation of cellular metabolism, especially in glioma cells[62]. In glioma IDH1/2 mutations are responsible for conversion of 2-oxoglutarate to 2-hydroxyglutarate, which, by inhibition of 2-oxoglutarate-dependent dioxygenases, affects: (1) proto-oncogene expression; (2) DNA and histone modification; and (3) alteration of extracellular matrix proteins (due to inhibition of collagen hydroxylation)[62]. This paper reviews the possible role of lipid metabolism in human cancers, particularly in PC biology, prognosis, and treatment.
Pancreatic ductal adenocarcinoma (PDAC) is the most common pancreatic neoplasm, comprising approximately 90% of all pancreatic malignancies, and the eight leading cause of cancer-associated death in the world[63]. The 5-year survival rate of PDAC patients is approximately about 5%[64]. Surgery is the primary treatment modality and the only available chance for recovery, however only approximately 10% of patients are eligible for surgical treatment. Other therapies have proven ineffective thus far.
Similar to other cancers, both activation of oncogenes and inactivation of tumor suppressor genes play key role in PDAC pathogenesis. The most frequent genetic alterations documented in PCs, including PDAC, are presented in Table 3. Other pancreatic tumors show different aberrations (Table 4).
| Gene | Protein | Mechanism of alteration in PDAC | Regulated processes in PDAC | Alteration in PDAC | Ref. |
| Oncogenes | |||||
| KRAS | KRAS | Point mutations | Cell proliferation and survival, motility, glucose transport, glycolysis, hexosamine synthesis, nonoxidative pentose phosphate pathway arm, glutaminolysis | > 95% | [73,94,259-261] |
| AKT | AKT | Mutations, amplification | Signal transduction, lipogenesis, glucose transport | 10%-20% | [73,79-81,262-264] |
| c-erbB2 | HER2 | Overexpression amplification | Proliferation, differentiation, survival | 20%-80% | [265-268] |
| Myc | MYC | Amplification overexpression | Glycolysis, glutaminolysis, PDH inhibition | 70% | [55,73,82,94,269] |
| Tumor suppressor genes | |||||
| TP53 | p53 | Mutation and second allele deletion | Cell cycle, apoptosis, DNA repair, glucose transport, glycolysis, lipogenesis, ppp oxidative arm, glutaminolysis | 50%-80% | [270-273] |
| Smad4/DPC4 | SMAD4 | Homozygous deletion, mutation and second allele deletion | Cell cycle, TGF-β signaling | 55% | [274-276] |
| STK/LKB1 | LKB1 | Homozygous deletion, mutation and second allele deletion | Apoptosis, lipogenesis, energy production, protein synthesis | 5% | [277-279] |
| CDKN2A/p16 | p16 | Homozygous deletion, mutation, hypermethylation | Cell cycle | 95% | [280-282] |
| PTEN | PTEN | Hypermethylation, inhibition by miRNA | PI3K/AKT signaling pathway | 30%-70% | [79,283,284] |
| Type of pancreatic cancer | Gene affected | Ref. |
| Pancreatic ductal adenocarcinoma (PDAC) | KRAS, AKT, MYC, TP53, SMAD4, CDKN2A, PTEN | [55,63,64,73,78,79,94,269,284-287] |
| (90% of all pancreatic cancers) | ||
| Acinar cell carcinoma (ACCA) | APC/β-catenin (CTNNB1), BRCA2, BCL10 | [288-290] |
| (< 1% of all pancreatic cancers) | ||
| Adenosquamous carcinoma (ASC) | TP53, CDKN2A, KRAS, E-cadherin, | [291,292] |
| (< 1% of all pancreatic cancers) | ||
| Intraductal papillary mucinous neoplasm (IPNM) | GNAS, KRAS, RNF4, STK11/LKB1, MUC1, MUC2, hTERT, COX2, Shh | [278,293,294] |
| (1%-3% of all pancreatic cancers) | ||
| Mucinous cystic neoplasm (MCN) | KRAS, RNF4, TP53, CDKN2A | [295] |
| (< 1% of all pancreatic cancers) | ||
| Serous cystadenoma (SCN) | VHL | [296] |
| (< 1% of all pancreatic cancers) | ||
| Solid-pseudopapillary neoplasm (SPN) | APC/β-catenin (CTNNB1), E-cadherin | [297,298] |
| (1%-2% of all pancreatic cancers) | ||
| Pancreatic neuroendocrine tumors (PanNET) | DAXX, ATRX, MEN1, TSC2, PTEN, PI3KCA, CHGA, CHGB, mTOR | [299-302] |
| (2%-5% of all pancreatic cancers) |
In addition to genetic and epigenetic alterations, development of PC involves significant alterations of cellular metabolism, supporting rapid proliferation of cancer cells. Reduced vascularity, leading to poor perfusion is characteristic for PC. This results in low availability of oxygen and nutrients[65,66]. The presence of hypoxia corresponds to highly aggressive character of PCs[67].
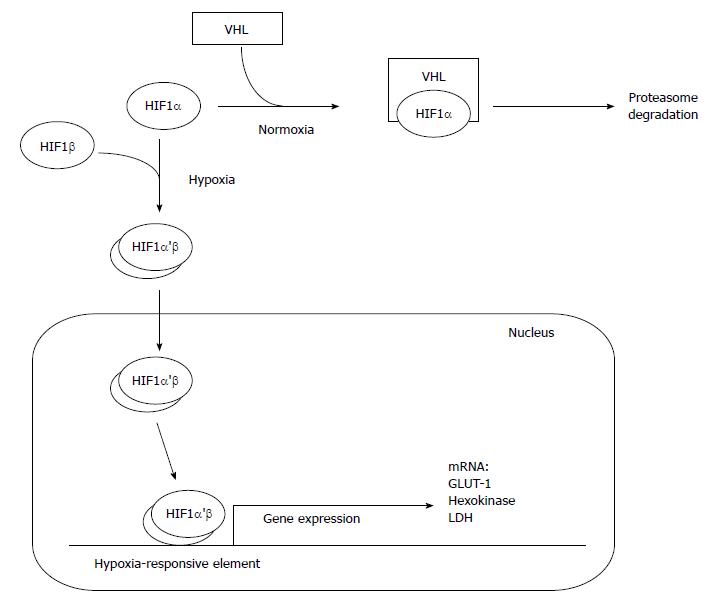
Oxygen deprivation of both non-cancer and cancer cells leads to the stabilization of hypoxia inducible factor 1α (HIF-1α), which dimerizes with HIF-1β, transfers into nucleus and binds with hypoxia-responsive elements present in DNA (Figure 4). This counteract the deleterious impact of decreased oxygen availability[68]. High level of HIF-1α is associated with increased glucose consumption due to activation of glucose transporter 1 (GLUT1), and glycolysis, especially hexokinase (1 and 2), and LDH[69-73] (Figure 4). Overexpression of HIF-1α in human PC cells makes this malignancy similar to other cancers[74]. Interestingly, the expression of HIF-1α in the hypoxic part of pancreatic tumor is at the same level as in its well-oxygenated fragments[75]. Some data indicate that phosphorylation of HIF-1α weakens the interaction of this protein with von Hippel-Lindau tumor suppressor (VHL) which normally stimulates degradation of HIF-1α during normoxia (Figure 4). The phosphorylation result from activation of MAPK or other protein kinase (putatively AKT) in cancer cells[76]. Both kinases are downstream effectors in various signaling pathways, including KRAS pathway. Continuous KRAS signaling and downstream activation of MAPK and AKT results from the mutation of KRAS (observed in 90% of PDACs), or can be stimulated by epidermal growth factor (EGF), prostaglandin E2 (PGE2), and some oxidants[73,77,78]. PI3K/Akt signaling pathway leads to overexpression of HIF-1α, and directly participates in glucose transport and metabolism by regulating GLUT1 gene expression in PC cells, especially when the function of PTEN, tumor suppressor inhibiting PI3K/AKT pathway, is lost[79-81]. Another oncogene, MYC, interacts with KRAS and HIF-1α in PDAC metabolic switch. MYC response elements are present in most glycolytic genes, thus, allowing MYC protein to regulate glucose metabolism[73,82]. Some data suggest that activation of HIF-1α, leading to metabolic reprogramming of pancreatic cells during normoxia, is also controlled by β-adrenergic receptors through the transactivation of epidermal growth factor receptor (EGFR, requiring PKA activity), and further activation of AKT[83]. Also insulin, causing activation of PI3K/AKT and MAPK pathways, can be potential stimulator of HIF-1α activity acting independently of oxygen availability[84].
Mucin 1 (MUC1), a transmembrane protein involved in stabilization of HIF-1α is one of the newly discovered activators of HIF-1α in PC. Directly interacting with HIF-1α and DNA, MUC1 induces expression of glycolytic genes[85]. High activity of MUC1 is correlated with intensive growth and metastasis of pancreatic tumors[86,87]. HIF-1α is coexpressed with Nupr1 (also known as p8 or Com, i.e. candidate of metastasis) in human PDAC[88]. Nupr 1 is a chromatin protein, structurally related to the high-mobility group (HMG) protein, it interacts with several other proteins in the regulation of cell cycle, apoptosis, autophagy, and gene transcription[89]. It is responsible for increased resistance of stress-exposed PDAC cells[90] and supposedly interacts and amplify the KRAS signaling in cancer cells, in order to overcome the activity of some tumor suppressors (such as p16) action[91].
The data presented above suggest that several proteins (mainly products of proto-oncogens or tumor suppressor genes) might affect conversion of glucose to pyruvate in PDAC cells.
Most of the pyruvate formed as a result of increased glycolysis in PDAC cells, is metabolized to lactate, some pyruvate is used to citrate, and further to FAs biosynthesis[92,93]. Acordingly, the activity of CS, one of the crucial enzymes involved in pyruvate to FA conversion (Figure 1), is elevated in PC[19,20]. Thus, it is likely that citrate is synthesized from glucose in PC cells, although glutamine seems to play an important role as well.
Son et al[94] suggested that KRAS directs glutamine carbons to Krebs cycle in PC cells, to export them to cytosol for cytosolic ME reaction. This results in the generation of NADPH, which is used for lipid biosynthesis and for redox state control. Deprivation of glutamine or inhibition of glutaminase activity are reflected by decreased production of ATP and higher levels of reactive oxygen species (ROS). Glutamine may also supply OAA, condensed with acetyl-CoA, to citrate synthesis, or be involved in citrate formation through reductive carboxylation of 2-oxoglutarate catalyzed by reverse IDH reaction. Although the involvement of glutamine was documented in some malignancies, its role in PC cells is still not completely understood[14,36-40,95]. Nevertheless, de novo biosynthesis of lipids (possibly from glucose and/or glutamine) is elevated in PDAC cells[96-98].
Gemcitabine, herceptin or irinotecan treatment has minimal impact on survival rates in patients with advanced PC[99,100]. In contrast treating PC patient with gemcitabine, α-lipoic acid, and hydroxycitrate yielded promising results[101]. Since hydroxycitrate is an inhibitor of ACLY, the activity of the latter lipogenic enzyme (splitting citrate to acetyl-CoA and OAA in cytosol) is likely elevated in PC cells as well, and, similar to other cancers, plays an important role in the development of this malignancy. The next stage of lipogenesis, leading to biosynthesis of malonyl-CoA (fatty acid synthase substrate), is catalyzed by acetyl-CoA carboxylase (ACCA). Phosphorylation by AMPK, leading to ACCA activity cessation, is one of the crucial stages of lipogenesis regulation in lipogenic tissues[102]. The activity of AMPK in PDAC cells is lower than in normal cells, mostly due to LKB1 tumor suppressor inhibition, leading to increased ACCA activity[103]. Fatty acid synthase (FASN) reaction constitutes the last step in palmitate synthesis. The significant role of FASN in cancer development was established approximately two decades ago, when the oncogenic antigen-519 (OA-519), a molecular marker, was identified in breast cancer patients[9]. FASN utilizes acetyl-CoA (supplied by ACLY), malonyl-CoA (supplied by ACCA) and NADPH as a reducing equivalent. In the case of PC cells, NADPH is a product of PPP or reaction catalyzed by ME during oxidative decarboxylation of malate formed from glutamine (i.e. during glutaminolysis)[94]. FASN is the most extensively studied lipogenic enzyme in PDAC cells. Elevated expression of FASN-encoding gene was documented in human PC[96,104,105] and high level of FASN protein, both in tumor cells and in serum is associated with poor prognosis[96,98]. Furthermore inhibition of FASN activity was revealed to induce apoptosis in several tumors[106-110]. Indeed, FASN is an oncogenic protein and its overexpression in non-transformed human breast epithelial cells, can produce their cancer-like phenotype, in a HER1/2 dependent process[111]. Similar phenomenon was reported in the case of colorectal cancer cells[112]. The expression of FASN is strongly induced in hypoxia, by MAPK or PI3K/AKT signaling pathways. This results in activation of SREBP1c transcription factor, which directly binds to FASN promoter (and promoters of other lipogenic genes)[113,114]. Similar effect can be observed in the absence of PTEN tumor suppressor, which normally inhibits PI3K/AKT signaling[114,115]. Moreover, SREBP1c-independent regulation of FASN, mediated by HER2 with PI3K or mTOR involvement was observed in breast cancer cells[116]. Furthermore strong acidic environment of breast cancer may promote epigenetic modification of FASN promoter, leading to increased expression of this gene[117]. As all those events take place in PC cells, the mechanism of FASN regulation in PDAC is probably similar as in the case of other malignancies.
Inhibited activity of FASN (or other lipogenic enzymes) is reflected by decreased tumor growth and may lead to apoptosis of some cancer cells. The inhibition of FASN was revealed to diminish proliferation of osteosarcoma and colorectal cancer cells, through decrease of HER2 activity, leading to down-regulation of PI3K/Akt signaling pathway[112,118]. Induction of apoptosis is likely to result from elevated concentration of malonyl-CoA, that is reflected by decreased oxidation of FA and increased ceramide concentration. Ceramide is a well-known activator of apoptosis, and its enhanced biosynthesis (along with inhibited ceramidase activity) leads to the death of PC cells[106,119]. Furthermore the altered composition of FAs in phospholipid structure (predominance of polyunsaturated acids over saturated and monounsaturated acids) increases the oxidative stress yielding the same result[120].
Glycolytic synthesis of ATP seems the most important pathway in hypoxic cancer cells. In the cases of normoxia, glucose is rather directed to PPP for NADPH and pentose synthesis, and KRAS acts as the main controlling factor supporting tumor cell proliferation[121,122]. Both oxidative and non-oxidative phases of PPP are up-regulated in PC cells. The non-oxidative phase is up-regulated by KRAS[73,123], whereas G6PDH activity (main enzyme of oxidative phase, controlling NADPH production) is increased putatively, due to p53 deficiency[19]. p53 inhibits G6PDH through direct binding, and its loss leads to the up-regulation of the oxidative PPP phase in cancer cells[73,124]. Taken together, these data suggest that similar to other malignancies, the increased glucose flux (both by glycolytic and pentose phosphate pathway) is integrated with the enhanced biosynthesis of lipids in PC cells. Pathways involved in the conversion of glucose to lipids in PC cells are presented in Figure 5.
The lipids formed in cancer cells play two important roles. Firstly, they are building blocks for cell membrane formation during cell proliferation (mainly cholesterol, phosphatidylcholine, phsphatidylserine, phosphatidylethanolamine). Secondly, they play an important role as signaling molecules (phosphatidylinositol, phosphatidic acid, diacylglycerol), or substrates for posttranslational protein modification, including palmitoylation and prenylation[125]. Mammalian cancer cells rely mostly on saturated (SFAs) or monounsaturated FAs (MUFAs). MUFAs are less susceptible to peroxidation, thus increasing the resistance of cancer cells to oxidative stress[126]. Elevated level of MUFAs is maintained mostly by stearoyl-CoA desaturase 1 (SCD1). Inhibition of SCD1 activity in some tumors (e.g., in prostate cancer) leads to inhibition of cancer cell growth. Diminished SCD1 activity is reflected by lower synthesis of phosphatidylinositol, which participates in AKT activation, crucial for cancer development and growth. Additionally inhibition of SCD1 blocks oncogenic transformation of KRAS necessary for activation of this gene and further tumor growth[127]. As SCD1 is very active in PDAC cells[128], and KRAS and AKT signaling pathway are important for their development and growth, SCD1 supposedly plays an essential role in pathogenesis of that malignancy via the same mechanism as in case of other tumors.
In the context of lipid synthesis, especially FASN activity, special attention should be paid to lipid rafts. Lipid rafts are cholesterol- and sphingolipid-rich membranous lipid domains, which contain several signaling and transport proteins. According to some authors, lipid rafts play an important role in health and disease, including carcinogenesis[129]. Lipid rafts rich in proteins of the caveolin family are referred to as caveolae. Caveolin-1 encoding gene expression is altered in some cancers including colon cancer[130,131], breast cancer[132], urothelial carcinoma[133], esophageal squamous cell carcinoma[134], and prostate cancer[135]. The overexpression of caveolin -1 in colon cancer cells is associated with elevated saturated to unsaturated FA ratio in cellular membrane[136]. Deregulation of caveolin-1 is also observed in PC cells[137]. Moreover, caveolin-1 and FASN are co-expressed in the cells of this malignancy. This phenomenon is consistent with histological grade and stage of the tumor (high expression of ceveolin-1 and FASN genes correspond with poor differentiation status)[104]. Thus, FASN and caveolin-1 were suggested as potential diagnostic and prognostic markers of PC and possible therapeutic targets[104].
Recently published data suggest that cancer cells do not rely solely on the de novo lipogenesis, but also utilize food-derived FAs for synthesis of phospholipids required for cell proliferation and lipid signaling[138,139]. This corroborates well with the evidence that a high dietary intake of fat constitutes potential risk factor of some malignancies[140]. Moreover, there is growing evidence that obesity, associated with elevated blood concentrations of FAs, modulates the risk and prognosis of certain cancers[141]. These findings suggest that, apart from lipogenesis, cancer cells can utilize FAs present in blood (derived from VLDL and chylomicrons or from adipose tissue) for their growth. This fact may partly explain why many promising lipogenic enzymes inhibitors tested succesfully in preclinical studies did not confirm their efficiacy in further clinical trials. Furthermore, apart from inhibition of lipogenesis, also reduced dietary lipid digestion and absorption, and decreased lipoprotein lipase and FAs uptake seem necessary for the control of cancer growth[139].
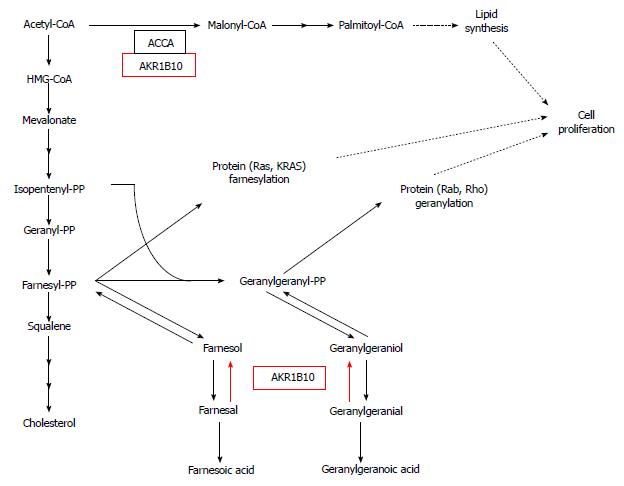
Recently, overexpression and oncogenic function of aldo-keto reductase family 1B10 (AKR1B10; A-aldo, K-keto, R-reductase), tightly associated with lipid metabolism in human PC cell lines, has been reported[142]. AKR protein family consists of enzymes which catalyze the reaction: alcohol + NADP+→ aldehyde (or ketone) + NADPH + H+. These enzymes are expressed in numerous human organs/tissues. AKR1B10, the enzyme specific to such substrates as farnesal, geranylgeranial, retinal, and carbonyls[143-146], is overexpressed in certain malignancies, especially in tobacco-related cancers, including non-small cell lung carcinoma[147] and PC[142]. Oncogenic function of AKR1B10 is associated with protein farnesylation and up-regulation of FA synthesis by stabilization of ACCA[142,148]. Farnesyl diphosphate is a precursor of cholesterol biosynthesis and a substrate for protein farnesylation, which plays an important role in carcinogenesis[149]. Conversion of farnesyl diphosphate to farnesol diminishes its intracellular level, and, consequently, protein farnesylation. Farnesol can be further converted to farnesal, then oxidized to farnesoic acid. If the activity of AKR1B10 is high (as in the case of PC cells), farnesal is reduced to farnesol, following the reaction pattern: farnesal + NADPH + H+→ farnesol + NADP+. Farnesol can be re-phosphorylated to farnesyl diphosphate, increasing the ability for protein farnesylation (Figure 6). Farnesyl diphosphate, together with isopentenyl diphosphate, is converted to geranylgeranyl diphosphate, a substrate for protein geranylation. A geranylgeranyl diphosphate, e.g., farnesyl diphosphate, can be converted to geranylgeranoic acid (via geranylgeraniol and geranylgeranial) (Figure 6). Similarly, high activity of AKR1B10 may cause the reversed conversion of geranylgeranial to geranylgeranyl diphospate, a substrate for protein geranylation (Figure 6). siRNA-mediated silencing of AKR1B10, knockdown of AKR1B10, or inhibition of the enzyme activity lead to decrease in protein prenylation[142]. Membrane-bound KRAS protein of PC cells, a product of point mutation in KRAS is activated by prenylation. If the expression of AKR1B10 is diminished, the activity of membrane-bound KRAS protein decreases in pancreatic cell lines[142]. Thus, the deactivation of AKR1B10 and resultant inhibition of the prenylation (fanezylation, geranylgeranylation) of protein (e.g., KRAS), may constitute a promising target for PC treatment.
3-hydroxy-methylglutaryl-CoA reductase (HMG-CoA reductase) is a key enzyme of cholesterol synthesis pathway (Figure 6), which is inhibited by statins, prescribed to treat hypercholesterolemia. Since the reaction catalysed by HMG-CoA reductase provides substrate for cholesterol synthesis (that is of great importance in rapidly proliferating cancer cells), and also for isoprenoids necessary for prenylation of proteins, the application of statins as an antiproliferative drugs have been studied. Numerous in vitro studies, also with the use of PC cancer cells, provided promising results[150].
Cyclooxygenase-2 (COX-2) is another enzyme which plays an important role in lipid metabolism, namely in the conversion of arachidonic acid (released from membrane phospholipids by phospholipase A2) to prostaglandins. COX-2 is overexpressed in many malignancies, including 45%-75% PCs[151-155]. This suggests, that this enzyme plays an important role in pancreatic carcinogenesis and chemoresistance of PC cells. Moreover, the overexpression of COX-2 in PC cells was postulated to be associated with greater invasiveness of this malignancy and promotion of angiogenesis. Recent data suggest that combination of COX-2 inhibitor (Celecoxib) with gemcitabine and irinotecan could be an active treatment for non-operable PC[152]. These clinical observations have been supported by the results of in vitro studies. Inhibition of COX-2 by non-steroidal anti-inflammatory drugs causes a dose-dependent block of pancreatic cell line proliferation[156]. According to recent reports, the anti-tumor activity of class I histone deacetylase (HDAC) inhibitors in human PC model is significantly improved by the simultaneous inhibition of COX-2[157]. Taken together, the results of clinical and in vitro observations suggest that COX-2 plays an important role in PC development. The up-regulation of COX-2 in PC cells and its role in carcinogenesis are probably related to inflammation. The anti-cancer action of COX-2 inhibitors is most likely associated with the reduction of inflammation that can contribute to cell proliferation. Several authors revealed that many malignancies, including PC, result from a chronic inflammatory process[158]. According to Jackson and Evers[151], several signaling pathways involving COX-2, NF-kappa B and phosphatidyl inositol 3-kinase may constitute a link between inflammation and carcinogenesis.
Overexpression of FASN is associated with significantly enhanced proliferation of non-tumorigenic mammary[111] and prostate[159] epithelial cells. On the other hand, siRNA-mediated silencing of FASN gene expression or inhibition of FASN activity by pharmacological (synthetic or natural) agents leads to growth arrest of some cancer and normal cells[160-162]. Moreover, FASN inhibitors suppress the synthesis of DNA and induce apoptosis in cancer cell lines[163]. Previous studies confirmed the association between FASN activity and cell cycle progression[161,164]. However, activity of FASN was not reflected by cell cycle progression in some experimental models, e.g. MCF7 cell line[165]. Also siRNA-mediated knockdown of FASN gene expression did not cause a significant growth arrest in PC cell line (Panc-1)[105]. Therefore, the results published thus far do not present sufficient evidence for the role of FASN in cell cycle regulation, especially in PC cells. Nevertheless, the FASN knockdown in Panc-1 cells were revealed to show reduced resistance to gemcitabine[105].
The results of in vitro studies and clinical observations suggest that elevated expression of FASN gene in cancer cells is related to markedly worse prognosis. Overexpression of FASN gene was proved to be associated with cancer progression, higher risk of recurrence and shorter survival of patients with breast cancer[5,166], prostate cancer[167], melanoma[168], nephroblastoma[169], renal cell carcinoma[170], endometrial carcinoma[171], colorectal carcinoma[172], ovarian cancer[173], squamous cell carcinoma of the lung[174], head and neck squamous cell carcinoma[175], and squamous cell carcinoma of the tongue[176]. CD44 is a transmembrane glycoprotein which is involved in tumor progression and metastasis[177]. Interaction between CD44 and c-MET (tyrosine kinase), a proto-oncogene involved in several processes (including tumor growth, invasion, and metastasis)[178], is essential for activation of the latter and down-stream signaling in some malignancies[179]. Interestingly, inhibition of FASN and ACLY in human colorectal cancer cell lines (KM20, HCT116) is associated with reduced expression of CD44. This is attributed to attenuated activation of c-MET, AKT, FAK, and paxillin, factors affecting adhesion, migration and invasion of cancer cells[180]. The abovementioned phenomenon was reflected by lower metastatic potential of colorectal cancer cells. The data suggest a direct link between lipogenic enzyme activity (FASN and ACLY) and tumor progression to a metastatic phenotype. As the inhibition of FASN is related to decreased phosphorylation of c-Met in diffuse large B-cell lymphoma[181] and prostate cancer[182], one can surmise that lipogenesis is feature of metastatic cancers, including PC. However, to date there are no evidence confirming this hypothesis.
Overexpression of FASN gene is associated with poor prognosis in PC patients[96,104,105]. As previously mentioned, the overexpression of FASN gene may be associated with gemcitabine resistance of PC cells[105], and the inhibition of FASN enhances the cytotoxicity of this agent[105]. Similar phenomenon was observed in the case of human breast cancer cells and ovarian cancer cells. According to Menendez group, the inhibition of FASN is associated with enhanced cytotoxicity of docetaxel, vinorelbine, paclitaxel, 5-fluorouracil, and herceptin in the Her-2 positive breast cancer cell lines and ovarian cancer cells[183-187].
At present there is no sufficiently specific and sensitive serum (plasma) marker of PC. Ca19-9, the most widely used marker of this malignancy (the sensitivity up to 80%), is also elevated in other conditions, including chronic pancreatitis and cholangitis, as well as in other tumors[188,189]. Moreover, Ca19-9 is not useful in detecting early stages of PC[190]. According to some authors, circulating micro-RNA (miR-21, mir-210, mir155, mir196a) could constitute novel diagnostic biomarkers of PC[191,192]. Proteomic analyses of human PCs revealed numerous differentially regulated proteins, which could be involved in the progression of this malignancy, and, consequently, could act as its biomarkers, determined in pancreatic juice and in serum[193]. Also up-regulation of numerous proteins, which can be used as biomarkers of PC, has been reported recently[194]. However, despite extensive studies, we still lack a valid approach for detection of PC, especially its early stages, and sufficiently specific and sensitive biomarkers of this malignancy.
The fact that cancer cells and the normal cells of surrounding tissues are characterized by differential expression patterns of FASN suggests that serum levels of FASN may constitute a good biomarker of malignancy. Indeed, up-regulation of FASN in cancer cells was proved to be associated with increased serum levels of this enzyme in patients with some malignancies. The serum FASN level measured by ELISA in breast, prostate, colon, and ovarian cancer patients was significantly higher than in healthy controls[195-197]. Moreover, an increase in the serum levels of FASN proved to be proportional to the clinical stage of colorectal cancer and breast cancer[196,198]. The ELISA-determined serum levels of FASN were also elevated in patients with PC and intraductal papillary mucinous neoplasm[98]. Interestingly, the serum FASN levels of most PC patients decreased after resection of this malignancy[98]. This suggests that the elevated serum level of FASN reflects its up-regulation in PC cells. However, increased levels of FASN were also found in sera of patients with chronic pancreatitis[98]. This suggests that this parameter is not a PC-specific biomarker. Nevertheless, the serum levels of FASN could potentially add to the panel of markers used in the monitoring of individuals at high risk of PC.
According to some authors, PC patients show increased proportion of total MUFA in all plasma lipid classes, a feature which is associated with increased delta 9 desaturase (SCD1) and delta 5 desaturase indices[128]. Moreover, the association between longer survival of PC patients and higher level of eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and with lower SCD1 index was demonstrated[128]. Recently, Yabushita et al[199] documented a significant decrease in serum (and pancreatic) level of palmitoleic acid in an experimental model of PDAC, and suggested that this FA could serve as a biomarker of human PC. Palmitoleic acid is a monounsaturated FA (16:1 n-9). It can be synthesized from palmitic acid, the main product of FASN, or may originate from diet. Conversion of palmitic acid to palmitoleic acid is catalyzed by SCD1, which is up-regulated in some malignancies including PC[128,200]. The reason for decrease in palmitoleic acid in patients with PC is not clear, as due to higher activity of SCD1, elevated level of this FA should be rather anticipated. Despite unknown molecular basis for the decreased serum and tissue concentration of palmitoleic acid the diagnostic value of this finding should be verified in patients with PC. Chavarro et al[201] showed that blood levels of some MUFAs including myristoleic acid (14:1 n-5), palmitoleic acid, and oleic acid (18:1 n-9), were associated with higher incidence of prostate cancer. This relationship was the strongest in the case of palmitoleic acid.
Recently Zhang et al[202] reported that PC can be diagnosed by means of 1H nuclear magnetic resonance (NMR)-based metabonomic profiles. These authors showed that numerous plasma metabolites, including lipids, are either elevated (e.g., VLDL) or decreased (e.g., HDL, LDL and 3-hydroxybutyrate) in patients with this malignancy.
Yabushita et al[199] revealed that serum chenodeoxycholic acid, a major constituent of bile acids (which play a key role in lipid digestion in the alimentary tract), is elevated in the experimental model of PDAC. Also Urayama et al[203] claimed on elevated serum levels of some bile acids (taurocholic acid and tauroursodeoxycholic acid) in PC patients.
Overall, several PC-characteristic features of lipids metabolism have been found: (1) elevated serum level of FASN; (2) elevated serum levels of EPA, DHA and VLDL; (3) decreased serum levels of palmitoleic acid, HDL, LDL and 3-hydroxybutyrate, and (4) elevated serum levels of bile acids. All these parameters could serve as additional markers of PC.
Up-regulation of lipogenic enzymes in PC cells and resultant enhanced synthesis of lipids[19,96,104,105] seem to occur early in tumorigenesis and can be associated with the progression of the disease. Therefore, metabolic imaging with lipid precursor tracers: 11C-acetate, 18F-fluoroacetate (as a substrates for FA synthesis), and 11C-choline, 18F-fluorocholine (as a substrate for fosphatidylcholine synthesis), may constitute a novel imaging technique for diagnosis of PC, even at the very early stages of this malignancy. It is of note that 11C-acetate and 11C-choline have been successfully used for detecting primary prostate cancer, as well as metastases and recurrence of this malignancy[204]. However, both 11C-acetate and 11C-choline cannot be used in case of small metastatic foci[205]. Moreover, the sensitivity of 11C-acetate in the detection of prostate cancer is decreased in patients whose PSA level is lower than 3 ng/mL[204]. Finally it should be remembered that the incorporation of 11C-acetate (or its analogue 18F-fluoroacetate) to lipids is determined not only by FASN activity, but also by the activity of acetyl-CoA synthetase[206].
Chemotherapy provides only modest improvement in pancreatic cancer patients. Effective molecular therapeutic strategy requires characteristic features of the disease to be identified. As previously mentioned the values of some parameters of lipids synthesis, namely the expression of FASN gene and resultant activity of FASN, are significantly higher in cancer cells than in adjacent normal cells. This suggests that inhibition of FASN could constitute a selective therapeutic approach in cancer patients. Possible application of FASN as a therapeutic target is sustained by the results of many studies which showed that pharmacological blockade of this enzyme exerted cytostatic and cytotoxic effects to several tumor cells[97,109,125,207-216]. Pharmacological blockade of other enzymes involved in lipogenic pathway such as ACLY[18,209,217,218], ACCA[219-221], SCD1[222-225], and acyl-CoA synthetase[209], could also be an effective strategy for cancer treatment. Table 5 lists lipogenic enzymes inhibitor which can be potential antitumor drugs.
| Enzyme name | Inhibitor | Type of neoplasm | Ref. |
| Fatty acid synthase (FASN) | Cerulenin | Breast cancer, | [303] |
| ovarian cancer | [304] | ||
| C75 | Breast cancer | [216] | |
| Pancreatic cancer | [232] | ||
| Epigallocatechin-3-gallate (EGG) | Prostate cancer | [228] | |
| C93 | Lung cancer | [305] | |
| Ovarian cancer | [306] | ||
| Luteolin | Breast cancer, ovarian cancer | [228] | |
| Pancreatic cancer | [232] | ||
| Orlistat | Prostate cancer | [307] | |
| ATP citrate lyase (ACLY) | SB-204990 hydroxycitrate | Lung cancer | [308] |
| Brest cancer | [18] | ||
| Pancreatic cancer | [101] | ||
| Acetyl-CoA carboxylase (ACCA) | Soraphen A TOFA | Prostate cancer | [309] |
| Lung cancer, colon cancer | [310] | ||
| Stearoyl-CoA desaturase (SCD1) | CVT-11127 TOFA | Lung cancer | [222] |
| Colon cancer | [225] | ||
| Acetyl-CoA synthetase (ACS) | Triacsin c | Various cancers cell lines | [311] |
Similar to other malignancies, the overexpression of FASN observed in PC cells is associated with poor prognosis[96,104]. This suggest that FASN is involved in PC cell survival and its inhibition could constitute an effective strategy for PC treatment. Irresponsiveness to chemotherapy and radiotherapy is an important feature of PC. According to Yang et al[105], overexpression of FASN can be associated with resistance to gemcitabine and radiotherapy in PC patients. The exact molecular mechanism by which FASN induce gemcitabine resistance of PC cells is unknown. As the elevated expression of this molecule was proved to protect breast cancer cells from drug-induced apoptosis[165], also the FASN-induced resistance of PC cells to gemcitabine can result from similar mechanism. C75 (trans-4-carboxy-5-octyl-3-methylenebutyrolactone), a synthetic analog of natural cerulenin (isolated from Cephalosporum caerulens), is an inhibitor of FASN most often used in experimental models. This antitumor activity of this agent was documented in the case of human breast cancer[109], prostate cancer[226], ovary cancer[227] and mesothelioma[215] cell lines. Also many green tea polyphenols (e.g., EGCG-epigallocatechin gallate or ECG-epicatechin gallate) and plant-derived flavonoids (such as luteoin) showed inhibitory effect to FASN[208]. Green tea polyphenols down-regulate FASN gene expression and induce apoptosis in human prostate cancer[228-230]. Luteolin (natural flawonoid) inhibits FASN in vitro and induces cytotoxic effects in breast, prostate cancer and hepatocelullar carcinoma cells[231]. Moreover, the consumption of flavonoid rich foods was revealed to decrease the incidence of some malignancies[105]. Harris et al[232] studied the effect of FASN inhibitors (C75 and some phytochemicals) on the in vitro proliferation of PC cells (MIA PaCa-2). They found that C75 and luteolin decreased proliferation of these cells at a similar dose. Also other tested phytochemicals, quercetin (flavonoid) and resveratrol (stilbenoid), inhibited the proliferation albeit, at significantly higher concentrations. The same authors revealed that the inhibitory effect of luteolin against PC cells results from three mechanisms: decreased synthesis of FA, and nucleic acids and decreased energy production. In contrast quercetin and resveratrol (natural inhibitors of FASN), which showed weaker inhibitory potential affect mainly glycogen metabolism. Collectively, the results published by Harris et al[232] suggest that the blockade of FASN by some flavonoids could lead to inhibition of pancreatic cells proliferation, similarly as in other cancer cells.
The results of clinical observations suggests that the incidence of cancer in diabetic patients, treated with metformin (an oral hypoglycemic drug, N,N’-dimethyl biguanide) is lower than in individuals with diabetes who do not receive this drug[233-236]. The anticancer properties of metformin were also confirmed by in vitro studies[237,238]. Recently, Nair et al[239] reported that metformin inhibits PC cell proliferation and tumor growth via down-regulation of Sp transcription factors and Sp regulated genes. Noticeably, FASN is one of the Sp regulated genes[240]. Thus, one can assume that the metformin induced blockade of PC cell proliferation and tumor growth is at least partially associated with indirect inhibition of FASN activity and lipid synthesis.
The anticancer potential of statins, inhibitors of HMG-CoA reductase also have been studied in vitro with various cancers cells lines. The antitumor effects of lipophilic statins (e.g., lovastatin, simvastatin) resulted mainly from suppression of proliferation and promotion of apoptosis[150]. The chemopreventive effects of statins have been also reported in PC cell lines[241-243], and in mouse model of PC[244]. Available data from analyses on large human populations show, that daily intake of statins, in doses for cardiovascular event prevention, is not associated with the risk of PC[245-247]. However some recent data suggests that in subgroup of male smokers statins use may reduce the odds of PC[248], and is associated with better survival in diabetic patients[249]. The combination of statins and a FASN inhibitors used in an anticancer therapy would be of particular interest, but until now there are no data published regarding such approach.
In summary, the results presented above suggest that inhibitors of FASN (and inhibitors of other lipogenic enzymes) constitute promising anticancer agents. However, most of the known FASN inhibitors which can be potentially used as anticancer drugs displayed some side effects[250]. Nevertheless, the evidence of PC cells proliferation blockade resulting from direct or indirect inhibition of FASN, and potential involvement of FASN in gemcitabine (chemotherapeutic) resistance, substantiate further research on the role of this molecule in the biology and therapy of pancreatic malignancies. Moreover, there is an urgent need for specific/selective, side effect free inhibitors of FASN, which can be used in treatment of PC.
Similar to other malignancies, the reprogramming of lipid metabolism in PDAC, is closely connected with tumor development, growth, and progression. Hypoxia, activity of oncogenic factors, or the loss of tumor suppressors lead to significant changes in lipid biosynthesis and metabolism. KRAS, together with MYC and HIF1α, either increase the use of glucose and glutamine as substrates for FA synthesis, or regulate the lipogenesis directly. SFA and MUFA, (produced by FASN and SCD1 or taken up from blood), enhance the tumor growth by up-regulation of some oncogenic factors. FA built into phospholipids (together with caveolin-1) participate in the remodeling of cancer cell membrane structure. Other products of altered lipid metabolism, such as isoprene derivatives (farnesyl diphosphate or geranylgeranyl diphosphate), influence the activity of some proteins involved in tumorigenesis (enzymes and regulatory proteins) through their prenylation. Up-regulation of prostaglandin biosynthesis (from arachidonic acids) by COX2 links inflammation to PC development.
FASN is the most extensively studied enzyme involved in the lipid metabolism of PDAC cells. Its high activity in PC cells is associated with poor prognosis and increased resistance to chemo- or radiotherapy. Elevated serum levels of FASN, EPA, DHA or VLDL, and decreased serum levels of palmitoleic acid, HDL, LDL, or 3-hydroxybutyrate could serve as additional markers of PDAC. As the lipogenic activity of PDAC cells is higher than in normal cells, pharmacological inhibition of FASN and other lipogenic enzymes seems a promising therapeutic target. C75, some flavonoids, and metformin are good candidates for anticancer agents, but further research is required prior to their implementation to PDAC treatment.
P- Reviewers: Heringdorf DMZ, Makishima M S- Editor: Cui XM L- Editor: A E- Editor: Zhang DN
| 1. | Suvà ML, Riggi N, Bernstein BE. Epigenetic reprogramming in cancer. Science. 2013;339:1567-1570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 517] [Cited by in RCA: 542] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 2. | Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19834] [Cited by in RCA: 19507] [Article Influence: 780.3] [Reference Citation Analysis (0)] |
| 3. | Tennant DA, Durán RV, Boulahbel H, Gottlieb E. Metabolic transformation in cancer. Carcinogenesis. 2009;30:1269-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 165] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 4. | Warburg O. The metabolism of tumors. London: Constable 1930; . |
| 5. | Alo’ PL, Visca P, Marci A, Mangoni A, Botti C, Di Tondo U. Expression of fatty acid synthase (FAS) as a predictor of recurrence in stage I breast carcinoma patients. Cancer. 1996;77:474-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Swinnen JV, Vanderhoydonc F, Elgamal AA, Eelen M, Vercaeren I, Joniau S, Van Poppel H, Baert L, Goossens K, Heyns W. Selective activation of the fatty acid synthesis pathway in human prostate cancer. Int J Cancer. 2000;88:176-179. [PubMed] |
| 7. | Turyn J, Schlichtholz B, Dettlaff-Pokora A, Presler M, Goyke E, Matuszewski M, Kmieć Z, Krajka K, Swierczynski J. Increased activity of glycerol 3-phosphate dehydrogenase and other lipogenic enzymes in human bladder cancer. Horm Metab Res. 2003;35:565-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Kuhajda FP, Piantadosi S, Pasternack GR. Haptoglobin-related protein (Hpr) epitopes in breast cancer as a predictor of recurrence of the disease. N Engl J Med. 1989;321:636-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 99] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Kuhajda FP, Jenner K, Wood FD, Hennigar RA, Jacobs LB, Dick JD, Pasternack GR. Fatty acid synthesis: a potential selective target for antineoplastic therapy. Proc Natl Acad Sci USA. 1994;91:6379-6383. [PubMed] |
| 10. | Alò PL, Visca P, Framarino ML, Botti C, Monaco S, Sebastiani V, Serpieri DE, Di Tondo U. Immunohistochemical study of fatty acid synthase in ovarian neoplasms. Oncol Rep. 2000;7:1383-1388. [PubMed] |
| 11. | Rashid A, Pizer ES, Moga M, Milgraum LZ, Zahurak M, Pasternack GR, Kuhajda FP, Hamilton SR. Elevated expression of fatty acid synthase and fatty acid synthetic activity in colorectal neoplasia. Am J Pathol. 1997;150:201-208. [PubMed] |
| 12. | Pizer ES, Lax SF, Kuhajda FP, Pasternack GR, Kurman RJ. Fatty acid synthase expression in endometrial carcinoma: correlation with cell proliferation and hormone receptors. Cancer. 1998;83:528-537. [PubMed] |
| 13. | Boren J, Cascante M, Marin S, Comín-Anduix B, Centelles JJ, Lim S, Bassilian S, Ahmed S, Lee WN, Boros LG. Gleevec (STI571) influences metabolic enzyme activities and glucose carbon flow toward nucleic acid and fatty acid synthesis in myeloid tumor cells. J Biol Chem. 2001;276:37747-37753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 143] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, Thompson CB. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci USA. 2007;104:19345-19350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1731] [Cited by in RCA: 2000] [Article Influence: 111.1] [Reference Citation Analysis (0)] |
| 15. | Lee WN, Byerley LO, Bassilian S, Ajie HO, Clark I, Edmond J, Bergner EA. Isotopomer study of lipogenesis in human hepatoma cells in culture: contribution of carbon and hydrogen atoms from glucose. Anal Biochem. 1995;226:100-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Marin-Valencia I, Yang C, Mashimo T, Cho S, Baek H, Yang XL, Rajagopalan KN, Maddie M, Vemireddy V, Zhao Z. Analysis of tumor metabolism reveals mitochondrial glucose oxidation in genetically diverse human glioblastomas in the mouse brain in vivo. Cell Metab. 2012;15:827-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 427] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 17. | Bauer DE, Hatzivassiliou G, Zhao F, Andreadis C, Thompson CB. ATP citrate lyase is an important component of cell growth and transformation. Oncogene. 2005;24:6314-6322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 398] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 18. | Hanai JI, Doro N, Seth P, Sukhatme VP. ATP citrate lyase knockdown impacts cancer stem cells in vitro. Cell Death Dis. 2013;4:e696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 19. | Schlichtholz B, Turyn J, Goyke E, Biernacki M, Jaskiewicz K, Sledzinski Z, Swierczynski J. Enhanced citrate synthase activity in human pancreatic cancer. Pancreas. 2005;30:99-104. [PubMed] |
| 20. | Simonnet H, Alazard N, Pfeiffer K, Gallou C, Béroud C, Demont J, Bouvier R, Schägger H, Godinot C. Low mitochondrial respiratory chain content correlates with tumor aggressiveness in renal cell carcinoma. Carcinogenesis. 2002;23:759-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 263] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 21. | Szutowicz A, Kwiatkowski J, Angielski S. Lipogenetic and glycolytic enzyme activities in carcinoma and nonmalignant diseases of the human breast. Br J Cancer. 1979;39:681-687. [PubMed] |
| 22. | Lu CW, Lin SC, Chen KF, Lai YY, Tsai SJ. Induction of pyruvate dehydrogenase kinase-3 by hypoxia-inducible factor-1 promotes metabolic switch and drug resistance. J Biol Chem. 2008;283:28106-28114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 263] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 23. | Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2552] [Cited by in RCA: 2895] [Article Influence: 152.4] [Reference Citation Analysis (0)] |
| 24. | Kim JW, Gao P, Liu YC, Semenza GL, Dang CV. Hypoxia-inducible factor 1 and dysregulated c-Myc cooperatively induce vascular endothelial growth factor and metabolic switches hexokinase 2 and pyruvate dehydrogenase kinase 1. Mol Cell Biol. 2007;27:7381-7393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 508] [Cited by in RCA: 504] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 25. | Hitosugi T, Fan J, Chung TW, Lythgoe K, Wang X, Xie J, Ge Q, Gu TL, Polakiewicz RD, Roesel JL. Tyrosine phosphorylation of mitochondrial pyruvate dehydrogenase kinase 1 is important for cancer metabolism. Mol Cell. 2011;44:864-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 274] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 26. | Vizán P, Alcarraz-Vizán G, Díaz-Moralli S, Solovjeva ON, Frederiks WM, Cascante M. Modulation of pentose phosphate pathway during cell cycle progression in human colon adenocarcinoma cell line HT29. Int J Cancer. 2009;124:2789-2796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 27. | Adrych K, Smoczynski M, Stojek M, Sledzinski T, Slominska E, Goyke E, Smolenski RT, Swierczynski J. Decreased serum essential and aromatic amino acids in patients with chronic pancreatitis. World J Gastroenterol. 2010;16:4422-4427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Gatenby RA, Gillies RJ. Glycolysis in cancer: a potential target for therapy. Int J Biochem Cell Biol. 2007;39:1358-1366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 210] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 29. | DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2779] [Cited by in RCA: 3039] [Article Influence: 178.8] [Reference Citation Analysis (0)] |
| 30. | Curi R, Lagranha CJ, Doi SQ, Sellitti DF, Procopio J, Pithon-Curi TC, Corless M, Newsholme P. Molecular mechanisms of glutamine action. J Cell Physiol. 2005;204:392-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 320] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 31. | Biswas S, Lunec J, Bartlett K. Non-glucose metabolism in cancer cells--is it all in the fat. Cancer Metastasis Rev. 2012;31:689-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 32. | Colombo SL, Palacios-Callender M, Frakich N, Carcamo S, Kovacs I, Tudzarova S, Moncada S. Molecular basis for the differential use of glucose and glutamine in cell proliferation as revealed by synchronized HeLa cells. Proc Natl Acad Sci USA. 2011;108:21069-21074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 130] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 33. | Choi SY, Collins CC, Gout PW, Wang Y. Cancer-generated lactic acid: a regulatory, immunosuppressive metabolite. J Pathol. 2013;230:350-355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 186] [Cited by in RCA: 238] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 34. | Le A, Lane AN, Hamaker M, Bose S, Gouw A, Barbi J, Tsukamoto T, Rojas CJ, Slusher BS, Zhang H. Glucose-independent glutamine metabolism via TCA cycling for proliferation and survival in B cells. Cell Metab. 2012;15:110-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 770] [Cited by in RCA: 879] [Article Influence: 67.6] [Reference Citation Analysis (0)] |
| 35. | Wellen KE, Lu C, Mancuso A, Lemons JM, Ryczko M, Dennis JW, Rabinowitz JD, Coller HA, Thompson CB. The hexosamine biosynthetic pathway couples growth factor-induced glutamine uptake to glucose metabolism. Genes Dev. 2010;24:2784-2799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 304] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 36. | Metallo CM, Gameiro PA, Bell EL, Mattaini KR, Yang J, Hiller K, Jewell CM, Johnson ZR, Irvine DJ, Guarente L. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature. 2012;481:380-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1359] [Cited by in RCA: 1438] [Article Influence: 102.7] [Reference Citation Analysis (0)] |
| 37. | Mullen AR, Wheaton WW, Jin ES, Chen PH, Sullivan LB, Cheng T, Yang Y, Linehan WM, Chandel NS, DeBerardinis RJ. Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature. 2012;481:385-388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 889] [Cited by in RCA: 1029] [Article Influence: 73.5] [Reference Citation Analysis (0)] |
| 38. | Wise DR, Ward PS, Shay JE, Cross JR, Gruber JJ, Sachdeva UM, Platt JM, DeMatteo RG, Simon MC, Thompson CB. Hypoxia promotes isocitrate dehydrogenase-dependent carboxylation of α-ketoglutarate to citrate to support cell growth and viability. Proc Natl Acad Sci USA. 2011;108:19611-19616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 705] [Cited by in RCA: 804] [Article Influence: 57.4] [Reference Citation Analysis (0)] |
| 39. | Yoo H, Antoniewicz MR, Stephanopoulos G, Kelleher JK. Quantifying reductive carboxylation flux of glutamine to lipid in a brown adipocyte cell line. J Biol Chem. 2008;283:20621-20627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 252] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 40. | Scott DA, Richardson AD, Filipp FV, Knutzen CA, Chiang GG, Ronai ZA, Osterman AL, Smith JW. Comparative metabolic flux profiling of melanoma cell lines: beyond the Warburg effect. J Biol Chem. 2011;286:42626-42634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 272] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 41. | Yuneva M, Zamboni N, Oefner P, Sachidanandam R, Lazebnik Y. Deficiency in glutamine but not glucose induces MYC-dependent apoptosis in human cells. J Cell Biol. 2007;178:93-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 469] [Cited by in RCA: 545] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 42. | Karbowska J, Kochan Z, Swierczynski J. Increase of lipogenic enzyme mRNA levels in rat white adipose tissue after multiple cycles of starvation-refeeding. Metabolism. 2001;50:734-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 43. | Korczynska J, Stelmanska E, Swierczynski J. Differential effect of long-term food restriction on fatty acid synthase and leptin gene expression in rat white adipose tissue. Horm Metab Res. 2003;35:593-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 44. | Stelmanska E, Korczynska J, Swierczynski J. Tissue-specific effect of refeeding after short- and long-term caloric restriction on malic enzyme gene expression in rat tissues. Acta Biochim Pol. 2004;51:805-814. [PubMed] |
| 45. | Stelmanska E, Sucajtys-Szulc E, Korczynska J, Adrych K, Swierczynski J. Diversity of SREBP-1 gene expression in rat adipose tissue depots in response to refeeding after food restriction. Biochim Biophys Acta. 2005;1733:130-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 46. | Nogalska A, Swierczynski J. Potential role of high serum leptin concentration in age-related decrease of fatty acid synthase gene expression in rat white adipose tissue. Exp Gerontol. 2004;39:147-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 47. | Nogalska A, Pankiewicz A, Goyke E, Swierczynski J. The age-related inverse relationship between ob and lipogenic enzymes genes expression in rat white adipose tissue. Exp Gerontol. 2003;38:415-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 48. | Nogalska A, Swierczynski J. The age-related differences in obese and fatty acid synthase gene expression in white adipose tissue of rat. Biochim Biophys Acta. 2001;1533:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 49. | Swierczynski J, Goyke E, Wach L, Pankiewicz A, Kochan Z, Adamonis W, Sledzinski Z, Aleksandrowicz Z. Comparative study of the lipogenic potential of human and rat adipose tissue. Metabolism. 2000;49:594-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 50. | Fritz V, Fajas L. Metabolism and proliferation share common regulatory pathways in cancer cells. Oncogene. 2010;29:4369-4377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 153] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 51. | Zhang XD, Qin ZH, Wang J. The role of p53 in cell metabolism. Acta Pharmacol Sin. 2010;31:1208-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 52. | Hu J, Liu Z, Wang X. Does TP53 mutation promote ovarian cancer metastasis to omentum by regulating lipid metabolism. Med Hypotheses. 2013;81:515-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 53. | Dang CV. MYC, metabolism, cell growth, and tumorigenesis. Cold Spring Harb Perspect Med. 2013;3:pii: a014217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 465] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 54. | Wise DR, DeBerardinis RJ, Mancuso A, Sayed N, Zhang XY, Pfeiffer HK, Nissim I, Daikhin E, Yudkoff M, McMahon SB. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci USA. 2008;105:18782-18787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1606] [Cited by in RCA: 1543] [Article Influence: 90.8] [Reference Citation Analysis (0)] |
| 55. | Gao P, Tchernyshyov I, Chang TC, Lee YS, Kita K, Ochi T, Zeller KI, De Marzo AM, Van Eyk JE, Mendell JT. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature. 2009;458:762-765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1736] [Cited by in RCA: 1694] [Article Influence: 105.9] [Reference Citation Analysis (0)] |
| 56. | Wang PY, Ma W, Park JY, Celi FS, Arena R, Choi JW, Ali QA, Tripodi DJ, Zhuang J, Lago CU. Increased oxidative metabolism in the Li-Fraumeni syndrome. N Engl J Med. 2013;368:1027-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 57. | He Z, Liu H, Agostini M, Yousefi S, Perren A, Tschan MP, Mak TW, Melino G, Simon HU. p73 regulates autophagy and hepatocellular lipid metabolism through a transcriptional activation of the ATG5 gene. Cell Death Differ. 2013;20:1415-1424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 58. | Porteiro B, Díaz-Ruíz A, Martínez G, Senra A, Vidal A, Serrano M, Gualillo O, López M, Malagón MM, Diéguez C. Ghrelin requires p53 to stimulate lipid storage in fat and liver. Endocrinology. 2013;154:3671-3679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 59. | Hu W, Zhang C, Wu R, Sun Y, Levine A, Feng Z. Glutaminase 2, a novel p53 target gene regulating energy metabolism and antioxidant function. Proc Natl Acad Sci USA. 2010;107:7455-7460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 672] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 60. | Suzuki S, Tanaka T, Poyurovsky MV, Nagano H, Mayama T, Ohkubo S, Lokshin M, Hosokawa H, Nakayama T, Suzuki Y. Phosphate-activated glutaminase (GLS2), a p53-inducible regulator of glutamine metabolism and reactive oxygen species. Proc Natl Acad Sci USA. 2010;107:7461-7466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 527] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 61. | Daye D, Wellen KE. Metabolic reprogramming in cancer: unraveling the role of glutamine in tumorigenesis. Semin Cell Dev Biol. 2012;23:362-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 285] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 62. | Zhang C, Moore LM, Li X, Yung WK, Zhang W. IDH1/2 mutations target a key hallmark of cancer by deregulating cellular metabolism in glioma. Neuro Oncol. 2013;15:1114-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 63. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11128] [Cited by in RCA: 11835] [Article Influence: 845.4] [Reference Citation Analysis (4)] |
| 64. | Tsutsumi K, Sato N, Tanabe R, Mizumoto K, Morimatsu K, Kayashima T, Fujita H, Ohuchida K, Ohtsuka T, Takahata S. Claudin-4 expression predicts survival in pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2012;19 Suppl 3:S491-S499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 65. | Sofuni A, Iijima H, Moriyasu F, Nakayama D, Shimizu M, Nakamura K, Itokawa F, Itoi T. Differential diagnosis of pancreatic tumors using ultrasound contrast imaging. J Gastroenterol. 2005;40:518-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 120] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 66. | Koong AC, Mehta VK, Le QT, Fisher GA, Terris DJ, Brown JM, Bastidas AJ, Vierra M. Pancreatic tumors show high levels of hypoxia. Int J Radiat Oncol Biol Phys. 2000;48:919-922. [PubMed] |
| 67. | Feig C, Gopinathan A, Neesse A, Chan DS, Cook N, Tuveson DA. The pancreas cancer microenvironment. Clin Cancer Res. 2012;18:4266-4276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 841] [Cited by in RCA: 1027] [Article Influence: 85.6] [Reference Citation Analysis (0)] |
| 68. | Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40:294-309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1923] [Cited by in RCA: 1817] [Article Influence: 121.1] [Reference Citation Analysis (0)] |
| 69. | Pizzi S, Porzionato A, Pasquali C, Guidolin D, Sperti C, Fogar P, Macchi V, De Caro R, Pedrazzoli S, Parenti A. Glucose transporter-1 expression and prognostic significance in pancreatic carcinogenesis. Histol Histopathol. 2009;24:175-185. [PubMed] |
| 70. | Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell. 2008;13:472-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1553] [Cited by in RCA: 1704] [Article Influence: 100.2] [Reference Citation Analysis (0)] |
| 71. | Natsuizaka M, Ozasa M, Darmanin S, Miyamoto M, Kondo S, Kamada S, Shindoh M, Higashino F, Suhara W, Koide H. Synergistic up-regulation of Hexokinase-2, glucose transporters and angiogenic factors in pancreatic cancer cells by glucose deprivation and hypoxia. Exp Cell Res. 2007;313:3337-3348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 72. | Rong Y, Wu W, Ni X, Kuang T, Jin D, Wang D, Lou W. Lactate dehydrogenase A is overexpressed in pancreatic cancer and promotes the growth of pancreatic cancer cells. Tumour Biol. 2013;34:1523-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 144] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 73. | Ying H, Kimmelman AC, Lyssiotis CA, Hua S, Chu GC, Fletcher-Sananikone E, Locasale JW, Son J, Zhang H, Coloff JL. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell. 2012;149:656-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1246] [Cited by in RCA: 1568] [Article Influence: 120.6] [Reference Citation Analysis (0)] |
| 74. | Dang CV, Semenza GL. Oncogenic alterations of metabolism. Trends Biochem Sci. 1999;24:68-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 775] [Cited by in RCA: 758] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 75. | Chun YS, Kim MS, Park JW. Oxygen-dependent and -independent regulation of HIF-1alpha. J Korean Med Sci. 2002;17:581-588. [PubMed] |
| 76. | Kwon SJ, Song JJ, Lee YJ. Signal pathway of hypoxia-inducible factor-1alpha phosphorylation and its interaction with von Hippel-Lindau tumor suppressor protein during ischemia in MiaPaCa-2 pancreatic cancer cells. Clin Cancer Res. 2005;11:7607-7613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 77. | di Magliano MP, Logsdon CD. Roles for KRAS in pancreatic tumor development and progression. Gastroenterology. 2013;144:1220-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 316] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 78. | Macgregor-Das AM, Iacobuzio-Donahue CA. Molecular pathways in pancreatic carcinogenesis. J Surg Oncol. 2013;107:8-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 79. | Foo WC, Rashid A, Wang H, Katz MH, Lee JE, Pisters PW, Wolff RA, Abbruzzese JL, Fleming JB, Wang H. Loss of phosphatase and tensin homolog expression is associated with recurrence and poor prognosis in patients with pancreatic ductal adenocarcinoma. Hum Pathol. 2013;44:1024-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 80. | Melstrom LG, Salabat MR, Ding XZ, Milam BM, Strouch M, Pelling JC, Bentrem DJ. Apigenin inhibits the GLUT-1 glucose transporter and the phosphoinositide 3-kinase/Akt pathway in human pancreatic cancer cells. Pancreas. 2008;37:426-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 121] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 81. | Zhang M, Ma Q, Hu H, Zhang D, Li J, Ma G, Bhat K, Wu E. Stem cell factor/c-kit signaling enhances invasion of pancreatic cancer cells via HIF-1α under normoxic condition. Cancer Lett. 2011;303:108-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 82. | Skoudy A, Hernández-Muñoz I, Navarro P. Pancreatic ductal adenocarcinoma and transcription factors: role of c-Myc. J Gastrointest Cancer. 2011;42:76-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 83. | Hu HT, Ma QY, Zhang D, Shen SG, Han L, Ma YD, Li RF, Xie KP. HIF-1alpha links beta-adrenoceptor agonists and pancreatic cancer cells under normoxic condition. Acta Pharmacol Sin. 2010;31:102-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 84. | Wang L, Zhou W, Gou S, Wang T, Liu T, Wang C. Insulin promotes proliferative vitality and invasive capability of pancreatic cancer cells via hypoxia-inducible factor 1alpha pathway. J Huazhong Univ Sci Technolog Med Sci. 2010;30:349-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 85. | Chaika NV, Gebregiworgis T, Lewallen ME, Purohit V, Radhakrishnan P, Liu X, Zhang B, Mehla K, Brown RB, Caffrey T. MUC1 mucin stabilizes and activates hypoxia-inducible factor 1 alpha to regulate metabolism in pancreatic cancer. Proc Natl Acad Sci USA. 2012;109:13787-13792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 191] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 86. | Singh R, Bandyopadhyay D. MUC1: a target molecule for cancer therapy. Cancer Biol Ther. 2007;6:481-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 136] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 87. | Tsutsumida H, Swanson BJ, Singh PK, Caffrey TC, Kitajima S, Goto M, Yonezawa S, Hollingsworth MA. RNA interference suppression of MUC1 reduces the growth rate and metastatic phenotype of human pancreatic cancer cells. Clin Cancer Res. 2006;12:2976-2987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 86] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 88. | Hamidi T, Cano CE, Grasso D, Garcia MN, Sandi MJ, Calvo EL, Dagorn JC, Lomberk G, Urrutia R, Goruppi S. Nupr1-aurora kinase A pathway provides protection against metabolic stress-mediated autophagic-associated cell death. Clin Cancer Res. 2012;18:5234-5246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 89. | Cano CE, Hamidi T, Sandi MJ, Iovanna JL. Nupr1: the Swiss-knife of cancer. J Cell Physiol. 2011;226:1439-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 90. | Hamidi T, Algül H, Cano CE, Sandi MJ, Molejon MI, Riemann M, Calvo EL, Lomberk G, Dagorn JC, Weih F. Nuclear protein 1 promotes pancreatic cancer development and protects cells from stress by inhibiting apoptosis. J Clin Invest. 2012;122:2092-2103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 91. | Cano CE, Hamidi T, Garcia MN, Grasso D, Loncle C, Garcia S, Calvo E, Lomberk G, Dusetti N, Bartholin L. Genetic inactivation of Nupr1 acts as a dominant suppressor event in a two-hit model of pancreatic carcinogenesis. Gut. 2013;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 92. | Boros LG, Bassilian S, Lim S, Lee WN. Genistein inhibits nonoxidative ribose synthesis in MIA pancreatic adenocarcinoma cells: a new mechanism of controlling tumor growth. Pancreas. 2001;22:1-7. [PubMed] |
| 93. | Boros LG, Lapis K, Szende B, Tömösközi-Farkas R, Balogh A, Boren J, Marin S, Cascante M, Hidvégi M. Wheat germ extract decreases glucose uptake and RNA ribose formation but increases fatty acid synthesis in MIA pancreatic adenocarcinoma cells. Pancreas. 2001;23:141-147. [PubMed] |
| 94. | Son J, Lyssiotis CA, Ying H, Wang X, Hua S, Ligorio M, Perera RM, Ferrone CR, Mullarky E, Shyh-Chang N. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature. 2013;496:101-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1227] [Cited by in RCA: 1523] [Article Influence: 126.9] [Reference Citation Analysis (0)] |
| 95. | Sabine JR, Kopelovich L, Abraham S, Morris HP. Control of lipid metabolism in hepatomas: conversion of glutamate carbon to fatty acid carbon via citrate in several transplantable hepatomas. Biochim Biophys Acta. 1973;296:493-498. [PubMed] |
| 96. | Alo PL, Amini M, Piro F, Pizzuti L, Sebastiani V, Botti C, Murari R, Zotti G, Di Tondo U. Immunohistochemical expression and prognostic significance of fatty acid synthase in pancreatic carcinoma. Anticancer Res. 2007;27:2523-2527. [PubMed] |
| 97. | Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7:763-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1925] [Cited by in RCA: 2205] [Article Influence: 122.5] [Reference Citation Analysis (0)] |
| 98. | Walter K, Hong SM, Nyhan S, Canto M, Fedarko N, Klein A, Griffith M, Omura N, Medghalchi S, Kuhajda F. Serum fatty acid synthase as a marker of pancreatic neoplasia. Cancer Epidemiol Biomarkers Prev. 2009;18:2380-2385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 99. | Safran H, Iannitti D, Ramanathan R, Schwartz JD, Steinhoff M, Nauman C, Hesketh P, Rathore R, Wolff R, Tantravahi U. Herceptin and gemcitabine for metastatic pancreatic cancers that overexpress HER-2/neu. Cancer Invest. 2004;22:706-712. [PubMed] |
| 100. | El-Rayes BF, Zalupski MM, Shields AF, Ferris AM, Vaishampayan U, Heilbrun LK, Venkatramanamoorthy R, Adsay V, Philip PA. A phase II study of celecoxib, gemcitabine, and cisplatin in advanced pancreatic cancer. Invest New Drugs. 2005;23:583-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 101. | Guais A, Baronzio G, Sanders E, Campion F, Mainini C, Fiorentini G, Montagnani F, Behzadi M, Schwartz L, Abolhassani M. Adding a combination of hydroxycitrate and lipoic acid (METABLOC™) to chemotherapy improves effectiveness against tumor development: experimental results and case report. Invest New Drugs. 2012;30:200-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 102. | Saha AK, Ruderman NB. Malonyl-CoA and AMP-activated protein kinase: an expanding partnership. Mol Cell Biochem. 2003;253:65-70. [PubMed] |
| 103. | Adachi S, Yasuda I, Kawaguchi J, Yamauchi T, Nakashima M, Itani M, Nakamura M, Yoshioka T, Moriwaki H, Kozawa O. Ultraviolet enhances the sensitivity of pancreatic cancer cells to gemcitabine by activation of 5’ AMP-activated protein kinase. Biochem Biophys Res Commun. 2011;414:53-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 104. | Witkiewicz AK, Nguyen KH, Dasgupta A, Kennedy EP, Yeo CJ, Lisanti MP, Brody JR. Co-expression of fatty acid synthase and caveolin-1 in pancreatic ductal adenocarcinoma: implications for tumor progression and clinical outcome. Cell Cycle. 2008;7:3021-3025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 105. | Yang Y, Liu H, Li Z, Zhao Z, Yip-Schneider M, Fan Q, Schmidt CM, Chiorean EG, Xie J, Cheng L. Role of fatty acid synthase in gemcitabine and radiation resistance of pancreatic cancers. Int J Biochem Mol Biol. 2011;2:89-98. [PubMed] |
| 106. | Bandyopadhyay S, Zhan R, Wang Y, Pai SK, Hirota S, Hosobe S, Takano Y, Saito K, Furuta E, Iiizumi M. Mechanism of apoptosis induced by the inhibition of fatty acid synthase in breast cancer cells. Cancer Res. 2006;66:5934-5940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 127] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 107. | De Schrijver E, Brusselmans K, Heyns W, Verhoeven G, Swinnen JV. RNA interference-mediated silencing of the fatty acid synthase gene attenuates growth and induces morphological changes and apoptosis of LNCaP prostate cancer cells. Cancer Res. 2003;63:3799-3804. [PubMed] |
| 108. | Lupu R, Menendez JA. Pharmacological inhibitors of Fatty Acid Synthase (FASN)--catalyzed endogenous fatty acid biogenesis: a new family of anti-cancer agents. Curr Pharm Biotechnol. 2006;7:483-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 146] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 109. | Pizer ES, Thupari J, Han WF, Pinn ML, Chrest FJ, Frehywot GL, Townsend CA, Kuhajda FP. Malonyl-coenzyme-A is a potential mediator of cytotoxicity induced by fatty-acid synthase inhibition in human breast cancer cells and xenografts. Cancer Res. 2000;60:213-218. [PubMed] |
| 110. | Zecchin KG, Rossato FA, Raposo HF, Melo DR, Alberici LC, Oliveira HC, Castilho RF, Coletta RD, Vercesi AE, Graner E. Inhibition of fatty acid synthase in melanoma cells activates the intrinsic pathway of apoptosis. Lab Invest. 2011;91:232-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 111. | Vazquez-Martin A, Colomer R, Brunet J, Lupu R, Menendez JA. Overexpression of fatty acid synthase gene activates HER1/HER2 tyrosine kinase receptors in human breast epithelial cells. Cell Prolif. 2008;41:59-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 159] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 112. | Li N, Lu H, Chen C, Bu X, Huang P. Loss of fatty acid synthase inhibits the “HER2-PI3K/Akt axis” activity and malignant phenotype of Caco-2 cells. Lipids Health Dis. 2013;12:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 113. | Choi WI, Jeon BN, Park H, Yoo JY, Kim YS, Koh DI, Kim MH, Kim YR, Lee CE, Kim KS. Proto-oncogene FBI-1 (Pokemon) and SREBP-1 synergistically activate transcription of fatty-acid synthase gene (FASN). J Biol Chem. 2008;283:29341-29354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 114. | Rosin RD. Perspectives on this issue of the IJS. Int J Surg. 2010;8:1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 316] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 115. | Bandyopadhyay S, Pai SK, Watabe M, Gross SC, Hirota S, Hosobe S, Tsukada T, Miura K, Saito K, Markwell SJ. FAS expression inversely correlates with PTEN level in prostate cancer and a PI 3-kinase inhibitor synergizes with FAS siRNA to induce apoptosis. Oncogene. 2005;24:5389-5395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 97] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 116. | Yoon S, Lee MY, Park SW, Moon JS, Koh YK, Ahn YH, Park BW, Kim KS. Up-regulation of acetyl-CoA carboxylase alpha and fatty acid synthase by human epidermal growth factor receptor 2 at the translational level in breast cancer cells. J Biol Chem. 2007;282:26122-26131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 180] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 117. | Menendez JA, Decker JP, Lupu R. In support of fatty acid synthase (FAS) as a metabolic oncogene: extracellular acidosis acts in an epigenetic fashion activating FAS gene expression in cancer cells. J Cell Biochem. 2005;94:1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 69] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 118. | Wang TF, Wang H, Peng AF, Luo QF, Liu ZL, Zhou RP, Gao S, Zhou Y, Chen WZ. Inhibition of fatty acid synthase suppresses U-2 OS cell invasion and migration via downregulating the activity of HER2/PI3K/AKT signaling pathway in vitro. Biochem Biophys Res Commun. 2013;440:229-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 119. | Morad SA, Messner MC, Levin JC, Abdelmageed N, Park H, Merrill AH, Cabot MC. Potential role of acid ceramidase in conversion of cytostatic to cytotoxic end-point in pancreatic cancer cells. Cancer Chemother Pharmacol. 2013;71:635-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 120. | Vandhana S, Coral K, Jayanthi U, Deepa PR, Krishnakumar S. Biochemical changes accompanying apoptotic cell death in retinoblastoma cancer cells treated with lipogenic enzyme inhibitors. Biochim Biophys Acta. 2013;1831:1458-1466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 121. | Vizan P, Boros LG, Figueras A, Capella G, Mangues R, Bassilian S, Lim S, Lee WN, Cascante M. K-ras codon-specific mutations produce distinctive metabolic phenotypes in NIH3T3 mice [corrected] fibroblasts. Cancer Res. 2005;65:5512-5515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 94] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 122. | Weinberg F, Hamanaka R, Wheaton WW, Weinberg S, Joseph J, Lopez M, Kalyanaraman B, Mutlu GM, Budinger GR, Chandel NS. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci USA. 2010;107:8788-8793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1138] [Cited by in RCA: 1368] [Article Influence: 91.2] [Reference Citation Analysis (0)] |
| 123. | Boros LG, Lerner MR, Morgan DL, Taylor SL, Smith BJ, Postier RG, Brackett DJ. [1,2-13C2]-D-glucose profiles of the serum, liver, pancreas, and DMBA-induced pancreatic tumors of rats. Pancreas. 2005;31:337-343. [PubMed] |
| 124. | Jiang P, Du W, Wang X, Mancuso A, Gao X, Wu M, Yang X. p53 regulates biosynthesis through direct inactivation of glucose-6-phosphate dehydrogenase. Nat Cell Biol. 2011;13:310-316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 621] [Cited by in RCA: 606] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 125. | Zadra G, Photopoulos C, Loda M. The fat side of prostate cancer. Biochim Biophys Acta. 2013;1831:1518-1532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 215] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 126. | Rysman E, Brusselmans K, Scheys K, Timmermans L, Derua R, Munck S, Van Veldhoven PP, Waltregny D, Daniëls VW, Machiels J. De novo lipogenesis protects cancer cells from free radicals and chemotherapeutics by promoting membrane lipid saturation. Cancer Res. 2010;70:8117-8126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 547] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 127. | Fritz V, Benfodda Z, Rodier G, Henriquet C, Iborra F, Avancès C, Allory Y, de la Taille A, Culine S, Blancou H. Abrogation of de novo lipogenesis by stearoyl-CoA desaturase 1 inhibition interferes with oncogenic signaling and blocks prostate cancer progression in mice. Mol Cancer Ther. 2010;9:1740-1754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 218] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 128. | Macášek J, Vecka M, Žák A, Urbánek M, Krechler T, Petruželka L, Staňková B, Zeman M. Plasma fatty acid composition in patients with pancreatic cancer: correlations to clinical parameters. Nutr Cancer. 2012;64:946-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 129. | Michel V, Bakovic M. Lipid rafts in health and disease. Biol Cell. 2007;99:129-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 236] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 130. | Patlolla JM, Swamy MV, Raju J, Rao CV. Overexpression of caveolin-1 in experimental colon adenocarcinomas and human colon cancer cell lines. Oncol Rep. 2004;11:957-963. [PubMed] |
| 131. | Kim HA, Kim KH, Lee RA. Expression of caveolin-1 is correlated with Akt-1 in colorectal cancer tissues. Exp Mol Pathol. 2006;80:165-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 132. | Fiucci G, Ravid D, Reich R, Liscovitch M. Caveolin-1 inhibits anchorage-independent growth, anoikis and invasiveness in MCF-7 human breast cancer cells. Oncogene. 2002;21:2365-2375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 205] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 133. | Fong A, Garcia E, Gwynn L, Lisanti MP, Fazzari MJ, Li M. Expression of caveolin-1 and caveolin-2 in urothelial carcinoma of the urinary bladder correlates with tumor grade and squamous differentiation. Am J Clin Pathol. 2003;120:93-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 134. | Kato K, Hida Y, Miyamoto M, Hashida H, Shinohara T, Itoh T, Okushiba S, Kondo S, Katoh H. Overexpression of caveolin-1 in esophageal squamous cell carcinoma correlates with lymph node metastasis and pathologic stage. Cancer. 2002;94:929-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 172] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 135. | Li L, Yang G, Ebara S, Satoh T, Nasu Y, Timme TL, Ren C, Wang J, Tahir SA, Thompson TC. Caveolin-1 mediates testosterone-stimulated survival/clonal growth and promotes metastatic activities in prostate cancer cells. Cancer Res. 2001;61:4386-4392. [PubMed] |
| 136. | Rakheja D, Kapur P, Hoang MP, Roy LC, Bennett MJ. Increased ratio of saturated to unsaturated C18 fatty acids in colonic adenocarcinoma: implications for cryotherapy and lipid raft function. Med Hypotheses. 2005;65:1120-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 137. | Suzuoki M, Miyamoto M, Kato K, Hiraoka K, Oshikiri T, Nakakubo Y, Fukunaga A, Shichinohe T, Shinohara T, Itoh T. Impact of caveolin-1 expression on prognosis of pancreatic ductal adenocarcinoma. Br J Cancer. 2002;87:1140-1144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 115] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 138. | Louie SM, Roberts LS, Mulvihill MM, Luo K, Nomura DK. Cancer cells incorporate and remodel exogenous palmitate into structural and oncogenic signaling lipids. Biochim Biophys Acta. 2013;1831:1566-1572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 111] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 139. | Kuemmerle NB, Rysman E, Lombardo PS, Flanagan AJ, Lipe BC, Wells WA, Pettus JR, Froehlich HM, Memoli VA, Morganelli PM. Lipoprotein lipase links dietary fat to solid tumor cell proliferation. Mol Cancer Ther. 2011;10:427-436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 218] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 140. | Zaidi N, Lupien L, Kuemmerle NB, Kinlaw WB, Swinnen JV, Smans K. Lipogenesis and lipolysis: the pathways exploited by the cancer cells to acquire fatty acids. Prog Lipid Res. 2013;52:585-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 387] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 141. | Swierczynski J, Sledzinski T. The role of adipokines and gastrointestinal tract hormones in obesity. Principles of metabolic surgery. Berlin Heidelberg: Spriger 2012; 53-79. |
| 142. | Chung YT, Matkowskyj KA, Li H, Bai H, Zhang W, Tsao MS, Liao J, Yang GY. Overexpression and oncogenic function of aldo-keto reductase family 1B10 (AKR1B10) in pancreatic carcinoma. Mod Pathol. 2012;25:758-766. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 143. | Gallego O, Ruiz FX, Ardèvol A, Domínguez M, Alvarez R, de Lera AR, Rovira C, Farrés J, Fita I, Parés X. Structural basis for the high all-trans-retinaldehyde reductase activity of the tumor marker AKR1B10. Proc Natl Acad Sci USA. 2007;104:20764-20769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 156] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 144. | Quinn AM, Harvey RG, Penning TM. Oxidation of PAH trans-dihydrodiols by human aldo-keto reductase AKR1B10. Chem Res Toxicol. 2008;21:2207-2215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 145. | Endo S, Matsunaga T, Mamiya H, Ohta C, Soda M, Kitade Y, Tajima K, Zhao HT, El-Kabbani O, Hara A. Kinetic studies of AKR1B10, human aldose reductase-like protein: endogenous substrates and inhibition by steroids. Arch Biochem Biophys. 2009;487:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 146. | Martin HJ, Maser E. Role of human aldo-keto-reductase AKR1B10 in the protection against toxic aldehydes. Chem Biol Interact. 2009;178:145-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 87] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 147. | Fukumoto S, Yamauchi N, Moriguchi H, Hippo Y, Watanabe A, Shibahara J, Taniguchi H, Ishikawa S, Ito H, Yamamoto S. Overexpression of the aldo-keto reductase family protein AKR1B10 is highly correlated with smokers’ non-small cell lung carcinomas. Clin Cancer Res. 2005;11:1776-1785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 233] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 148. | Ma J, Yan R, Zu X, Cheng JM, Rao K, Liao DF, Cao D. Aldo-keto reductase family 1 B10 affects fatty acid synthesis by regulating the stability of acetyl-CoA carboxylase-alpha in breast cancer cells. J Biol Chem. 2008;283:3418-3423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 131] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 149. | Novelli G, D’Apice MR. Protein farnesylation and disease. J Inherit Metab Dis. 2012;35:917-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 150. | Osmak M. Statins and cancer: current and future prospects. Cancer Lett. 2012;324:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 144] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 151. | Jackson L, Evers BM. Chronic inflammation and pathogenesis of GI and pancreatic cancers. Cancer Treat Res. 2006;130:39-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 152. | Lipton A, Campbell-Baird C, Witters L, Harvey H, Ali S. Phase II trial of gemcitabine, irinotecan, and celecoxib in patients with advanced pancreatic cancer. J Clin Gastroenterol. 2010;44:286-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 153. | Merati K, said Siadaty M, Andea A, Sarkar F, Ben-Josef E, Mohammad R, Philip P, Shields AF, Vaitkevicius V, Grignon DJ. Expression of inflammatory modulator COX-2 in pancreatic ductal adenocarcinoma and its relationship to pathologic and clinical parameters. Am J Clin Oncol. 2001;24:447-452. [PubMed] |
| 154. | Yip-Schneider MT, Barnard DS, Billings SD, Cheng L, Heilman DK, Lin A, Marshall SJ, Crowell PL, Marshall MS, Sweeney CJ. Cyclooxygenase-2 expression in human pancreatic adenocarcinomas. Carcinogenesis. 2000;21:139-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 225] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 155. | Ding XZ, Hennig R, Adrian TE. Lipoxygenase and cyclooxygenase metabolism: new insights in treatment and chemoprevention of pancreatic cancer. Mol Cancer. 2003;2:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 111] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 156. | Molina MA, Sitja-Arnau M, Lemoine MG, Frazier ML, Sinicrope FA. Increased cyclooxygenase-2 expression in human pancreatic carcinomas and cell lines: growth inhibition by nonsteroidal anti-inflammatory drugs. Cancer Res. 1999;59:4356-4362. [PubMed] |
| 157. | Peulen O, Gonzalez A, Peixoto P, Turtoi A, Mottet D, Delvenne P, Castronovo V. The anti-tumor effect of HDAC inhibition in a human pancreas cancer model is significantly improved by the simultaneous inhibition of cyclooxygenase 2. PLoS One. 2013;8:e75102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 158. | Wu Y, Antony S, Meitzler JL, Doroshow JH. Molecular mechanisms underlying chronic inflammation-associated cancers. Cancer Lett. 2013;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 205] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 159. | Migita T, Ruiz S, Fornari A, Fiorentino M, Priolo C, Zadra G, Inazuka F, Grisanzio C, Palescandolo E, Shin E. Fatty acid synthase: a metabolic enzyme and candidate oncogene in prostate cancer. J Natl Cancer Inst. 2009;101:519-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 306] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 160. | Gao Y, Lin LP, Zhu CH, Chen Y, Hou YT, Ding J. Growth arrest induced by C75, A fatty acid synthase inhibitor, was partially modulated by p38 MAPK but not by p53 in human hepatocellular carcinoma. Cancer Biol Ther. 2006;5:978-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 161. | Knowles LM, Axelrod F, Browne CD, Smith JW. A fatty acid synthase blockade induces tumor cell-cycle arrest by down-regulating Skp2. J Biol Chem. 2004;279:30540-30545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 111] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 162. | Morikawa K, Ikeda C, Nonaka M, Suzuki I. Growth arrest and apoptosis induced by quercetin is not linked to adipogenic conversion of human preadipocytes. Metabolism. 2007;56:1656-1665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 163. | Pizer ES, Chrest FJ, DiGiuseppe JA, Han WF. Pharmacological inhibitors of mammalian fatty acid synthase suppress DNA replication and induce apoptosis in tumor cell lines. Cancer Res. 1998;58:4611-4615. [PubMed] |
| 164. | Knowles LM, Smith JW. Genome-wide changes accompanying knockdown of fatty acid synthase in breast cancer. BMC Genomics. 2007;8:168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 165. | Liu H, Liu Y, Zhang JT. A new mechanism of drug resistance in breast cancer cells: fatty acid synthase overexpression-mediated palmitate overproduction. Mol Cancer Ther. 2008;7:263-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 164] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 166. | Alò PL, Visca P, Trombetta G, Mangoni A, Lenti L, Monaco S, Botti C, Serpieri DE, Di Tondo U. Fatty acid synthase (FAS) predictive strength in poorly differentiated early breast carcinomas. Tumori. 1999;85:35-40. [PubMed] |
| 167. | Shurbaji MS, Kalbfleisch JH, Thurmond TS. Immunohistochemical detection of a fatty acid synthase (OA-519) as a predictor of progression of prostate cancer. Hum Pathol. 1996;27:917-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 159] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 168. | Innocenzi D, Alò PL, Balzani A, Sebastiani V, Silipo V, La Torre G, Ricciardi G, Bosman C, Calvieri S. Fatty acid synthase expression in melanoma. J Cutan Pathol. 2003;30:23-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 109] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 169. | Camassei FD, Jenkner A, Ravà L, Bosman C, Francalanci P, Donfrancesco A, Alò PL, Boldrini R. Expression of the lipogenic enzyme fatty acid synthase (FAS) as a predictor of poor outcome in nephroblastoma: an interinstitutional study. Med Pediatr Oncol. 2003;40:302-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 170. | Horiguchi A, Asano T, Asano T, Ito K, Sumitomo M, Hayakawa M. Pharmacological inhibitor of fatty acid synthase suppresses growth and invasiveness of renal cancer cells. J Urol. 2008;180:729-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 171. | Sebastiani V, Visca P, Botti C, Santeusanio G, Galati GM, Piccini V, Capezzone de Joannon B, Di Tondo U, Alo PL. Fatty acid synthase is a marker of increased risk of recurrence in endometrial carcinoma. Gynecol Oncol. 2004;92:101-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 172. | Ogino S, Nosho K, Meyerhardt JA, Kirkner GJ, Chan AT, Kawasaki T, Giovannucci EL, Loda M, Fuchs CS. Cohort study of fatty acid synthase expression and patient survival in colon cancer. J Clin Oncol. 2008;26:5713-5720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 148] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 173. | Gansler TS, Hardman W, Hunt DA, Schaffel S, Hennigar RA. Increased expression of fatty acid synthase (OA-519) in ovarian neoplasms predicts shorter survival. Hum Pathol. 1997;28:686-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 223] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 174. | Piyathilake CJ, Frost AR, Manne U, Bell WC, Weiss H, Heimburger DC, Grizzle WE. The expression of fatty acid synthase (FASE) is an early event in the development and progression of squamous cell carcinoma of the lung. Hum Pathol. 2000;31:1068-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 112] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 175. | Silva SD, Agostini M, Nishimoto IN, Coletta RD, Alves FA, Lopes MA, Kowalski LP, Graner E. Expression of fatty acid synthase, ErbB2 and Ki-67 in head and neck squamous cell carcinoma. A clinicopathological study. Oral Oncol. 2004;40:688-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 176. | Silva SD, Perez DE, Nishimoto IN, Alves FA, Pinto CA, Kowalski LP, Graner E. Fatty acid synthase expression in squamous cell carcinoma of the tongue: clinicopathological findings. Oral Dis. 2008;14:376-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 177. | Orian-Rousseau V. CD44, a therapeutic target for metastasising tumours. Eur J Cancer. 2010;46:1271-1277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 370] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 178. | Trusolino L, Bertotti A, Comoglio PM. MET signalling: principles and functions in development, organ regeneration and cancer. Nat Rev Mol Cell Biol. 2010;11:834-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 850] [Cited by in RCA: 968] [Article Influence: 64.5] [Reference Citation Analysis (0)] |
| 179. | Orian-Rousseau V, Chen L, Sleeman JP, Herrlich P, Ponta H. CD44 is required for two consecutive steps in HGF/c-Met signaling. Genes Dev. 2002;16:3074-3086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 406] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 180. | Zaytseva YY, Rychahou PG, Gulhati P, Elliott VA, Mustain WC, O’Connor K, Morris AJ, Sunkara M, Weiss HL, Lee EY. Inhibition of fatty acid synthase attenuates CD44-associated signaling and reduces metastasis in colorectal cancer. Cancer Res. 2012;72:1504-1517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 161] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 181. | Uddin S, Hussain AR, Ahmed M, Bu R, Ahmed SO, Ajarim D, Al-Dayel F, Bavi P, Al-Kuraya KS. Inhibition of fatty acid synthase suppresses c-Met receptor kinase and induces apoptosis in diffuse large B-cell lymphoma. Mol Cancer Ther. 2010;9:1244-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 182. | Coleman DT, Bigelow R, Cardelli JA. Inhibition of fatty acid synthase by luteolin post-transcriptionally down-regulates c-Met expression independent of proteosomal/lysosomal degradation. Mol Cancer Ther. 2009;8:214-224. [PubMed] |
| 183. | Menendez JA, Colomer R, Lupu R. Inhibition of tumor-associated fatty acid synthase activity enhances vinorelbine (Navelbine)-induced cytotoxicity and apoptotic cell death in human breast cancer cells. Oncol Rep. 2004;12:411-422. [PubMed] |
| 184. | Menendez JA, Lupu R, Colomer R. Inhibition of tumor-associated fatty acid synthase hyperactivity induces synergistic chemosensitization of HER -2/ neu -overexpressing human breast cancer cells to docetaxel (taxotere). Breast Cancer Res Treat. 2004;84:183-195. [PubMed] |
| 185. | Menendez JA, Vellon L, Colomer R, Lupu R. Pharmacological and small interference RNA-mediated inhibition of breast cancer-associated fatty acid synthase (oncogenic antigen-519) synergistically enhances Taxol (paclitaxel)-induced cytotoxicity. Int J Cancer. 2005;115:19-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 86] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 186. | Vazquez-Martin A, Ropero S, Brunet J, Colomer R, Menendez JA. Inhibition of Fatty Acid Synthase (FASN) synergistically enhances the efficacy of 5-fluorouracil in breast carcinoma cells. Oncol Rep. 2007;18:973-980. [PubMed] |
| 187. | Menendez JA, Vellon L, Lupu R. The antiobesity drug Orlistat induces cytotoxic effects, suppresses Her-2/neu (erbB-2) oncogene overexpression, and synergistically interacts with trastuzumab (Herceptin) in chemoresistant ovarian cancer cells. Int J Gynecol Cancer. 2006;16:219-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 188. | Haglund C, Roberts PJ, Kuusela P, Scheinin TM, Mäkelä O, Jalanko H. Evaluation of CA 19-9 as a serum tumour marker in pancreatic cancer. Br J Cancer. 1986;53:197-202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 100] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 189. | Duffy MJ. CA 19-9 as a marker for gastrointestinal cancers: a review. Ann Clin Biochem. 1998;35:364-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 79] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 190. | Sandblom G, Granroth S, Rasmussen IC. TPS, CA 19-9, VEGF-A, and CEA as diagnostic and prognostic factors in patients with mass lesions in the pancreatic head. Ups J Med Sci. 2008;113:57-64. [PubMed] |
| 191. | Wang J, Chen J, Chang P, LeBlanc A, Li D, Abbruzzesse JL, Frazier ML, Killary AM, Sen S. MicroRNAs in plasma of pancreatic ductal adenocarcinoma patients as novel blood-based biomarkers of disease. Cancer Prev Res (Phila). 2009;2:807-813. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 434] [Cited by in RCA: 443] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 192. | Yabushita S, Fukamachi K, Tanaka H, Sumida K, Deguchi Y, Sukata T, Kawamura S, Uwagawa S, Suzui M, Tsuda H. Circulating microRNAs in serum of human K-ras oncogene transgenic rats with pancreatic ductal adenocarcinomas. Pancreas. 2012;41:1013-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 193. | Sitek B, Sipos B, Alkatout I, Poschmann G, Stephan C, Schulenborg T, Marcus K, Lüttges J, Dittert DD, Baretton G. Analysis of the pancreatic tumor progression by a quantitative proteomic approach and immunhistochemical validation. J Proteome Res. 2009;8:1647-1656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 194. | Yabushita S, Fukamachi K, Kikuchi F, Ozaki M, Miyata K, Sukata T, Deguchi Y, Tanaka H, Kakehashi A, Kawamura S. Twenty-one proteins up-regulated in human H-ras oncogene transgenic rat pancreas cancers are up-regulated in human pancreas cancer. Pancreas. 2013;42:1034-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 195. | Wang Y, Kuhajda FP, Sokoll LJ, Chan DW. Two-site ELISA for the quantitative determination of fatty acid synthase. Clin Chim Acta. 2001;304:107-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 196. | Wang Y, Kuhajda FP, Li JN, Pizer ES, Han WF, Sokoll LJ, Chan DW. Fatty acid synthase (FAS) expression in human breast cancer cell culture supernatants and in breast cancer patients. Cancer Lett. 2001;167:99-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 197. | Wang YY, Kuhajda FP, Li J, Finch TT, Cheng P, Koh C, Li T, Sokoll LJ, Chan DW. Fatty acid synthase as a tumor marker: its extracellular expression in human breast cancer. J Exp Ther Oncol. 2004;4:101-110. [PubMed] |
| 198. | Notarnicola M, Tutino V, Calvani M, Lorusso D, Guerra V, Caruso MG. Serum levels of fatty acid synthase in colorectal cancer patients are associated with tumor stage. J Gastrointest Cancer. 2012;43:508-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 199. | Yabushita S, Fukamachi K, Tanaka H, Fukuda T, Sumida K, Deguchi Y, Mikata K, Nishioka K, Kawamura S, Uwagawa S. Metabolomic and transcriptomic profiling of human K-ras oncogene transgenic rats with pancreatic ductal adenocarcinomas. Carcinogenesis. 2013;34:1251-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 200. | von Roemeling CA, Marlow LA, Wei JJ, Cooper SJ, Caulfield TR, Wu K, Tan WW, Tun HW, Copland JA. Stearoyl-CoA desaturase 1 is a novel molecular therapeutic target for clear cell renal cell carcinoma. Clin Cancer Res. 2013;19:2368-2380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 220] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 201. | Chavarro JE, Kenfield SA, Stampfer MJ, Loda M, Campos H, Sesso HD, Ma J. Blood levels of saturated and monounsaturated fatty acids as markers of de novo lipogenesis and risk of prostate cancer. Am J Epidemiol. 2013;178:1246-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 202. | Zhang L, Jin H, Guo X, Yang Z, Zhao L, Tang S, Mo P, Wu K, Nie Y, Pan Y. Distinguishing pancreatic cancer from chronic pancreatitis and healthy individuals by (1)H nuclear magnetic resonance-based metabonomic profiles. Clin Biochem. 2012;45:1064-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 203. | Urayama S, Zou W, Brooks K, Tolstikov V. Comprehensive mass spectrometry based metabolic profiling of blood plasma reveals potent discriminatory classifiers of pancreatic cancer. Rapid Commun Mass Spectrom. 2010;24:613-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 109] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 204. | Czernin J, Benz MR, Allen-Auerbach MS. PET Imaging of Prostate Cancer Using C-Acetate. PET Clin. 2009;4:163-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 205. | Fox JJ, Schöder H, Larson SM. Molecular imaging of prostate cancer. Curr Opin Urol. 2012;22:320-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 206. | Yun M, Bang SH, Kim JW, Park JY, Kim KS, Lee JD. The importance of acetyl coenzyme A synthetase for 11C-acetate uptake and cell survival in hepatocellular carcinoma. J Nucl Med. 2009;50:1222-1228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 207. | Zhao Y, Butler EB, Tan M. Targeting cellular metabolism to improve cancer therapeutics. Cell Death Dis. 2013;4:e532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 668] [Cited by in RCA: 794] [Article Influence: 66.2] [Reference Citation Analysis (0)] |
| 208. | Liu H, Liu JY, Wu X, Zhang JT. Biochemistry, molecular biology, and pharmacology of fatty acid synthase, an emerging therapeutic target and diagnosis/prognosis marker. Int J Biochem Mol Biol. 2010;1:69-89. [PubMed] |
| 209. | Mashima T, Seimiya H, Tsuruo T. De novo fatty-acid synthesis and related pathways as molecular targets for cancer therapy. Br J Cancer. 2009;100:1369-1372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 335] [Cited by in RCA: 411] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 210. | Little JL, Kridel SJ. Fatty acid synthase activity in tumor cells. Subcell Biochem. 2008;49:169-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 211. | Kridel SJ, Lowther WT, Pemble CW. Fatty acid synthase inhibitors: new directions for oncology. Expert Opin Investig Drugs. 2007;16:1817-1829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 212. | Kuhajda FP. Fatty acid synthase and cancer: new application of an old pathway. Cancer Res. 2006;66:5977-5980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 495] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 213. | Thupari JN, Pinn ML, Kuhajda FP. Fatty acid synthase inhibition in human breast cancer cells leads to malonyl-CoA-induced inhibition of fatty acid oxidation and cytotoxicity. Biochem Biophys Res Commun. 2001;285:217-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 148] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 214. | Li JN, Gorospe M, Chrest FJ, Kumaravel TS, Evans MK, Han WF, Pizer ES. Pharmacological inhibition of fatty acid synthase activity produces both cytostatic and cytotoxic effects modulated by p53. Cancer Res. 2001;61:1493-1499. [PubMed] |
| 215. | Gabrielson EW, Pinn ML, Testa JR, Kuhajda FP. Increased fatty acid synthase is a therapeutic target in mesothelioma. Clin Cancer Res. 2001;7:153-157. [PubMed] |
| 216. | Kuhajda FP, Pizer ES, Li JN, Mani NS, Frehywot GL, Townsend CA. Synthesis and antitumor activity of an inhibitor of fatty acid synthase. Proc Natl Acad Sci USA. 2000;97:3450-3454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 374] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 217. | Zu XY, Zhang QH, Liu JH, Cao RX, Zhong J, Yi GH, Quan ZH, Pizzorno G. ATP citrate lyase inhibitors as novel cancer therapeutic agents. Recent Pat Anticancer Drug Discov. 2012;7:154-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 218. | Hanai J, Doro N, Sasaki AT, Kobayashi S, Cantley LC, Seth P, Sukhatme VP. Inhibition of lung cancer growth: ATP citrate lyase knockdown and statin treatment leads to dual blockade of mitogen-activated protein kinase (MAPK) and phosphatidylinositol-3-kinase (PI3K)/AKT pathways. J Cell Physiol. 2012;227:1709-1720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 134] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 219. | Wang C, Rajput S, Watabe K, Liao DF, Cao D. Acetyl-CoA carboxylase-a as a novel target for cancer therapy. Front Biosci (Schol Ed). 2010;2:515-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 220. | Zu X, Zhong J, Luo D, Tan J, Zhang Q, Wu Y, Liu J, Cao R, Wen G, Cao D. Chemical genetics of acetyl-CoA carboxylases. Molecules. 2013;18:1704-1719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 221. | Poteet E, Choudhury GR, Winters A, Li W, Ryou MG, Liu R, Tang L, Ghorpade A, Wen Y, Yuan F. Reversing the Warburg effect as a treatment for glioblastoma. J Biol Chem. 2013;288:9153-9164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 222. | Scaglia N, Chisholm JW, Igal RA. Inhibition of stearoylCoA desaturase-1 inactivates acetyl-CoA carboxylase and impairs proliferation in cancer cells: role of AMPK. PLoS One. 2009;4:e6812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 160] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 223. | Hess D, Chisholm JW, Igal RA. Inhibition of stearoylCoA desaturase activity blocks cell cycle progression and induces programmed cell death in lung cancer cells. PLoS One. 2010;5:e11394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 156] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 224. | Minville-Walz M, Pierre AS, Pichon L, Bellenger S, Fèvre C, Bellenger J, Tessier C, Narce M, Rialland M. Inhibition of stearoyl-CoA desaturase 1 expression induces CHOP-dependent cell death in human cancer cells. PLoS One. 2010;5:e14363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 225. | Mason P, Liang B, Li L, Fremgen T, Murphy E, Quinn A, Madden SL, Biemann HP, Wang B, Cohen A. SCD1 inhibition causes cancer cell death by depleting mono-unsaturated fatty acids. PLoS One. 2012;7:e33823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 139] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 226. | Pizer ES, Pflug BR, Bova GS, Han WF, Udan MS, Nelson JB. Increased fatty acid synthase as a therapeutic target in androgen-independent prostate cancer progression. Prostate. 2001;47:102-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 114] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 227. | Wang HQ, Altomare DA, Skele KL, Poulikakos PI, Kuhajda FP, Di Cristofano A, Testa JR. Positive feedback regulation between AKT activation and fatty acid synthase expression in ovarian carcinoma cells. Oncogene. 2005;24:3574-3582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 148] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 228. | Brusselmans K, De Schrijver E, Heyns W, Verhoeven G, Swinnen JV. Epigallocatechin-3-gallate is a potent natural inhibitor of fatty acid synthase in intact cells and selectively induces apoptosis in prostate cancer cells. Int J Cancer. 2003;106:856-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 143] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 229. | Yeh CW, Chen WJ, Chiang CT, Lin-Shiau SY, Lin JK. Suppression of fatty acid synthase in MCF-7 breast cancer cells by tea and tea polyphenols: a possible mechanism for their hypolipidemic effects. Pharmacogenomics J. 2003;3:267-276. [PubMed] |
| 230. | Vergote D, Cren-Olivé C, Chopin V, Toillon RA, Rolando C, Hondermarck H, Le Bourhis X. (-)-Epigallocatechin (EGC) of green tea induces apoptosis of human breast cancer cells but not of their normal counterparts. Breast Cancer Res Treat. 2002;76:195-201. [PubMed] |
| 231. | Brusselmans K, Vrolix R, Verhoeven G, Swinnen JV. Induction of cancer cell apoptosis by flavonoids is associated with their ability to inhibit fatty acid synthase activity. J Biol Chem. 2005;280:5636-5645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 302] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 232. | Harris DM, Li L, Chen M, Lagunero FT, Go VL, Boros LG. Diverse mechanisms of growth inhibition by luteolin, resveratrol, and quercetin in MIA PaCa-2 cells: a comparative glucose tracer study with the fatty acid synthase inhibitor C75. Metabolomics. 2012;8:201-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 233. | Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330:1304-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1692] [Cited by in RCA: 1821] [Article Influence: 91.1] [Reference Citation Analysis (0)] |
| 234. | Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JM. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care. 2009;32:1620-1625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 839] [Cited by in RCA: 809] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 235. | Li D, Yeung SC, Hassan MM, Konopleva M, Abbruzzese JL. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology. 2009;137:482-488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 476] [Cited by in RCA: 467] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 236. | Ben Sahra I, Le Marchand-Brustel Y, Tanti JF, Bost F. Metformin in cancer therapy: a new perspective for an old antidiabetic drug. Mol Cancer Ther. 2010;9:1092-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 368] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 237. | Ben Sahra I, Laurent K, Loubat A, Giorgetti-Peraldi S, Colosetti P, Auberger P, Tanti JF, Le Marchand-Brustel Y, Bost F. The antidiabetic drug metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene. 2008;27:3576-3586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 633] [Cited by in RCA: 678] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 238. | Zhuang Y, Miskimins WK. Metformin induces both caspase-dependent and poly(ADP-ribose) polymerase-dependent cell death in breast cancer cells. Mol Cancer Res. 2011;9:603-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 239. | Nair V, Pathi S, Jutooru I, Sreevalsan S, Basha R, Abdelrahim M, Samudio I, Safe S. Metformin inhibits pancreatic cancer cell and tumor growth and downregulates Sp transcription factors. Carcinogenesis. 2013;34:2870-2879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 240. | Lu S, Archer MC. Sp1 coordinately regulates de novo lipogenesis and proliferation in cancer cells. Int J Cancer. 2010;126:416-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 241. | Mistafa O, Stenius U. Statins inhibit Akt/PKB signaling via P2X7 receptor in pancreatic cancer cells. Biochem Pharmacol. 2009;78:1115-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 242. | Müller C, Bockhorn AG, Klusmeier S, Kiehl M, Roeder C, Kalthoff H, Koch OM. Lovastatin inhibits proliferation of pancreatic cancer cell lines with mutant as well as with wild-type K-ras oncogene but has different effects on protein phosphorylation and induction of apoptosis. Int J Oncol. 1998;12:717-723. [PubMed] |
| 243. | Ura H, Obara T, Nishino N, Tanno S, Okamura K, Namiki M. Cytotoxicity of simvastatin to pancreatic adenocarcinoma cells containing mutant ras gene. Jpn J Cancer Res. 1994;85:633-638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 244. | Liao J, Chung YT, Yang AL, Zhang M, Li H, Zhang W, Yan L, Yang GY. Atorvastatin inhibits pancreatic carcinogenesis and increases survival in LSL-KrasG12D-LSL-Trp53R172H-Pdx1-Cre mice. Mol Carcinog. 2013;52:739-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 245. | Cui X, Xie Y, Chen M, Li J, Liao X, Shen J, Shi M, Li W, Zheng H, Jiang B. Statin use and risk of pancreatic cancer: a meta-analysis. Cancer Causes Control. 2012;23:1099-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 246. | Chiu HF, Chang CC, Ho SC, Wu TN, Yang CY. Statin use and the risk of pancreatic cancer: a population-based case-control study. Pancreas. 2011;40:669-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 247. | Bradley MC, Hughes CM, Cantwell MM, Murray LJ. Statins and pancreatic cancer risk: a nested case-control study. Cancer Causes Control. 2010;21:2093-2100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 248. | Carey FJ, Little MW, Pugh TF, Ndokera R, Ing H, Clark A, Dennison A, Metcalfe MS, Robinson RJ, Hart AR. The differential effects of statins on the risk of developing pancreatic cancer: a case-control study in two centres in the United Kingdom. Dig Dis Sci. 2013;58:3308-3312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 249. | Nakai Y, Isayama H, Sasaki T, Mizuno S, Sasahira N, Kogure H, Kawakubo K, Yamamoto N, Hirano K, Ijichi H. Clinical outcomes of chemotherapy for diabetic and nondiabetic patients with pancreatic cancer: better prognosis with statin use in diabetic patients. Pancreas. 2013;42:202-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 250. | Flavin R, Peluso S, Nguyen PL, Loda M. Fatty acid synthase as a potential therapeutic target in cancer. Future Oncol. 2010;6:551-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 422] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 251. | Migita T, Narita T, Nomura K, Miyagi E, Inazuka F, Matsuura M, Ushijima M, Mashima T, Seimiya H, Satoh Y. ATP citrate lyase: activation and therapeutic implications in non-small cell lung cancer. Cancer Res. 2008;68:8547-8554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 308] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 252. | Yancy HF, Mason JA, Peters S, Thompson CE, Littleton GK, Jett M, Day AA. Metastatic progression and gene expression between breast cancer cell lines from African American and Caucasian women. J Carcinog. 2007;6:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 253. | Varis A, Wolf M, Monni O, Vakkari ML, Kokkola A, Moskaluk C, Frierson H, Powell SM, Knuutila S, Kallioniemi A. Targets of gene amplification and overexpression at 17q in gastric cancer. Cancer Res. 2002;62:2625-2629. [PubMed] |
| 254. | Halliday KR, Fenoglio-Preiser C, Sillerud LO. Differentiation of human tumors from nonmalignant tissue by natural-abundance 13C NMR spectroscopy. Magn Reson Med. 1988;7:384-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 84] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 255. | Yahagi N, Shimano H, Hasegawa K, Ohashi K, Matsuzaka T, Najima Y, Sekiya M, Tomita S, Okazaki H, Tamura Y. Co-ordinate activation of lipogenic enzymes in hepatocellular carcinoma. Eur J Cancer. 2005;41:1316-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 192] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 256. | Milgraum LZ, Witters LA, Pasternack GR, Kuhajda FP. Enzymes of the fatty acid synthesis pathway are highly expressed in in situ breast carcinoma. Clin Cancer Res. 1997;3:2115-2120. [PubMed] |
| 257. | Cao Y, Pearman AT, Zimmerman GA, McIntyre TM, Prescott SM. Intracellular unesterified arachidonic acid signals apoptosis. Proc Natl Acad Sci USA. 2000;97:11280-11285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 316] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 258. | Mashima T, Sato S, Sugimoto Y, Tsuruo T, Seimiya H. Promotion of glioma cell survival by acyl-CoA synthetase 5 under extracellular acidosis conditions. Oncogene. 2009;28:9-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 259. | Collins MA, Bednar F, Zhang Y, Brisset JC, Galbán S, Galbán CJ, Rakshit S, Flannagan KS, Adsay NV, Pasca di Magliano M. Oncogenic Kras is required for both the initiation and maintenance of pancreatic cancer in mice. J Clin Invest. 2012;122:639-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 500] [Cited by in RCA: 601] [Article Influence: 46.2] [Reference Citation Analysis (0)] |
| 260. | Hruban RH, van Mansfeld AD, Offerhaus GJ, van Weering DH, Allison DC, Goodman SN, Kensler TW, Bose KK, Cameron JL, Bos JL. K-ras oncogene activation in adenocarcinoma of the human pancreas. A study of 82 carcinomas using a combination of mutant-enriched polymerase chain reaction analysis and allele-specific oligonucleotide hybridization. Am J Pathol. 1993;143:545-554. [PubMed] |
| 261. | Smit VT, Boot AJ, Smits AM, Fleuren GJ, Cornelisse CJ, Bos JL. KRAS codon 12 mutations occur very frequently in pancreatic adenocarcinomas. Nucleic Acids Res. 1988;16:7773-7782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 462] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 262. | Cheng JQ, Ruggeri B, Klein WM, Sonoda G, Altomare DA, Watson DK, Testa JR. Amplification of AKT2 in human pancreatic cells and inhibition of AKT2 expression and tumorigenicity by antisense RNA. Proc Natl Acad Sci USA. 1996;93:3636-3641. [PubMed] |
| 263. | Nicholson KM, Anderson NG. The protein kinase B/Akt signalling pathway in human malignancy. Cell Signal. 2002;14:381-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1197] [Cited by in RCA: 1265] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 264. | Ruggeri BA, Huang L, Wood M, Cheng JQ, Testa JR. Amplification and overexpression of the AKT2 oncogene in a subset of human pancreatic ductal adenocarcinomas. Mol Carcinog. 1998;21:81-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 265. | Aumayr K, Soleiman A, Sahora K, Schindl M, Werba G, Schoppmann SF, Birner P. HER2 Gene Amplification and Protein Expression in Pancreatic Ductal Adenocarcinomas. Appl Immunohistochem Mol Morphol. 2014;22:146-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 266. | Chou A, Waddell N, Cowley MJ, Gill AJ, Chang DK, Patch AM, Nones K, Wu J, Pinese M, Johns AL. Clinical and molecular characterization of HER2 amplified-pancreatic cancer. Genome Med. 2013;5:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 267. | Walsh N, Kennedy S, Larkin A, Corkery B, O’Driscoll L, Clynes M, Crown J, O’Donovan N. EGFR and HER2 inhibition in pancreatic cancer. Invest New Drugs. 2013;31:558-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 268. | Yamanaka Y, Friess H, Kobrin MS, Büchler M, Kunz J, Beger HG, Korc M. Overexpression of HER2/neu oncogene in human pancreatic carcinoma. Hum Pathol. 1993;24:1127-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 138] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 269. | Dang CV, Le A, Gao P. MYC-induced cancer cell energy metabolism and therapeutic opportunities. Clin Cancer Res. 2009;15:6479-6483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 590] [Cited by in RCA: 710] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 270. | Oren M. Decision making by p53: life, death and cancer. Cell Death Differ. 2003;10:431-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 808] [Cited by in RCA: 802] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 271. | Redston MS, Caldas C, Seymour AB, Hruban RH, da Costa L, Yeo CJ, Kern SE. p53 mutations in pancreatic carcinoma and evidence of common involvement of homocopolymer tracts in DNA microdeletions. Cancer Res. 1994;54:3025-3033. [PubMed] |
| 272. | Scarpa A, Capelli P, Mukai K, Zamboni G, Oda T, Iacono C, Hirohashi S. Pancreatic adenocarcinomas frequently show p53 gene mutations. Am J Pathol. 1993;142:1534-1543. [PubMed] |
| 273. | Shen L, Sun X, Fu Z, Yang G, Li J, Yao L. The fundamental role of the p53 pathway in tumor metabolism and its implication in tumor therapy. Clin Cancer Res. 2012;18:1561-1567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 274. | Hahn SA, Schutte M, Hoque AT, Moskaluk CA, da Costa LT, Rozenblum E, Weinstein CL, Fischer A, Yeo CJ, Hruban RH. DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science. 1996;271:350-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1723] [Cited by in RCA: 1669] [Article Influence: 57.6] [Reference Citation Analysis (0)] |
| 275. | Iacobuzio-Donahue CA, Song J, Parmiagiani G, Yeo CJ, Hruban RH, Kern SE. Missense mutations of MADH4: characterization of the mutational hot spot and functional consequences in human tumors. Clin Cancer Res. 2004;10:1597-1604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 276. | Liu F. SMAD4/DPC4 and pancreatic cancer survival. Commentary re: M. Tascilar et al., The SMAD4 protein and prognosis of pancreatic ductal adenocarcinoma. Clin. Cancer Res., 7: 4115-4121, 2001. Clin Cancer Res. 2001;7:3853-3856. [PubMed] |
| 277. | Katajisto P, Vallenius T, Vaahtomeri K, Ekman N, Udd L, Tiainen M, Mäkelä TP. The LKB1 tumor suppressor kinase in human disease. Biochim Biophys Acta. 2007;1775:63-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 278. | Sato N, Rosty C, Jansen M, Fukushima N, Ueki T, Yeo CJ, Cameron JL, Iacobuzio-Donahue CA, Hruban RH, Goggins M. STK11/LKB1 Peutz-Jeghers gene inactivation in intraductal papillary-mucinous neoplasms of the pancreas. Am J Pathol. 2001;159:2017-2022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 207] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 279. | Su GH, Hruban RH, Bansal RK, Bova GS, Tang DJ, Shekher MC, Westerman AM, Entius MM, Goggins M, Yeo CJ. Germline and somatic mutations of the STK11/LKB1 Peutz-Jeghers gene in pancreatic and biliary cancers. Am J Pathol. 1999;154:1835-1840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 281] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 280. | Caldas C, Hahn SA, da Costa LT, Redston MS, Schutte M, Seymour AB, Weinstein CL, Hruban RH, Yeo CJ, Kern SE. Frequent somatic mutations and homozygous deletions of the p16 (MTS1) gene in pancreatic adenocarcinoma. Nat Genet. 1994;8:27-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 785] [Cited by in RCA: 754] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 281. | Schutte M, Hruban RH, Geradts J, Maynard R, Hilgers W, Rabindran SK, Moskaluk CA, Hahn SA, Schwarte-Waldhoff I, Schmiegel W. Abrogation of the Rb/p16 tumor-suppressive pathway in virtually all pancreatic carcinomas. Cancer Res. 1997;57:3126-3130. [PubMed] |
| 282. | Ueki T, Toyota M, Sohn T, Yeo CJ, Issa JP, Hruban RH, Goggins M. Hypermethylation of multiple genes in pancreatic adenocarcinoma. Cancer Res. 2000;60:1835-1839. [PubMed] |
| 283. | Okami K, Wu L, Riggins G, Cairns P, Goggins M, Evron E, Halachmi N, Ahrendt SA, Reed AL, Hilgers W. Analysis of PTEN/MMAC1 alterations in aerodigestive tract tumors. Cancer Res. 1998;58:509-511. [PubMed] |
| 284. | Ying H, Elpek KG, Vinjamoori A, Zimmerman SM, Chu GC, Yan H, Fletcher-Sananikone E, Zhang H, Liu Y, Wang W. PTEN is a major tumor suppressor in pancreatic ductal adenocarcinoma and regulates an NF-κB-cytokine network. Cancer Discov. 2011;1:158-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 197] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 285. | Hong SM, Park JY, Hruban RH, Goggins M. Molecular signatures of pancreatic cancer. Arch Pathol Lab Med. 2011;135:716-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 286. | Iacobuzio-Donahue CA, Velculescu VE, Wolfgang CL, Hruban RH. Genetic basis of pancreas cancer development and progression: insights from whole-exome and whole-genome sequencing. Clin Cancer Res. 2012;18:4257-4265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 111] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 287. | Schleger C, Verbeke C, Hildenbrand R, Zentgraf H, Bleyl U. c-MYC activation in primary and metastatic ductal adenocarcinoma of the pancreas: incidence, mechanisms, and clinical significance. Mod Pathol. 2002;15:462-469. [PubMed] |
| 288. | Abraham SC, Wu TT, Hruban RH, Lee JH, Yeo CJ, Conlon K, Brennan M, Cameron JL, Klimstra DS. Genetic and immunohistochemical analysis of pancreatic acinar cell carcinoma: frequent allelic loss on chromosome 11p and alterations in the APC/beta-catenin pathway. Am J Pathol. 2002;160:953-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 190] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 289. | Hosoda W, Sasaki E, Murakami Y, Yamao K, Shimizu Y, Yatabe Y. BCL10 as a useful marker for pancreatic acinar cell carcinoma, especially using endoscopic ultrasound cytology specimens. Pathol Int. 2013;63:176-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 290. | Skoulidis F, Cassidy LD, Pisupati V, Jonasson JG, Bjarnason H, Eyfjord JE, Karreth FA, Lim M, Barber LM, Clatworthy SA. Germline Brca2 heterozygosity promotes Kras(G12D) -driven carcinogenesis in a murine model of familial pancreatic cancer. Cancer Cell. 2010;18:499-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 131] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 291. | Brody JR, Costantino CL, Potoczek M, Cozzitorto J, McCue P, Yeo CJ, Hruban RH, Witkiewicz AK. Adenosquamous carcinoma of the pancreas harbors KRAS2, DPC4 and TP53 molecular alterations similar to pancreatic ductal adenocarcinoma. Mod Pathol. 2009;22:651-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 292. | Trikudanathan G, Dasanu CA. Adenosquamous carcinoma of the pancreas: a distinct clinicopathologic entity. South Med J. 2010;103:903-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 293. | Nissim S, Idos GE, Wu B. Genetic markers of malignant transformation in intraductal papillary mucinous neoplasm of the pancreas: a meta-analysis. Pancreas. 2012;41:1195-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 294. | Wu J, Matthaei H, Maitra A, Dal Molin M, Wood LD, Eshleman JR, Goggins M, Canto MI, Schulick RD, Edil BH. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci Transl Med. 2011;3:92ra66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 596] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 295. | Fukushima N, Fukayama M. Mucinous cystic neoplasms of the pancreas: pathology and molecular genetics. J Hepatobiliary Pancreat Surg. 2007;14:238-242. [PubMed] |
| 296. | Kosmahl M, Wagner J, Peters K, Sipos B, Klöppel G. Serous cystic neoplasms of the pancreas: an immunohistochemical analysis revealing alpha-inhibin, neuron-specific enolase, and MUC6 as new markers. Am J Surg Pathol. 2004;28:339-346. [PubMed] |
| 297. | Kobayashi T, Ozasa M, Miyashita K, Saga A, Miwa K, Saito M, Morioka M, Takeuchi M, Takenouchi N, Yabiku T. Large solid-pseudopapillary neoplasm of the pancreas with aberrant protein expression and mutation of β-catenin: a case report and literature review of the distribution of β-catenin mutation. Intern Med. 2013;52:2051-2056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 298. | Vassos N, Agaimy A, Klein P, Hohenberger W, Croner RS. Solid-pseudopapillary neoplasm (SPN) of the pancreas: case series and literature review on an enigmatic entity. Int J Clin Exp Pathol. 2013;6:1051-1059. [PubMed] |
| 299. | Weisbrod AB, Zhang L, Jain M, Barak S, Quezado MM, Kebebew E. Altered PTEN, ATRX, CHGA, CHGB, and TP53 expression are associated with aggressive VHL-associated pancreatic neuroendocrine tumors. Horm Cancer. 2013;4:165-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 300. | Zhang J, Francois R, Iyer R, Seshadri M, Zajac-Kaye M, Hochwald SN. Current understanding of the molecular biology of pancreatic neuroendocrine tumors. J Natl Cancer Inst. 2013;105:1005-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 301. | Heaphy CM, de Wilde RF, Jiao Y, Klein AP, Edil BH, Shi C, Bettegowda C, Rodriguez FJ, Eberhart CG, Hebbar S. Altered telomeres in tumors with ATRX and DAXX mutations. Science. 2011;333:425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 915] [Cited by in RCA: 849] [Article Influence: 60.6] [Reference Citation Analysis (0)] |
| 302. | Jiao Y, Shi C, Edil BH, de Wilde RF, Klimstra DS, Maitra A, Schulick RD, Tang LH, Wolfgang CL, Choti MA. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science. 2011;331:1199-1203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1432] [Cited by in RCA: 1330] [Article Influence: 95.0] [Reference Citation Analysis (0)] |
| 303. | Pizer ES, Jackisch C, Wood FD, Pasternack GR, Davidson NE, Kuhajda FP. Inhibition of fatty acid synthesis induces programmed cell death in human breast cancer cells. Cancer Res. 1996;56:2745-2747. [PubMed] |
| 304. | Pizer ES, Wood FD, Heine HS, Romantsev FE, Pasternack GR, Kuhajda FP. Inhibition of fatty acid synthesis delays disease progression in a xenograft model of ovarian cancer. Cancer Res. 1996;56:1189-1193. [PubMed] |
| 305. | Orita H, Coulter J, Lemmon C, Tully E, Vadlamudi A, Medghalchi SM, Kuhajda FP, Gabrielson E. Selective inhibition of fatty acid synthase for lung cancer treatment. Clin Cancer Res. 2007;13:7139-7145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 91] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 306. | Zhou W, Han WF, Landree LE, Thupari JN, Pinn ML, Bililign T, Kim EK, Vadlamudi A, Medghalchi SM, El Meskini R. Fatty acid synthase inhibition activates AMP-activated protein kinase in SKOV3 human ovarian cancer cells. Cancer Res. 2007;67:2964-2971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 118] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 307. | Kridel SJ, Axelrod F, Rozenkrantz N, Smith JW. Orlistat is a novel inhibitor of fatty acid synthase with antitumor activity. Cancer Res. 2004;64:2070-2075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 465] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 308. | Hatzivassiliou G, Zhao F, Bauer DE, Andreadis C, Shaw AN, Dhanak D, Hingorani SR, Tuveson DA, Thompson CB. ATP citrate lyase inhibition can suppress tumor cell growth. Cancer Cell. 2005;8:311-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 723] [Cited by in RCA: 813] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 309. | Beckers A, Organe S, Timmermans L, Scheys K, Peeters A, Brusselmans K, Verhoeven G, Swinnen JV. Chemical inhibition of acetyl-CoA carboxylase induces growth arrest and cytotoxicity selectively in cancer cells. Cancer Res. 2007;67:8180-8187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 244] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 310. | Wang C, Xu C, Sun M, Luo D, Liao DF, Cao D. Acetyl-CoA carboxylase-alpha inhibitor TOFA induces human cancer cell apoptosis. Biochem Biophys Res Commun. 2009;385:302-306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 86] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 311. | Mashima T, Oh-hara T, Sato S, Mochizuki M, Sugimoto Y, Yamazaki K, Hamada J, Tada M, Moriuchi T, Ishikawa Y. p53-defective tumors with a functional apoptosome-mediated pathway: a new therapeutic target. J Natl Cancer Inst. 2005;97:765-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 95] [Article Influence: 4.8] [Reference Citation Analysis (0)] |